Abstract
Ripened foods of animal origin comprise meat products and dairy products, being transformed by the wild microbiota which populates the raw materials, generating highly appreciated products over the world. Together with this beneficial microbiota, both pathogenic and toxigenic microorganisms such as Listeria monocytogenes, Salmonella enterica, Staphylococcus aureus, Clostridium botulinum, Escherichia coli, Candida spp., Penicillium spp. and Aspergillus spp., can contaminate these products and pose a risk for the consumers. Thus, effective strategies to hamper these hazards are required. Additionally, consumer demand for clean label products is increasing. Therefore, the manufacturing sector is seeking new efficient, natural, low-environmental impact and easy to apply strategies to counteract these microorganisms. This review gathers different approaches to maximize food safety and discusses the possibility of their being applied or the necessity of new evidence, mainly for validation in the manufacturing product and its sensory impact, before being implemented as preventative measures in the Hazard Analysis and Critical Control Point programs.
Keywords:
dry-cured meat; ripened cheese; pathogens; molds; yeasts; lactic acid bacteria; essential oils 1. Introduction
Ripened foods of animal origin can be divided into two main groups: meat products (dry-cured pieces and dry-cured fermented products, the latter commonly made via mincing and stuffing) and dairy products (mainly, ripened cheeses). These traditional foodstuffs are well-known and highly appreciated all over the world.
The environmental conditions throughout the ripening process of which these animal-derived products undergo favor the growth of diverse microbial populations that deeply contribute to their transformation. Most of these microorganisms, such as some molds, yeasts, gram-positive catalase-positive cocci (GCC+) and lactic acid bacteria (LAB) [1,2,3,4,5,6] have a positive impact on the development of the required sensory characteristics. The presence of this beneficial microbiota is not unique in these products since they are generally exposed to the wild microbiota of the processing environment. Additionally, this processing rarely entails any sanitizing step; pasteurization in artisanal dairy products could be performed, although it is not common; for meat products, however, it is negligible. Thus, the contamination of these products with pathogenic or toxigenic microorganisms usually leads to their development during the industrial ripening of these meat products. In this respect, bacteria such as Listeria monocytogenes, Salmonella spp., Staphylococcus aureus, Clostridium perfringens, Clostridium botulinum, Bacillus cereus and Escherichia coli are of most concern for ripened foods of animal origin [7,8,9,10,11,12,13,14,15,16,17,18]. In addition, fungal growth on their surface is also common, especially on dry-cured meat products [19,20,21]. Although these fungi similarly lead to the development of positive sensory characteristics [4,22], some of them can produce mycotoxins such as ochratoxin A (OTA), aflatoxins (AFs), cyclopiazonic acid (CPA), sterigmatocystin (STG) and citrinin (CIT) [23,24,25].
Since 2020, and through the Rapid Alert System Feed and Food (RASFF) developed by the European Union, a total of 345 notifications of pathogenic microorganisms in milk and dairy products as well as in meat and meat products were detected, along with 1 notification of the presence of mycotoxins in raw milk. Among all of them, 234 related to meat products, (28 of which were in dry-cured meat products), and 111 related to pathogenic microorganisms detected in cheese [26]. Therefore, it is crucial to understand and apply strategies that allow the survival of beneficial microorganisms in order to transform the products to be capable of hindering pathogenic or spoilage microorganisms. Furthermore, these strategies are considered as preventive measures within the Hazard Analysis and Critical Control Point (HACCP) programs of the industry.
There are several effective treatments that could be applied in ripened foods of animal origin, including physical methods (heat treatments, ionizing radiation, high hydrostatic pressures) and chemical preservatives (organic acids, antifungal compounds, nitrates and nitrites). However, these methods are not always compatible with the ripening process and can have a negative impact on the organoleptic characteristics of the final product since they are not selective, i.e., they can damage the beneficial microbiota of these ripened foods [27]. In addition, it has been reported that the inappropriate or continuous application of different synthetic compounds to control the pathogenic microorganisms could favor their resistance [28]. Moreover, consumers currently demand clean label products that are free from chemical additives and preservatives [29]. Therefore, preventive measures, such as strategies to control pathogenic or spoilage microorganism in ripened foods of animal origin, are currently based on the use of biocontrol agents (BCAs) of either microbial [30,31,32,33,34,35,36,37,38,39] or plant-based origin, such as essential oils (EOs) and spices [40,41,42,43,44,45]. These treatments should have a low environmental impact and a neutral or positive influence on the sensory properties of the ripened foods of animal origin, since their organoleptic characteristics are well defined and highly appreciated by consumers.
In the present work, the main pathogenic and toxigenic microorganisms that are able to develop in the different types of ripened foods of animal origin will be studied. Promising BCAs that are being used to reduce or eliminate them, as well as the challenges that their application entails and remain to be solved, are considered.
2. Pathogenic Bacteria in Ripened Foods and Biocontrol Strategies
2.1. Pathogenic Bacteria
It is important to monitor the growth of the microorganisms described below as they can grow in ripened food of animal origin that has not undergone any sanitizing process. Thus, these products will be mostly considered as ready-to-eat (RTE). The presence of the following described pathogens in these foods pose a considerable risk which must be controlled using different strategies, with BCAs being among the most appropriate ones.
2.1.1. Listeria monocytogenes
L. monocytogenes is a gram-positive, motile, facultative anaerobic bacterium that causes listeriosis. This foodborne pathogen can present flu-like symptoms such as fever, fatigue and gastrointestinal symptoms (nausea, vomiting and diarrhea). However, it can cause more severe life-threatening infections in high-risk population groups, such as septicemia, meningitis, meningoencephalitis, spontaneous abortion, still birth or fetal infection [46,47,48]. This pathogen has the capacity to pass three important barriers in the human host, namely the intestinal epithelium, the blood–brain barrier and the placenta, and subsequently disseminate to other organs [49].
There have been sixty-seven notifications since 2020 for milk and milk products in the whole of the European Union according to RASFF, of which about 50% were notifications in France for raw milk cheeses. Only eight notifications about the presence of L. monocytogenes in dry-cured meat products arose from diverse countries from the European Union [26]. In 2021 and 2022, among the total risks identified via the RASFF system, notifications due to the presence of L. monocytogenes in soft cheeses comprised 33.5% of the cases [26]. Specifically, in 2022, within biological contaminants, notifications due to L. monocytogenes accounted for 31.5%, with cheeses made with raw milk being involved in 57% of cases, with 100% of them originating in Europe and with France being the origin in 45.5% of cases.
2.1.2. Salmonella enterica
S. enterica is a gram-negative, motile bacterium that causes salmonellosis. Clinical manifestations are usually fever, weight loss, headache, lethargy, malaise, gastrointestinal bleeding, decreased white blood cells and platelets, and even neurological complications [46].
S. enterica invades the intestinal epithelium due to virulence factors that allow for intracellular multiplication in intestinal and immune cells such as macrophages. In this way, the bacteria reach the bloodstream, evading immune activity, and eventually reach the liver, spleen and bone marrow, where they continue to proliferate.
Eight cases of Salmonella in ripened cheese and sixteen cases in dry-cured meat products have been identified from 2020 to the present in the European Union [26].
2.1.3. Staphylococcus aureus
S. aureus is a gram-positive, nonmotile, coagulase-positive and ubiquitous bacterium. It can grow and produce thermostable enterotoxins resistant to digestive enzymes, which are responsible for staphylococcal food poisoning (SFP) [50,51]. The main symptoms of SFP are nausea, vomiting, diarrhea and abdominal pain [52], although more serious and life-threatening infections such as sepsis, necrotizing fasciitis, infective endocarditis, necrotizing pneumonia and toxic shock syndrome [53], although by far less common, are provoked directly by S. aureus invasion.
In fact, S. aureus has the ability to colonize the skin and mucous membranes of humans and warm-blooded animals. This bacterium has been isolated from a wide range of foods, such as fermented meat and cheese [54,55]. In addition, S. aureus has many virulence factors that enable for colonization, increasing its ability to trigger diseases [56].
2.1.4. Clostridium botulinum
C. botulinum is a gram-positive, obligatory anaerobic and spore-forming rod bacterium [57]. Ingestion of this preformed toxins can cause botulism with symptoms such as paralysis, nausea, vomiting, abdominal cramps, irritability, drooping eyelids, fatigue, difficulty feeding and swallowing [58].
Among the seven types of botulinum toxins (A to G), types A, B, E and F are well known to cause human illness. The severe form of food poisoning is rare, but it has a significant mortality rate [59].
One case of C. botulinum in ripened cheese has been reported since 2020 in the European Union [26].
2.1.5. Escherichia coli
E. coli is a gram-negative bacterium that is part of the intestinal microbiota. Symptoms are bloody diarrhea, abdominal pain, vomiting, hemorrhagic colitis, hemolytic uremic syndrome with acute renal failure, thrombotic thrombocytopenia purpura and even septicemia [60,61,62]. E. coli O157:H7 is one of the best-known serotypes to contain pathotypes that can cause food-borne infection in humans [46].
Since 2020, thirty-one reports of E. coli in milk and dairy products, with 90% coming from ripened cheese, have been made. Only two cases were reported for the presence of E. coli in cured and matured-meat products, namely in dry-cured sausages in Italy [26].
2.2. Microbial Biocontrol Strategies
In this section, biocontrol strategies for all pathogenic bacteria gathered in Section 2.1 are presented jointly, since most of them are used to control more than one pathogen, as displayed in Table 1, and this is how it is addressed in most of the reported studies [63,64].

Table 1.
Reduction of pathogenic bacteria viability using biocontrol agents in ripening food matrices of animal origin.
LAB are one of the most interesting groups of microorganisms that can be used as BCAs [74], mainly due to their recognized key role in fermented foods. Additionally, several strains belonging to species that are part of the LAB group have acquired QPS (Qualified Presumption of Safety) status [75]. Members of the genera Lactobacilli and members of the genera Lactococcus and Pediococcus were the most commonly explored, namely Latilactobacillus curvatus, L. sakei, Lactiplantibacillus plantarum, Limosilactobacillus fermentum, Lactococcus lactis and Pediococcus acidilactici [71,76,77,78]. Furthermore, LAB have frequently been used as starters or protective cultures due to their natural ability to dominate the microbial population of many foods. In fact, they are naturally found due to their ability to produce antimicrobial compounds, such as lactic acid and other organic acids, ethanol, diacetyl, carbon dioxide, hydrogen peroxide or bacteriocins [79,80]. The only approved bacteriocin for its use in certain ripened foods in the European Union is nisin, produced by L. lactis [81]. Pediocin, produced by Pediococcus is another type of bacteriocin which effectively preserves fermented food products such as meat, sausage products and cheeses [82].
Most of these metabolites act both individually and synergistically against pathogens [83]. It is known that LAB have a higher inhibition efficacy against pathogenic gram-positive bacteria [84]. Martín et al. [85] reported in vitro antibacterial activity of LAB isolated from traditional Spanish dry-cured fermented sausages and cheeses against L. monocytogenes (Table 1). When the biocontrol capacity of these strains was tested in traditional RTE ripened foods models, two of them were selected (Lacticaseibacillus casei 116 and L. sakei 205) because they provoked reductions higher than 2 log cycles of L. monocytogenes in cheeses and dry-cured fermented sausages models, respectively. In addition, when L. sakei 205 was inoculated into dry-cured fermented sausages, L. monocytogenes was reduced by 1.77 log CFU g−1 at the end of the ripening process of these products [65]. Furthermore, Martín et al. [86] demonstrated that the inoculation of this strain did not modify the sensory characteristics of dry-cured fermented sausages. L. casei 116 caused a decrease in L. monocytogenes of 2.2 log CFU g−1 when it was inoculated in short-ripening traditional cheeses without modifying their sensory properties [66,87]. Margalho et al. [67] indicated that greater than 3 log reduction in L. monocytogenes in Brazilian artisanal cheeses can be achieved using L. plantarum (1QB77) after 21 days of ripening. However, there have been no studies of sensory modifications due to the addition of this bacterium.
Campagnollo et al. [36] evaluated the inhibitory activity of LAB strains (Levilactobacillus brevis 2-392, L. plantarum 1-399 and 4 strains of E. faecalis (1-37, 2-49, 2-388 and 1-400)) against L. monocytogenes in microscale “Minas” Frescal and semi-hard cheese models. They demonstrated that the addition of a pool of LAB with antimicrobial properties resulted in bacteriostatic effects and inactivation of L. monocytogenes in “Minas” Frescal and “Minas” semi-hard cheeses, respectively. However, there are no available studies that display whether the inoculation of this pool of bacteria modifies the sensory characteristics of these products.
In dry-fermented Greek sausages, Pragalaki et al. [68] observed a significant inhibition of L. monocytogenes in the treatments with L. sakei 8416 and L. sakei 4413 compared to the untreated control. Furthermore, sausages produced with these LAB cultures obtained the highest scores for all sensory attributes in the study conducted by Baka et al. [88].
Kačániová et al. [69] studied the inhibitory capacity of 130 LAB isolated from “Bryndza” cheese. About 84% of the LAB isolates presented an inhibitory effect against S. enterica subsp. enterica and S. aureus subsp. aureus, with L. lactis subsp. lactis and Lactiplantibacillus paraplantarum being the most effective LAB strains to inhibit S. enterica subsp. enterica. Pragalaki et al. [68] observed a 2.2 log reduction in the E. coli O157:H7 population when L. sakei 4413 was inoculated in dry-fermented Greek sausage.
E. coli and S. aureus have been frequently studied as pathogens to be inhibited in ripened foods of animal origin. Inhibition of S. aureus growth by LAB has been related with the production of different compounds such as organic acids and bacteriocins, changes in redox potential, or combined effects of environmental stressors [89]. In a traditional dry-fermented sausage “suçuk”, the presence of L. sakei and Staphylococcus carnosus decreased S. aureus numbers from the first day on, and no S. aureus growth was observed [70]. In the presence of P. acidilactici, L. curvatus and Staphylococcus xylosus, a sub-inoculation level of S. aureus counts was determined from day 3 onwards. These results show that both starter culture preparations can reduce the growth of S. aureus at the initial ripening temperature [70]. Margalho et al. [67] studied a strain of L. plantarum (1QB77) with production of antimicrobial compounds isolated from a Brazilian artisan cheese (Minas Gerais), which showed an inhibition of S. aureus of approximately 2.3 log CFU g−1 in a microscale Cheeses model (microcheese).
The inoculation of L. plantarum PCS20 and L. delbrueckii DSM 20074 in fermented salami was able to reduce the levels of C. perfringens and Clostridium spp. by 2.0 and 1.5 log CFU g−1, respectively [71].
Excluding this report, there are scarce studies of BCAs being used against C. botulinum in these products; this is probably because of its development requirements—it is relatively easy to control with an HACCP using obstacle theory with a combination of aw, temperature and pH in each of the phases of the processing of these products [90]. Additionally, in meat products, the usual inclusion of nitrifying salts (sodium nitrate, potassium nitrate, potassium nitrite and sodium nitrite) confers a high protection against Clostridium species [91]. A novel and interesting field deserves to be explored in relation to the substitution of these salts via efficient clean-label strategies, since these preservatives have been related to colorectal cancer [92].
Concerning the use of yeasts as BCAs against pathogenic bacteria, some studies have shown the inhibitory effect of Debaryomyces hansenii, Candida spp., Geotrichum candidum and Pichia spp. against certain pathogenic microorganisms and, specifically, against L. monocytogenes [93,94]. However, most studies about yeasts with antibacterial activity are not carried out on dry-cured fermented food matrices. D. hansenii is one of the main yeasts found in dry-cured fermented meat products and cheeses because it is salt tolerant [3,95]. Nevertheless, Alía et al. [33] showed that D. hansenii 258H presented a limited action and even boosted L. monocytogenes growth in dry-cured ham slices. They also observed the upregulation of some key virulence genes and an unpleasant appearance of the product. Additionally, most of the research about the antibacterial activity of yeasts in ripened dairy products was executed using commercial culture media [96,97,98]. One study focusing on “Tilsit” cheese showed that the yeast Pichia norvegensis achieved a reduction of 1.5 log units (CFU cm−2) of L. monocytogenes in the product [95]. To summarize, the lack of studies on food matrices shows the need for further works focusing on how the yeasts act during processing and if they alter the technological or sensory characteristics.
2.3. Plant-Derivative Biocontrol Agents
Another biocontrol method to prevent the presence of pathogens is the use of plant derivatives, of which the most reported are EOs [99]. These EOs are aromatic and volatile oily extracts obtained from plant materials, including flowers, buds, roots, bark and leaves; they are composed of a mixture of phenylpropenes, terpenes and other volatile components such as thymol, carvacrol and eugenol, which are known for their high antimicrobial capacity [100,101]. The majority of EOs are safe for consumer use when used at the proper concentrations and have been generally recognized as safe (GRAS) [102]. The hydrophilic or lipophilic properties of EO constituents, type of microorganism studied and structure of their cell wall are the factors that affect the Eos’ antimicrobial activity [99]. Their application depends on the sensory impact of EOs that has been reported as one of the most negative aspects of their use [42].
The presence of Juniperus communis L. EO (0.01; 0.05 and 0.10 µL g−1) in fermented sausages inhibited the growth of foodborne pathogens such as E. coli, L. monocytogenes and Salmonella spp. and sulfite-reducing clostridia, although concentrations of more than 0.10 µL g−1 had an untypical flavor [72]. In another dry-cured sausage, ‘Chouriço de vinho’, the antimicrobial effect of EOs from herbs and spices traditionally used in seasoning against Salmonella spp., L. monocytogenes and S. aureus was assessed [42]. The bay, garlic, nutmeg, oregano, rosemary and thyme EOs at 0.005% (sensory-acceptable as assessed by consumers) reduced the counts of Salmonella spp. and L. monocytogenes in the first steps of drying. However, the thyme EO was the only one that completely inhibited the presence of S. aureus after 21 days [42]. Cinnamon, pomegranate and strawberry extracts were able to reduce the growth of L. monocytogenes in a study conducted on a cured ham-based medium. Cinnamon at 1% concentration was the most effective, reducing up to 3 log CFU mL−1 [73]. The incorporation of oregano EO in alginate films applied to slices of ham resulted in a reduction of up to 2.5 log CFU g−1 of L. monocytogenes [103]. Tea tree oil at a concentration of 0.25% was able to completely inhibit the growth of E. coli through several mechanisms of action [104]. On the other hand, S. aureus growth has been shown to be reduced, even at low concentrations of T-cadinol, which is present in a variety of essential plant oils and can lead to disintegration of the cell envelope and leakage of cytoplasm [105]. Ethanolic extract of rosemary leaves showed antimicrobial activity against Shigella sonnei, Salmonella Typhimurium and L. monocytogenes bacteria [106]. Rosemary EO showed inhibition and a bactericidal effect in vitro against S. aureus with a minimum bactericidal concentration of 5 μL mL−1 [107].
BCAs for the control of pathogenic bacteria in dry-cured ham and jerky have been little studied since these products are considered safe products due to their low water activity (aw) and salt content [108]. The inactivation of pathogens during cured ham processing has been demonstrated [109].
3. Pathogenic Yeasts in Ripened Foods and Biocontrol Strategies
3.1. Pathogenic Yeasts
Increasing interest exists in the biodiversity and ecology of yeasts in relation to various food products. This has been driven by the realization that yeasts can interact with other yeast species, as well as with other microorganisms in different ecosystems. These interactions may affect the roles that these fungi play in food [110]. The presence of yeasts in ripened foods, as well as their interaction with their autochthonous microbiota, is in most cases directly related to the improvement in the organoleptic characteristics of the final product [111,112]. In dry-cured meat products, both the proliferation of yeasts during the curing process and the addition of yeasts as starter cultures lead to improvements in texture and the production of pleasant volatile compounds [113]. All this is due to yeast metabolic processes of meat constituents, such as lipids and proteins [114]. As in meat products, yeasts are capable of deploying high metabolic activity in dairy products. Due to the variety of cheeses made from different types of milk with different maturation times, there are great physicochemical differences, which means that the microbiota, and in particular the kind of yeasts, vary from cheese to cheese.
D. hansenii is the predominant yeast species observed during the ripening of most cheeses [114], being present in 79% of all the cheeses in the study performed by Banjara et al. [115]. However, strains of Saccharomyces cerevisiae, Yarrowia lipolytica, G. candidum and Kluyveromyces marxianus have also been reported in cheese, although to a lower extent [113,114,115]. This is due to the aptitude of dairy yeasts for growing in the presence of a high salt concentration, low pH and low aw as well as its ability to metabolize lactic and citric acids [116,117,118]. On the other hand, the 91.9% of strains isolated from high moisture soft cheeses were classified as Geotrichum species. [119] (Table 2).
D. hansenii is also the dominant species in sausage manufacturing, being found at every manufacturing stage. Trichosporon ovoides, Y. lipolytica, Candida intermedia/curvata, C. parapsilosis, C. zeylanoides and Citeromyces matritensis are also present in Spanish fermented sausages, with most of them being psychrotrophic. C. intermedia/curvata, C. matritensis, C. zeylanoides and T. ovoides were detected only at the first stages of the sausage manufacturing process [120]. In Parma dry-cured ham, yeast species such as D. hansenii, Torulopsis candida and Torulopsis famata have been proved to be the predominant species. Studies of Spanish dry-cured ham showed that the yeast population profile changes significantly during processing. C. zeylanoides was the main species at the fresh stage (more than the 90% of isolates), but D. hansenii dominated the yeast population after the post-salting stage [121,122].
While yeasts are rarely associated with foodborne infections, a few studies have shown the presence of medically relevant yeast species in various cheeses. The presence of these fungi in some types of cheeses might be a regular cause of both economic and public health problems. Examples of yeasts with the ability to cause these problems include Candida spp., K. marxianus, G. candidum, D. hansenii and Pichia spp. [123], although we focused on a narrow population segment. Candida spp. are part of the normal human microbiota. They are commensal in healthy individuals but become pathogenic when the host’s defence system is compromised, causing conditions ranging from superficial mucosal to life-threatening systemic infections [117]. The genus Candida contains 163 species found in different ecosystems. Diseases are caused by some species such as C. albicans, C. tropicalis, C. krusei, C. glabrata, C. guilliermondii and C. parapsilosis [124]. To the general population, yeasts do not cause serious infections, though some species, such as C. albicans and Cryptococcus neoformans, are opportunistic pathogens that may cause infections in various organ systems, as well as general fungemia [116]. C. albicans, C. glabrata, C. parapsilosis and C. tropicalis are responsible for about 95% of Candida blood stream infections, although the vast majority of Candida spp. are not pathogenic [125]. It is known that contamination can occur due to a lack of attention to proper hand hygiene during milk production, or even due to improper cleaning of tools used to process milk and its derivatives [110,126].
Pathogenic species belonging to the genus Candida rarely occur in cheese. For instance, several Candida species, including C. albicans, have been found in cheese brine [127], but never in ripened cheese. However, Issatchenkia orientalis (teleomorph of C. krusei), Clavispora lusitaniae (teleomorph of C. lusitaniae) and Candida rugosa were seldom detected in cheese. There are some rare occurrences of species such as Candida famata/D. hansenii or C. krusei/I. orientalis in cheese [118]. When 120 samples from traditional Egyptian dairy products were analyzed, yeasts belonging to the Candida genus were identified [110] (Table 2). This raises the possibility that dairy products may be carrying pathogenic yeasts [110]. C. albicans was not reported in other analyses of yeast populations in cheese, but another opportunistic pathogenic yeast was reported in “feta” cheese [128] (Table 2). Although it has been found in brine [127], the source of these pathogenic yeasts in dairy products is not well known.
In a study analysing the microbial population of cheese, yeast species isolated from cheese were identified as C. parapsilosis, Candida catenulata, Y. lipolytica, Rhodotorula glutinis and Trichosporon species. C. parapsilosis, C. catenulata and Trichosporon spp. were also found in raw milk from different species and in several types of cheese. This is a public health concern, as it suggests that these species may survive some kind of cheese-making treatments and spread within the human population [129]. From the analysis of 45 artisanal cheese samples, a total of 251 Candida strains were isolated [126] (Table 2).
Candida non-albicans species were responsible for an increase in the proportion of cases of fungemia and other complex cases of candidiasis [130,131,132]. C. parapsilosis, isolated from some cheeses, is an emerging human pathogen capable of causing invasive candidiasis, but infection due to consumption of contaminated food has not been documented (Table 2). Y. lipolytica, isolated from cheese, is also an emerging opportunistic pathogen, although cases are rare [115] (Table 2). Some large-scale studies confirm that Y. lipolytica seldom causes infections. Only 4 isolates of Y. lipolytica were present among 6082 isolates from blood stream infections in 250 medical centers from 32 countries between 1992 and 2001 [133]. C. intermedia, rarely reported as a human pathogen, has been reported as one of the most predominant yeast species in some cheeses in which NaCl concentrations range from 2% to 8% (w/v) (Table 2).

Table 2.
The pathogenic capacity of yeasts isolated from different types of cheeses.
Table 2.
The pathogenic capacity of yeasts isolated from different types of cheeses.
| Type of Cheese | Yeast | Pathogenic Capacity | Reference |
|---|---|---|---|
| Cottage and cream cheese | Geotrichum species | No | [119] |
| Domiati and Kariesh cheese | Candida albicans Candida lusitaniae (teleomorph of Clavispora lusitaniae) Candida catenulata | Yes | [110] |
| Feta cheese | Candida tropicalis | No | [128] |
| Smeared cheese | Issatchenkia orientalis (teleomorph of Candida krusei) C. lusitaniae (teleomorph of C. lusitaniae) Candida rugosa Candida famata (teleomorph of Debaryomyces hansenii) | Yes | [134] |
| Several types of cheeses | Candida parapsilosis, C. catenulata, Yarrowia lipolytica, Rhodotorula glutinis and Trichosporon species | Yes | [129] |
| Artisanal cheese | C. albicans and 97.6% as Candida non-albicans, distributed in 79.3% of C. krusei, 12.3% of C. glabrata and 6.0% of C. tropicalis | No | [126] |
| Swiss-type blue cheese, Goat’s milk Cheddar | C. parapsilosis | No | [115] |
| Manteca (Italian cheese) | C. parapsilosis | No | [115] |
| Washed rind cheese | Y. lipolytica | No | [115] |
| Camembert Blue-veined cheeses | Candida intermedia | No | [135] |
According to the source of contamination of these potentially pathogenic yeasts, and considering the population segment to be protected, the first preventive measure within the HACCP plan should be the highest level of hygiene in the industries. This fact should be maximized in those kinds of cheeses that have not undergone thermal treatment, or in those industrial stages after this treatment.
3.2. Yeast Biocontrol Strategies Using Microorganisms
One of the most commonly used strategies is to exploit the capacity of some yeasts with QPS status, able to grow in ripened foods and endowed with antagonistic activity against other yeasts. Among these yeasts, D. hansenii is used in the production of ripened food of animal origin. This antagonistic capacity may be caused by the production of toxic proteins or glycoproteins called killer toxins or mycocins, which can kill sensitive yeast, but can be innocuous for consumers. Mycocin activity has been reported in more than 90 yeast species, and their presence is directly related with the presence of chromosomal or extrachromosomal genes (linear plasmids or viruses) [136]. This behaviour is not uniform among these species, nor can it be linked to sources of isolation [137]. Additionally, this killer phenotype is markedly affected by the substrate physicochemical characteristic where the yeast grows [117].
Several studies have reported the ability of mycocins from foodborne yeasts to kill pathogenic yeasts in vitro [138]. Killer activity by some yeasts against C. albicans was reported many years ago. Mycocins from D. hansenii have shown activity against opportunistic pathogenic including Candida species. [137]. The killer activity of D. hansenii against C. albicans and C. tropicalis in ripened cheeses was demonstrated. Therefore, these observations raise the possibility that D. hansenii could hamper Candida survival [117]. On the other hand, K. marxianus and Kluyveromyces lactis inhibited the growth of C. albicans isolated from “Tomme d’orchies” cheese [139].
LAB have long been used in dairy and meat products, providing microbial safety and organoleptic benefits. There are patented microorganisms, such as Lacticaseibacillus rhamnosus, for use as “yeast and mold control”, as well as L. plantarum. Some other LAB species, for e.g., Lactobacillus acidophilus and Limosilactobacillus reuteri, also show the capacity to inhibit yeasts producing metabolites with antifungal activity [116]. In a study conducted by Makki et al. [140], a protective culture combining a mixture of Lacticaseibacillus spp. and Lactiplantibacillus spp. had an effect on the outgrowth of D. hansenii, Meyerozyma guilliermondii and Torulaspora delbrueckii in “cottage” cheese.
3.3. Plant-Derivative Biocontrol Strategies
Concerning the use of plant-derived biocontrol agents, a leaf extract of Lawsonia inermis, an Indian herb, showed a very effective anti-candidal activity with differents sites of action, such as germ tube inhibition, protease, phospholipases and aspartate dehydrogenase inhibitory activity [141]. Solanum lycopersicum shows high levels of fungistatic activity against Candida spp., with its suggested mode of action being the targeting of the C. albicans ergosterol pathway via the upregulation of ergosterol genes [142]. A four percent Jugulans nigra extract is effective in eradicating C. albicans as clotrimazole due to juglone, an active component found in the black walnut tree [143]. The highest antimicrobial activity of clove (Syzygium aromaticum) against C. albicans was achieved at the concentration of 0.2% by causing damage to fungal membranes and cell walls [144,145]. Papaya seed extracts also cause apoptosis in Candida cells due to the oxidative stress created [146], with a similar effect to garlic oil (Allium sativum) against C. albicans [147]. Aloe vera, oregano leaf and grapefruit seed extract have all been shown to inhibit the growth of Candida species as well [116]. The inhibitory effect of Eugenia caryophyllata thumb leaf EOs on contaminating microorganisms of “Coalho” cheese was investigated, with the lowest minimum inhibitory concentration (MIC) level (200 µL mL−1) against C. albicans, C. parapsilosis and C. krusei being obtained [148].
Although these results are promising, it would be necessary to validate these effects of BCAs in food matrices. This should be performed in order to take into account the possible interaction of these agents with each of the ingredients of the cured products, which may modify their antimicrobial capacity. With regard to the effect on the organoleptic characteristics of the product, none of the above studies evaluated the effect on the sensory characteristics of the final product. If these extracts were to be used on an industrial scale, it would be necessary to assess these effects, as these are extracts and plants with a high organoleptic impact.
4. Pathogenic and Toxigenic Molds in Ripened Foods and Biocontrol Strategies
Some molds can cause a wide variety of human diseases such as allergic or invasive infections due to excessive inhalation of spores (mainly from Aspergillus spp.) or their transmission through infected wounds, as well as through the smoking of contaminated plants [149]. However, these infections are infrequent, and from the food safety view, the main problem associated with the mold contamination of ripened animal products is the production of mycotoxins, which are secondary metabolites with a wide range of toxic effects. The most important mycotoxins in dry-cured meat products are the OTA and AFs, due to their frequency and their toxicity, although other mycotoxins can be detected in these products, such as CPA, STG and CIT [24,25]. Similarly, the abovementioned mycotoxins have also been described in cheeses as well as PR toxin, roquefortine C and patulin [23,150]. The main molds that produce mycotoxins in animal-origin ripening foods are described below.
4.1. Biocontrol Strategies against Ochratoxin A-Producing Molds
OTA can be produced by different species of Penicillium and Aspergillus, such as Penicillium nordicum, Penicillium verrucosum, Aspergillus westerdijkiae and Aspergillus carbonarius. Within these species, P. nordicum has been described as the main OTA producer in dry-cured meat products and cheeses [151]. This mycotoxin is nephrotoxic, hepatotoxic, teratogenic, immunotoxic and has been classified as a possible human carcinogen (group 2B) by the International Agency for Research on Cancer (IARC) [152,153,154]. Preserved meats and cheeses are the main contributors to dietary exposure to OTA in several European countries [154].
The biocontrol of ochratoxigenic molds employed in dry-cured meat products includes the use of starter and protective cultures which contain LAB, GCC+, yeasts and non-toxigenic molds, as displayed in Table 3 [31,37,155,156,157,158,159,160,161]. Different strains from Enterococcus faecium were demonstrated to control OTA production via growing P. nordicum in a dry-cured fermented sausage based medium, although they did not affect the OTA produced by P. verrucosum [157]. GCC+, as S. xylosus, successfully decreased the OTA content using different strains of P. nordicum (Pn15, Pn92 and Pn856) a in dry-cured ham-based medium, although no effect was detected in sausages inoculated with the same strain of S. xylosus and Pn15 [31,160]. Fermented extracts developed from the fermentation of a meat model system (BFS) by L. plantarum and P. pentosaceus were able to totally eliminate the presence of P. nordicum and P. verrucosum using different concentrations depending on the bacterium and the mold strain tested [162]. Meftah et al. [159] revealed the ability of the yeasts C. zeylanoides and Rhodotorula mucilaginosa to reduce the OTA concentration produced by P. nordicum and A. westerdijkiae in three matrices (ham, and dry-cured sausages with industrial and traditional processing). Other yeasts, such as D. hansenii and Saccharomycopsis fibuligera, were able to completely inhibit the OTA produced by P. nordicum and A. ochraceus in speck, a typical meat product in the European Alpine area [37]. D. hansenii has also been displayed as an effective BCA against P. nordicum, P. verrucosum and A. westerdijkiae tested in other studies in dry-cured meat products or meat-model systems [31,159,161,163,164]. Additionally, the strain of D. hansenii used in some of these studies did not negatively modify the sensorial quality of dry-cured fermented sausages which contained it [30]. The non-toxigenic mold Penicillium chrysogenum, producer of the antifungal protein PgAFP, was proposed as a BCA against P. nordicum in a dry-cured ham-based medium [158] and in a meat-model system [165], showing in both studies possible nutrient competition. Similarly, this strain of P. chrysogenum controlled the growth of potentially ochratoxigenic molds, reducing the OTA accumulation in dry-cured Iberian hams which had undergone industrial processing [166]. This strain was also proposed as a good protective culture with no technological drawbacks during the ripening of dry-cured fermented sausages [30]. The protective potential of a commercial starter culture of Penicillium nalgiovense was displayed by the decrease in the OTA concentration produced by P. verrucosum in the dry-cured fermented sausage “salchichón” [167].

Table 3.
Main effects of biocontrol agents on toxigenic molds in ripening food matrices of animal origin.
Regarding the use of plant derivatives as biocontrol agents, some studies demonstrated the efficiency of reducing OTA using ingredients from dry-cured meat sausages such as rosemary, oregano and smoked paprika “pimentón” [41,44]. Oregano and rosemary leaves added to a dry-cured fermented-sausage medium and “pimentón” to a meat-based medium decreased the amount of OTA produced by P. nordicum [41,44]. Rosemary leaves were able to decrease the mycotoxin produced by P. nordicum during dry-cured sausage ripening and, together with their essential oil, the OTA produced by A. westerdijkiae in a dry-cured fermented sausage based medium [40,163]. However, the sensorial impact of these BCAs was not checked, although the concentrations of herb leaves used were expected to have no negative influence on consumer perceptions [41,163]. Additionally, other EOs such as basil EO, sage EO and oregano EO, and plant derivatives such as carvacrol and eugenol, have also been described as BCAs against ochratoxigenic molds in commercial culture media, but not in meat-based matrices [175,176], therefore its effectiveness in ripening products is not known yet.
On the other hand, there are few studies focused on the biocontrol of ochratoxigenic molds in cheeses, despite the fact that all kinds of ripened cheeses can be contaminated with this mycotoxin [150,168,177,178]. The use of LAB such as Lactobacillus buchneri and L. casei against P. nordicum in cheeses covered with films with the bacteria incorporated reached OTA reductions of up to 94%, although no sensory study was carried out to confirm their applicability [168]. Another study, which employed twenty-five strains of L. plantarum, one Lacticaseibacillus paracasei, one L. casei and one L. rhamnosus isolated from a Brazilian artisanal “Serrano Catarinense” cheese, showed the ability of these LAB to decrease the growth of P. nordicum in MRS agar, suggesting a possible future use as preservative agents during cheese manufacturing [179]. The lack of studies about the biocontrol of ochratoxigenic molds in cheeses opens up a new field of study necessary to reduce the risk posed by OTA presence in these ripened products.
4.2. Biocontrol Strategies against Aflatoxin-Producing Molds
AFs are highly toxic secondary metabolites produced by molds such as Aspergillus flavus, Aspergillus parasiticus and Aspergillus nomius [180]. Although these fungi are more frequent in cereal crops, they can colonize the surface of ripening products of animal origin [181,182,183]. The most important AFs are B1, B2, G1 and G2. These mycotoxins are carcinogenic (Group 1) and mutagenic for animals and humans according to the IARC [152].
Similar to the strategies employed against ochratoxigenic molds, different LAB, GCC+ and yeasts have been studied as BCAs against AF producers in dry-cured meat products. S. xylosus Sx8 was able to control the growth and the AFB1 produced by A. flavus and AFB1 and AF G1 produced by A. parasiticus in a dry-cured ham-based medium at three different temperatures (15, 20 and 25 °C) [160]. The BFS extract from L. plantarum and P. pentosaceus reduced the growth of A. flavus and A. parasiticus in a meat model system by up to 50% using concentrations between 21 and 43 g L−1 [162]. D. hansenii combined with the antifungal protein PgAFP and P. acidilactici on slices of dry-cured fermented sausages successfully diminished AFB1 and AFG1 amounts produced by A. parasiticus and the mold counts [169]. In another study, D. hansenii was tested against A. parasiticus and decreased the AF B1 in more than 53.85% and the AFG1 by up to 59.06% in dry-fermented sausages, while the AFG1 was below the limit of quantification in dry-cured ham [39].
Regarding the agents of plant origin, the smoked paprika “pimentón” reduced the AFB1 and AFG1 production by A. parasiticus in a dry-cured meat model system, although they did not decrease the mold’s growth [44].
The presence of AFs in cheeses has been described worldwide. In addition to the AFM1 that can be present in the milk used for cheese manufacturing, common aflatoxins have been detected due to the surface colonization of the product by aflatoxigenic molds [23,184,185,186]. Despite the risk that AFs pose in cheeses, there are only a few studies based on the biocontrol of AFs in this matrix. In cheese slices, the use of the protein PgAFP combined with D. hansenii in the presence or absence of P. acidilactici decreased the A. parasiticus growth and its AFG1 production below the method’s limit of detection [169]. On the other hand, the addition of Oreganum vulgare EO (0.02% v/v) to the Minas cheese formulation inhibited the germination of spores of A. flavus for up to 15 days of ripening, and was the cheese flavor and taste accepted by the panelists [170]. Moreover, Vitalini et al. [171] demonstrated that parsley EO applied on cheese slices was effective in preventing A. flavus growth. Tatlisu et al. [45] demonstrated the antifungal activity of thymol (main component of numerous EOs) and nanofibers with thymol applied to “kashar” cheese cube surfaces against A. parasiticus, although no sensory analyses was performed [45].
4.3. Biocontrol Strategies against Cyclopiazonic Acid-Producing Molds
CPA can be produced by different molds, such as A. flavus, A. parasiticus and Penicillium griseofulvum, in ripened meats, and mainly P. commune, P. roqueforti and P. camemberti in cheeses [24,182,187]. Due to the little amount of toxicological data, the IARC has not declared an acceptable CPA toxicity level yet, but it is well known that it includes sever gastrointestinal and neurological disorders and organ necrosis [188]. Therefore, this lack of data and legal limits results in a shortage of studies about the biocontrol of molds that only produce CPA.
Concerning the biocontrol studies in dry-cured meat products, the bacterium S. xylosus Sx8 decreased CPA production using two strains of P. griseofulvum grown in a dry-cured ham-based medium after 30 days of incubation at 25 °C [160]. Concentrations of 85 g L−1 of BFS extract from L. plantarum and between 21 and 85 g L−1 from P. pentosaceus did not allow for the growth of P. griseofulvum and P. commune in a meat model system [162]. Moreover, Delgado et al. [34] showed that P. chrysogenum, producer of the antifungal protein PgAFP, was able to diminish CPA amounts produced by P. griseofulvum under the limit of detection on a dry-fermented sausage-based medium and more than 97% on dry-cured fermented sausages after 21 days following industrial ripening [34].
In Edam cheeses, the clove, thyme, red thyme and litsea EOs completely inhibited the growth of two CPA producer strains of P. commune, while cumin and marjoram EOs showed high antifungal activity, although they did not totally inhibit the growth of the molds [43]. In this study, the evaluators recognized some EOs in sensory evaluation via the triangle test, but they did not have a negative effect on the taste and smell of the treated cheeses [43].
4.4. Biocontrol Strategies against Sterigmatocystin-Producing Molds
STG is a precursor of AFB1, so the producing molds mainly include different Aspergillus species, although there are other ones such as Eurotium, Fusarium and Podospora spp., which demonstrate an ability to produce this mycotoxin [189]. Concerning its toxicity, it has been found that STG induces tumors in animals and humans [189]. In spite of this evidence, the IARC only classified STG into Group 2B (possible human carcinogen) [190].
Regarding the biocontrol strategies, the BCA against AF producers could be applied for the toxigenic molds which produce both mycotoxins (AFs and STG), but STG production deserves to be further studied. Within the ripened animal products, STG has been mainly described in a wide range of cheeses contaminated with Aspergillus versicolor, A. flavus or A. parasiticus [189,191]. However, there are no studies of ripened matrices of animal origin based on the biocontrol of molds that only produce STG, although EOs from tarragon, oregano and savory showed the inhibition of two producers of STG isolated from cheeses, Aspergillus puulaauensis and Aspergillus jenseii [192].
4.5. Biocontrol Strategies against Citrinin-Producing Molds
CIT is produced by different species of Penicillium and Aspergillus, including P. cambemberti, Penicillium expansum, P. verrucosum, P. citrinum, P. viridicatum Aspergillus carneus and Aspergillus niveus [23]. Several studies have shown frequent cooccurrence of OTA and CIT in dry-cured meat products and cheeses [25,193]. CIT is nephrotoxic and hepatotoxic to humans and has been classified into Group 3 by the IARC [190] due to evidence of its in vivo carcinogenicity [194,195].
Given that some toxigenic strains can produce both OTA and CIT, the strategies to prevent OTA producers may also be effective for CIT accumulation. However, it must be considered that some molds can switch the production of OTA to CIT or vice versa to deal with different stressful environments [196,197]. Therefore, different strategies might be needed for reducing both mycotoxins. To our knowledge, there are no studies only focused on the biocontrol of this mycotoxin in dry-cured meat products. However, in cheeses a concentration of 150 µg mL−1 of eugenol and thymol inhibited CIT production by P. citrinum in “Arzúa-Ulloa” cheese, while in “Cabreriro” cheese these antifungal agents did not affect the CIT amounts [172]. In other studies, the Zataria multiflora Boiss EO decreased the growth and CIT production by P. citrinum in Iranian cheese and mozzarella [173,174]. Despite the use of EOs and compounds with a strong flavor in the above-mentioned studies, only the organoleptic effect of Z. multiflora Boiss EO was tested. Concentrations over 600 ppm, which were more effective against CIT production, were disliked by the consumers and, consequently, their applications were limited [174].
Despite the studies about the presence of mycotoxins in ripened products of animal origin, no notifications were made regarding the presence of mycotoxins in both meat and dairy products (RASFF).
5. Challenges in the Application of Microorganisms as Biocontrol Agents in Animal Origin Ripened Foods
The successful utilization of microorganisms as BCAs requires their effective growth and development in/on the food matrix, where it will be confronted with the targeted pathogenic microorganisms.
The isolation of autochthonous microorganisms from these products to be used as protective cultures is then of utmost interest since these strains are well adapted to these specific ecological niches. This ensures their survival and competition under processing and/or storage conditions. If these microorganisms are not isolated from the product intended to be inoculated, they should be examined to check their survival and development under the specific ripening conditions to select or discard them.
In order to maximize the correct implantation of microorganisms previously isolated from ripened products to be used as biocontrol strategies, prior in vitro tests are required. These in vitro tests are carried out by simulating the environmental conditions given in the different stages in which these microorganisms are inoculated during the ripening process [71]. Within these conditions we find intrinsic factors of the product, such as aw [164], pH [198], salt concentration [199] and interaction with other ingredients [165,200]; and conditions given in the ripening chambers, such as temperature [157] and relative humidity. All these assays should be performed with the aim of identifying the most appropriate stage, throughout the ripening process, for their inoculation as BCAs.
After this, the next step aims to evaluate its biocontrol potential via co-inoculation with the target pathogen microorganism in food models simulating temperature, NaCl concentration, aw and pH conditions of ripened foods, as displayed in Figure 1. This can be achieved by using culture media which simulates dry-cured ham, dry-fermented sausage or ripened cheese [39,41,85,160].
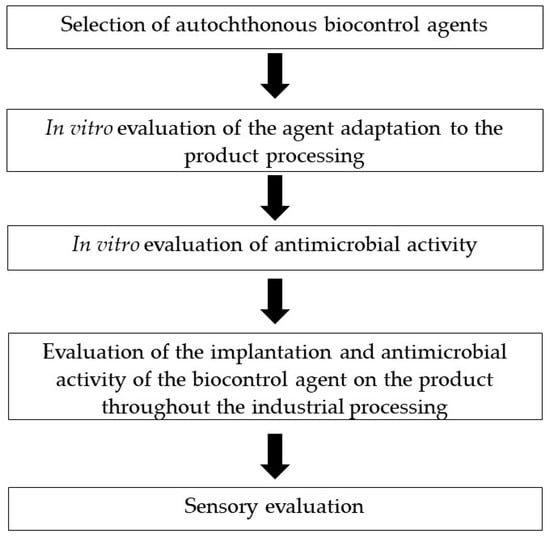
Figure 1.
Flow chart of the biocontrol agents’ selection and evaluation, a process to be used for ripened foods of animal origin.
Furthermore, the use of a model system to study the mechanisms and modes of action of BCAs is a powerful tool to avoid the effect of external factors, which could hide or modify the metabolic routes involved in their antimicrobial effect [201,202]
Once the inhibitory capacity of a BCA in a culture medium has been proven, it is necessary to evaluate it in the ripened foods of animal origin following their industrial processing. For this purpose, different studies have, firstly, inoculated the BCAs at the beginning of the product manufacturing and tested their antimicrobial activity [34,38,68,163] and, secondly, tested the BCA implantation at the end of the ripening [31]. Different molecular techniques are available to check whether the protective microorganisms have been correctly implanted in the product, such as analysis of chromosomal DNA via pulsed field gel electrophoresis (PFGE). This methodology has been reported as appropriate for the differentiation of LAB, GCC+ and yeast strains [31,203]. In the same way, RAPD-PCR analysis or the use of mitochondrial DNA restriction patterns have also been used to discriminate yeasts at the strain level [204,205]. In addition, RAPD-PCR analysis has also been proven to be suitable for the identification of mold strains [206].
Finally, to evaluate the suitability of protective microorganisms as preventative measure for HACCP, the performance of challenge tests during industrial ripening could give additional information in order to assess the actual efficiency of any BCA against the targeted pathogenic/toxigenic microorganism under the real ripening conditions in foods of animal origin [34,77].
6. Conclusions
The current microbial hazards found in ripened foods of animal origin require constant control from the food industry, since food safety is a primary requirement for the global food market. Not only are safe products demanded by consumers, but the avoiding of the use of chemical preservatives to manage these pathogenic and toxin-producer microorganisms is also wanted. The successful effect of microorganisms as BCAs requires their correct development on the foodstuffs throughout the industrial ripening process. Different strategies to counteract worrying foodborne pathogens have been evaluated, displaying apparent positive results. Their ability to inhibit pathogenic microorganisms or their toxins has been tested to different extents. Finally, their minimal or absent sensory impact must be also fulfilled prior to being included as preventive measures in the HACCP programs.
Author Contributions
Conceptualization, J.D. and M.R.; methodology, M.Á., E.C., I.M. and E.R.; writing—original draft preparation, M.Á., E.C., I.M. and E.R.; writing—review and editing, J.D. and M.R.; supervision, J.D. and M.R.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant PID2019-104260GB-I00 funded by MCIN/AEI/10.13039/501100011033. Grant GR21130 funded by Junta de Extremadura and by “European Union ERDF A way of making Europe”. E. Cebrián is recipient of the grant PRE2020-093605 funded by MCIN/AEI/10.13039/501100011033 and by “ESF Investing in your future”. M. Álvarez and I. Martín are recipient of the grants Margarita Salas MS-14 and MS-13 respectively, funded by Ministerio de Universidades and European Union NextGenerationEU.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andrade, M.J.; Córdoba, J.J.; Casado, E.M.; Córdoba, M.G.; Rodríguez, M. Effect of selected strains of Debaryomyces hansenii on the volatile compound production of dry fermented sausage “salchichón”. Meat Sci. 2010, 85, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.C.; de Araújo, J.P.A.; Fusieger, A.; de Carvalho, A.F.; Nero, L.A. Microbiological quality and safety of Brazilian artisanal cheeses. Braz. J. Microbiol. 2021, 52, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Cocolin, L.; Urso, R.; Rantsiou, K.; Cantoni, C.; Comi, G. Dynamics and characterization of yeasts during natural fermentation of Italian sausages. FEMS Yeast Res. 2006, 6, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Córdoba, J.J.; Aranda, E.; Córdoba, M.G.; Asensio, M.A. Contribution of a selected fungal population to the volatile compounds on dry-cured ham. Int. J. Food Microbiol. 2006, 110, 8–18. [Google Scholar] [CrossRef]
- Martín, B.; Garriga, M.; Hugas, M.; Bover-Cid, S.; Veciana-Nogués, M.T.; Aymerich, T. Molecular, technological and safety characterization of Gram-positive catalase-positive cocci from slightly fermented sausages. Int. J. Food Microbiol. 2006, 107, 148–158. [Google Scholar] [CrossRef]
- Prpich, N.Z.P.; Camprubí, G.E.; Cayré, M.E.; Castro, M.P. Indigenous microbiota to leverage traditional dry sausage production. Int. J. Food Sci. 2021, 2015, 6696856. [Google Scholar]
- Benito, M.J.; Martín, A.; Aranda, E.; Pérez-Nevado, F.; Ruiz-Moyano, S.; Córdoba, M.G. Characterization and selection of autochthonous lactic acid bacteria isolated from traditional Iberian dry-fermented salchichón and chorizo sausages. J. Food Sci. 2007, 72, 193–201. [Google Scholar] [CrossRef]
- Casquete, R.; Benito, M.J.; Martín, A.; Ruiz-Moyano, S.; Aranda, E.; Córdoba, M.G. Microbiological quality of salchichón and chorizo, traditional Iberian dry-fermented sausages from two different industries, inoculated with autochthonous starter cultures. Food Control 2012, 24, 191–198. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, S.; Choi, K.H. Microbial benefits and risks of raw milk cheese. Food Control 2016, 63, 201–215. [Google Scholar] [CrossRef]
- Hospital, X.F.; Hierro, E.; Stringer, S.; Fernández, M. A study on the toxigenesis by Clostridium botulinum in nitrate and nitrite-reduced dry fermented sausages. Int. J. Food Microbiol. 2016, 218, 66–70. [Google Scholar] [CrossRef]
- Chaidoutis, E.; Keramydas, D.; Papalexis, P.; Migdanis, A.; Migdanis, I.; Lazaris, A.C.; Kavantzas, N. Foodborne botulism: A brief review of cases transmitted by cheese products. Biomed. Rep. 2022, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Falardeau, J.; Trmčić, A.; Wang, S. The occurrence, growth, and biocontrol of Listeria monocytogenes in fresh and surface-ripened soft and semisoft cheeses. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4019–4048. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Barbosa, J.; Silva, J.; Felício, M.T.; Mena, C.; Hogg, T.; Gibbs, P.; Teixeira, P. Characterisation of alheiras, traditional sausages produced in the North of Portugal, with respect to their microbiological safety. Food Control 2007, 18, 436–440. [Google Scholar] [CrossRef]
- Gonçalves, M.T.P.; Benito, M.J.; Córdoba, M.d.G.; Egas, C.; Merchán, A.V.; Galván, A.I.; Ruiz-Moyano, S. Bacterial communities in Serpa cheese by culture dependent techniques, 16S rRNA gene sequencing and high-throughput sequencing analysis. J. Food Sci. 2018, 83, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; Nauta, M.; et al. Microbiological safety of aged meat. EFSA J. 2023, 21, 7745. [Google Scholar]
- Lobacz, A.; Zulewska, J. Fate of Salmonella spp. in the fresh soft raw milk cheese during storage at different temperatures. Microorganisms 2021, 9, 938. [Google Scholar] [CrossRef]
- Meloni, D. Presence of Listeria monocytogenes in Mediterranean-style dry fermented sausages. Foods 2015, 4, 34–50. [Google Scholar] [CrossRef]
- Possas, A.; Bonilla-Luque, O.M.; Valero, A. From cheese-making to consumption: Exploring the microbial safety of cheeses through predictive microbiology models. Foods 2021, 10, 355. [Google Scholar] [CrossRef]
- Ertas Onmaz, N.; Gungor, C.; Al, S.; Dishan, A.; Hizlisoy, H.; Yildirim, Y.; Kasap Tekinsen, F.; Disli, H.B.; Barel, M.; Karadal, F. Mycotoxigenic and phylogenetic perspective to the yeasts and filamentous moulds in mould-matured Turkish cheese. Int. J. Food Microbiol. 2021, 357, 109385. [Google Scholar] [CrossRef]
- Battilani, P.; Pietri, A.; Giorni, P.; Formenti, S.; Bertuzzi, T.; Toscani, T.; Virgili, R.; Kozakiewicz, Z. Penicillium populations in dry-cured ham manufacturing plants. J. Food. Prot. 2007, 70, 975–980. [Google Scholar] [CrossRef]
- López-Díaz, T.M.; Santos, J.A.; García-López, M.L.; Otero, A. Surface mycoflora of a Spanish fermented meat sausage and toxigenicity of Penicillium isolates. Int. J. Food Microbiol. 2001, 68, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Martı́n, A.; Córdoba, J.J.; Núñez, F.; Benito, M.J.; Asensio, M.A. Contribution of a selected fungal population to proteolysis on dry-cured ham. Int. J. Food Microbiol. 2004, 94, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.D.W. Mycotoxins in Cheese. In Cheese. Chemistry, Physics & Microbiology; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: Cambridge, MA, USA; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 1, ISBN 9780122636530. [Google Scholar]
- Lešić, T.; Vulić, A.; Vahčić, N.; Šarkanj, B.; Hengl, B.; Kos, I.; Polak, T.; Kudumija, N.; Pleadin, J. The Occurrence of five unregulated mycotoxins most important for traditional dry-cured meat products. Toxins 2022, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Markov, K.; Pleadin, J.; Bevardi, M.; Vahčić, N.; Sokolić-Mihalak, D.; Frece, J. Natural occurrence of aflatoxin B1, ochratoxin A and citrinin in Croatian fermented meat products. Food Control 2013, 34, 312–317. [Google Scholar] [CrossRef]
- European Commission Rapid Alert System for Food and Feed (RASFF). Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 26 March 2023).
- Asensio, M.A.; Núñez, F.; Delgado, J.; Bermúdez, E. Control of toxigenic molds in food processing. In Microbial Food Safety and Preservation Techniques; Rai, V.R., Bai, A.J., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 329–357. [Google Scholar]
- Alcano, M.J.; Jahn, R.C.; Scherer, C.D.; Wigmann, É.F.; Moraes, V.M.; Garcia, M.V.; Mallmann, C.A.; Copetti, M.V. Susceptibility of Aspergillus spp. to acetic and sorbic acids based on pH and effect of sub-inhibitory doses of sorbic acid on ochratoxin A production. Food Res. Int. 2016, 81, 25–30. [Google Scholar] [CrossRef]
- Román, S.; Sánchez-Siles, L.; Siegrist, M. The importance of food naturalness for consumers: Results of a systematic review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Álvarez, M.; Andrade, M.J.; García, C.; Rondán, J.J.; Núñez, F. Effects of preservative agents on quality attributes of dry-cured fermented sausages. Foods 2020, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, E.; Núñez, F.; Álvarez, M.; Roncero, E.; Rodríguez, M. Biocontrol of ochratoxigenic Penicillium nordicum in dry-cured fermented sausages by Debaryomyces hansenii and Staphylococcus xylosus. Int. J. Food Microbiol. 2022, 375, 109744. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, E.; Rodríguez, M.; Peromingo, B.; Bermúdez, E.; Núñez, F. Efficacy of the combined protective cultures of Penicillium chrysogenum and Debaryomyces hansenii for the control of ochratoxin A hazard in dry-cured ham. Toxins 2019, 11, 710. [Google Scholar] [CrossRef]
- Alía, A.; Córdoba, J.J.; Rodríguez, A.; García, C.; Andrade, M.J. Evaluation of the efficacy of Debaryomyces hansenii as protective culture for controlling Listeria monocytogenes in sliced dry-cured ham. LWT—Food Sci. Technol. 2020, 119, 108886. [Google Scholar] [CrossRef]
- Delgado, J.; Peromingo, B.; Rodríguez, A.; Rodríguez, M. Biocontrol of Penicillium griseofulvum to reduce cyclopiazonic acid contamination in dry-fermented sausages. Int. J. Food Microbiol. 2019, 293, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.T.T.; Lo, R.; Bansal, N.; Turner, M.S. Characterisation of Lactococcus lactis isolates from herbs, fruits and vegetables for use as biopreservatives against Listeria monocytogenes in cheese. Food Control 2018, 85, 472–483. [Google Scholar] [CrossRef]
- Campagnollo, F.B.; Pedrosa, G.T.S.; Kamimura, B.A.; Furtado, M.M.; Baptista, R.C.; Nascimento, H.M.; Alvarenga, V.O.; Magnani, M.; Sant’Ana, A.S. Growth potential of three strains of Listeria monocytogenes and Salmonella enterica in Frescal and semi-hard artisanal Minas microcheeses: Impact of the addition of lactic acid bacteria with antimicrobial activity. LWT—Food Sci. Technol. 2022, 158, 113169. [Google Scholar] [CrossRef]
- Iacumin, L.; Manzano, M.; Andyanto, D.; Comi, G. Biocontrol of ochratoxigenic moulds (Aspergillus ochraceus and Penicillium nordicum) by Debaryomyces hansenii and Saccharomycopsis fibuligera during speck production. Food Microbiol. 2017, 62, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Martín, I.; Alía, A.; Rodríguez, A.; Gómez, F.; Córdoba, J.J. Growth and expression of virulence genes of Listeria monocytogenes during the processing of dry-cured fermented “salchichón” manufactured with a selected Lactilactobacillus sakei. Biology 2021, 10, 1258. [Google Scholar] [CrossRef]
- Peromingo, B.; Andrade, M.J.; Delgado, J.; Sánchez-Montero, L.; Núñez, F. Biocontrol of aflatoxigenic Aspergillus parasiticus by native Debaryomyces hansenii in dry-cured meat products. Food Microbiol. 2019, 82, 269–276. [Google Scholar] [CrossRef]
- Álvarez, M.; Andrade, M.J.; Delgado, J.; Núñez, F.; Román, Á.C.; Rodrigues, P. Rosmarinus officinalis reduces the ochratoxin A production by Aspergillus westerdijkiae in a dry-cured fermented sausage-based medium. Food Control 2023, 145, 109436. [Google Scholar] [CrossRef]
- Álvarez, M.; Rodríguez, A.; Núñez, F.; Silva, A.; Andrade, M.J. In vitro antifungal effects of spices on ochratoxin A production and related gene expression in Penicillium nordicum on a dry-cured fermented sausage medium. Food Control 2020, 114, 107222. [Google Scholar] [CrossRef]
- García-Díez, J.; Alheiro, J.; Pinto, A.L.; Soares, L.; Falco, V.; Fraqueza, M.J.; Patarata, L. Behaviour of food-borne pathogens on dry cured sausage manufactured with herbs and spices essential oils and their sensorial acceptability. Food Control 2016, 59, 262–270. [Google Scholar] [CrossRef]
- Hlebová, M.; Foltinová, D.; Vešelényiová, D.; Medo, J.; Šramková, Z.; Tančinová, D.; Mrkvová, M.; Hleba, L. The vapor phase of selected essential oils and their antifungal activity in vitro and in situ against Penicillium commune, a common contaminant of cheese. Foods 2022, 11, 3517. [Google Scholar] [CrossRef]
- Sánchez-Montero, L.; Córdoba, J.J.; Alía, A.; Peromingo, B.; Núñez, F. Effect of Spanish smoked paprika “Pimentón de La Vera” on control of ochratoxin A and aflatoxins production on a dry-cured meat model system. Int. J. Food Microbiol. 2019, 308, 108303. [Google Scholar] [CrossRef] [PubMed]
- Tatlisu, N.B.; Yilmaz, M.T.; Arici, M. Fabrication and characterization of thymol-loaded nanofiber mats as a novel antimould surface material for coating cheese surface. Food Packag. Shelf Life 2019, 21, 100347. [Google Scholar] [CrossRef]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on major food-borne zoonotic bacterial pathogens. J. Trop Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef] [PubMed]
- Schlech, W.F. Epidemiology and clinical manifestations of Listeria monocytogenes infection. Microbiol. Spectr. 2019, 7, 793–802. [Google Scholar] [CrossRef]
- Mateus, T.; Silva, J.; Maia, R.L.; Teixeira, P. Listeriosis during pregnancy: A Public Health Concern. ISRN Obstet. Gynecol. 2013, 2013, 851712. [Google Scholar] [CrossRef]
- Chen, J.; Luo, X.; Jiang, L.; Jin, P.; Wei, W.; Liu, D.; Fang, W. Molecular characteristics and virulence potential of Listeria monocytogenes isolates from Chinese food systems. Food Microbiol. 2009, 26, 103–111. [Google Scholar] [CrossRef]
- Aljasir, S.F.; D’Amico, D.J. The effect of protective cultures on Staphylococcus aureus growth and enterotoxin production. Food Microbiol. 2020, 91, 103541. [Google Scholar] [CrossRef]
- Le, H.H.T.; Dalsgaard, A.; Andersen, P.S.; Nguyen, H.M.; Ta, Y.T.; Nguyen, T.T. Large-scale Staphylococcus aureus foodborne disease poisoning outbreak among primary school children. Microbiol. Res. 2021, 12, 43–52. [Google Scholar] [CrossRef]
- Pal, M. Staphylococcus aureus: A Major pathogen of food poisoning. Nutr. Food Process 2022, 5, 1–3. [Google Scholar] [CrossRef]
- Hamzah, A.M.C.; Yeo, C.C.; Puah, S.M.; Chua, K.H.; Chew, C.H. Staphylococcus aureus infections in Malaysia: A review of antimicrobial resistance and characteristics of the clinical isolates, 1990–2017. Antibiotics 2019, 8, 1990–2017. [Google Scholar] [CrossRef]
- Irlinger, F. Safety assessment of dairy microorganisms: Coagulase-negative staphylococci. Int. J. Food Microbiol. 2008, 126, 302–310. [Google Scholar] [CrossRef]
- Soares, J.C.; Marques, M.R.; Tavaria, F.K.; Pereira, J.O.; Malcata, F.X.; Pintado, M.M. Biodiversity and characterization of Staphylococcus species isolated from a small manufacturing dairy plant in Portugal. Int. J. Food Microbiol. 2011, 146, 123–129. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Doyle, M. Foodborne Bacterial Pathogens (Food Science and Technology), 1st ed.; CRC Press: Boca Raton, FL, USA, 1989; ISBN 978-0824778668. [Google Scholar]
- Alizadeh, A.M.; Hashempour-Baltork, F.; Alizadeh-Sani, M.; Maleki, M.; Azizi-Lalabadi, M.; Khosravi-Darani, K. Inhibition of Clostridium botulinum and its toxins by probiotic bacteria and their metabolites: An update review. Qual. Assur. Saf. Crop. Foods 2020, 12, 59–68. [Google Scholar] [CrossRef]
- Hamad, G.; Ombarak, R.A.; Eskander, M.; Mehany, T.; Anees, F.R.; Elfayoumy, R.A.; Omar, S.A.; Lorenzo, J.M.; Abou-Alella, S.A.E. Detection and inhibition of Clostridium botulinum in some Egyptian fish products by probiotics cell-free supernatants as bio-preservation agents. LWT—Food Sci. Technol. 2022, 163, 113603. [Google Scholar] [CrossRef]
- Shah, M.K. A review on pathogenic Escherichia coli in Malaysia. Adv. Anim. Vet. Sci. 2018, 6, 95–107. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Aljuffali, I.A.; Fang, J.Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Mersha, G.; Asrat, D.; Zewde, B.M.; Kyule, M. Occurrence of Escherichia coli O157:H7 in faeces, skin and carcasses from sheep and goats in Ethiopia. Lett. Appl. Microbiol. 2010, 50, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Castellano, P.; Ibarreche, M.P.; Massani, M.B.; Fontana, C.; Vignolo, G.M. Strategies for pathogen biocontrol using lactic acid bacteria and their metabolites: A focus on meat ecosystems and industrial environments. Microorganisms 2017, 5, 38. [Google Scholar] [CrossRef]
- Abdelhamid, A.G.; El-Dougdoug, N.K. Controlling foodborne pathogens with natural antimicrobials by biological control and antivirulence strategies. Heliyon 2020, 6, e05020. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Sánchez-Montero, L.; Padilla, P.; Córdoba, J.J. Effect of the dry-cured fermented sausage “salchichón” processing with a selected Lactobacillus sakei in Listeria monocytogenes and microbial population. Foods 2021, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Martín, I.; Rodríguez, A.; Córdoba, J.J. Application of selected lactic-acid bacteria to control Listeria monocytogenes in soft-ripened “Torta del Casar” cheese. LWT—Food Sci. Technol. 2022, 168, 113873. [Google Scholar] [CrossRef]
- Margalho, L.P.; Jorge, G.P.; Noleto, D.A.P.; Silva, C.E.; Abreu, J.S.; Piran, M.V.F.; Brocchi, M.; Sant’Ana, A.S. Biopreservation and probiotic potential of a large set of lactic acid bacteria isolated from Brazilian artisanal cheeses: From screening to in product approach. Microbiol. Res. 2021, 242, 126622. [Google Scholar] [CrossRef] [PubMed]
- Pragalaki, T.; Bloukas, J.G.; Kotzekidou, P. Inhibition of Listeria monocytogenes and Escherichia coli O157: H7 in liquid broth medium and during processing of fermented sausage using autochthonous starter cultures. Meat Sci. 2013, 95, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Borotová, P.; Terentjeva, M.; Kunová, S.; Felšöciová, S.; Haščík, P.; Lopašovský, Ľ.; Štefániková, J. Bryndza cheese of Slovak origin as potential resources of probiotic bacteria. Potravin Slovak J. Food Sci. 2020, 14, 641–646. [Google Scholar] [CrossRef]
- Kaban, G.; Kaya, M. Effect of starter culture on growth of Staphylococcus aureus in sucuk. Food Control 2006, 17, 797–801. [Google Scholar] [CrossRef]
- Di Gioia, D.; Mazzola, G.; Nikodinoska, I.; Aloisio, I.; Langerholc, T.; Rossi, M.; Raimondi, S.; Melero, B.; Rovira, J. Lactic acid bacteria as protective cultures in fermented pork meat to prevent Clostridium spp. growth. Int. J. Food Microbiol. 2016, 235, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tomović, V.; Šojić, B.; Savanović, J.; Kocić-Tanackov, S.; Pavlić, B.; Jokanović, M.; Dordević, V.; Parunović, N.; Martinović, A.; Vujadinović, D. New formulation towards healthier meat products: Juniperus communis L. essential oil as alternative for sodium nitrite in dry fermented sausages. Foods 2020, 9, 1066. [Google Scholar] [CrossRef]
- Dos Santos, L.R.; Alía, A.; Martin, I.; Gottardo, F.M.; Rodrigues, L.B.; Borges, K.A.; Furian, T.Q.; Córdoba, J.J. Antimicrobial activity of essential oils and natural plant extracts against Listeria monocytogenes in a dry-cured ham-based model. J. Sci. Food Agric. 2022, 102, 1729–1735. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Ramos, I.M.; Rodríguez-Pérez, M.; Poveda, J.M.; Seseña, S.; Palop, M.L. Lactic acid bacteria as biocontrol agents to reduce Staphylococcus aureus growth, enterotoxin production and virulence gene expression. LWT—Food Sci. Technol. 2022, 170, 114025. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel. Updated list of QPS recommended biological agents for safety risk assessments carried out by EFSA. EFSA J. 2023, 21, e07747. [Google Scholar]
- Carvalho, M.I.P.; Albano, H.C.P.; Teixeira, P.C.M. Influence of oregano essential oil on the inhibition of selected pathogens in “Alheira” during storage. Acta Sci. Pol. Technol. Aliment. 2019, 18, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Albano, H.; Oliveira, M.; Aroso, R.; Cubero, N.; Hogg, T.; Teixeira, P. Antilisterial activity of lactic acid bacteria isolated from “Alheiras” (traditional Portuguese fermented sausages): In situ assays. Meat Sci. 2007, 76, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Lozano, J.C.; Reguera-Useros, J.I.; Peláez-Martínez, M.d.C.; Sacristán-Pérez-Minayo, G.; Gutiérrez-Fernández, Á.J.; Hardisson de la Torre, A. The effect of the pediocin PA-1 produced by Pediococcus acidilactici against Listeria monocytogenes and Clostridium perfringens in Spanish dry-fermented sausages and frankfurters. Food Control 2010, 21, 679–685. [Google Scholar] [CrossRef]
- Kasra-Kermanshahi, R.; Mobarak-Qamsari, E. Inhibition effect of lactic acid bacteria against food born pathogen, Listeria monocytogenes. Appl. Food Biotechnol. 2015, 2, 11–19. [Google Scholar]
- Martín, I.; Rodríguez, A.; Delgado, J.; Córdoba, J.J. Strategies for biocontrol of Listeria monocytogenes using lactic acid bacteria and their metabolites in ready-to-eat meat-and dairy-ripened products. Foods 2022, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives Text with EEA relevance. Off. J. Eir. Union 2013, 045, 131–307. [Google Scholar]
- Darbandi, A.; Asadi, A.; Ari, M.M.; Ohadi, E.; Talebi, M.; Zadeh, M.H.; Emamie, A.D.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potencial use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Koohestani, M.; Moradi, M.; Tajik, H.; Badali, A. Effects of cell-free supernatant of Lactobacillus acidophilus LA5 and Lactobacillus casei 431 against planktonic form and biofilm of Staphylococcus aureus. Vet. Res. Forum. 2018, 9, 301–306. [Google Scholar]
- Araújo-Rodrigues, H.; dos Santos, M.T.P.G.; Ruiz-Moyano, S.; Tavaria, F.K.; Martins, A.P.L.; Alvarenga, N.; Pintado, M.E. Technological and protective performance of LAB isolated from Serpa PDO cheese: Towards selection and development of an autochthonous starter culture. LWT—Food Sci. Techno. 2021, 150, 112079. [Google Scholar] [CrossRef]
- Martín, I.; Cordoba, J.J.; Alía, A.; Martínez, R.; Rodríguez, A. Selection and characterization of lactic acid bacteria from traditional ripened foods with activity against Listeria monocytogenes. LWT—Food Sci. Techno. 2022, 163, 113579. [Google Scholar] [CrossRef]
- Martín, I.; García, C.; Rodríguez, A.; Córdoba, J.J. Effect of a selected protective culture of Lactilactobacillus sakei on the evolution of volatile compounds and on the final sensorial characteristics of traditional dry-cured fermented “Salchichón”. Biology 2023, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Martín, I.; Rodríguez, A.; García, C.; Córdoba, J.J. Evolution of volatile vompounds during ripening and final sensory changes of traditional raw Ewe’s milk cheese “Torta del Casar” maturated with selected protective lactic acid bacteria. Foods 2022, 11, 2658. [Google Scholar] [CrossRef] [PubMed]
- Baka, A.M.; Papavergou, E.J.; Pragalaki, T.; Bloukas, J.G.; Kotzekidou, P. Effect of selected autochthonous starter cultures on processing and quality characteristics of Greek fermented sausages. LWT—Food Sci. Technol. 2011, 44, 54–61. [Google Scholar] [CrossRef]
- Zdenkova, K.; Alibayov, B.; Karamonova, L.; Purkrtova, S.; Karpiskova, R.; Demnerova, K. Transcriptomic and metabolic responses of Staphylococcus aureus in mixed culture with Lactobacillus plantarum, Streptococcus thermophilus and Enterococcus durans in milk. J. Ind Microbiol. Biotechnol. 2016, 43, 1237–1247. [Google Scholar] [CrossRef]
- Wareing, P. Controlling Clostridium botulinum—Using challenge testing to create safe chilled foods. Leatherhead Food Res. 2017. Available online: https://www.leatherheadfood.com/wp-content/uploads/2017/04/White-paper-45-Controlling-Clostridium-botulinum.pdf (accessed on 24 March 2023).
- Govari, M.; Pexara, A. Nitrates and nitrites in meat products. J. Hell Vet. Med. Soc. 2015, 66, 127–140. [Google Scholar] [CrossRef]
- Cantwell, M.; Elliot, C. Nitrates, Nitrites and Nitrosamines from processed meat intake and colorectal cancer risk. J. Clin. Nutr. Diet. 2017, 3, 27. [Google Scholar] [CrossRef]
- Hatoum, R.; Labrie, S.; Fliss, I. Identification and partial characterization of antilisterial compounds produced by dairy yeasts. Probiotics Antimicrob. Proteins 2013, 5, 8–17. [Google Scholar] [CrossRef]
- Muccilli, S.; Restuccia, C. Bioprotective role of yeasts. Microorganisms 2015, 3, 588–611. [Google Scholar] [CrossRef]
- Goerges, S.; Koslowsky, M.; Velagic, S.; Borst, N.; Bockelmann, W.; Heller, K.J.; Scherer, S. Anti-listerial potential of food-borne yeasts in red smear cheese. Int. Dairy J. 2011, 21, 83–89. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Shahryari, S.; Kharazmi, M.S.; Jafari, S.M. Food applications of probiotic yeasts; focusing on their techno-functional, postbiotic and protective capabilities. Trends Food Sci. Technol. 2022, 128, 278–295. [Google Scholar] [CrossRef]
- de Lima, M.D.S.F.; de Souza, K.M.S.; Albuquerque, W.W.C.; Teixeira, J.A.C.; Cavalcanti, M.T.H.; Porto, A.L.F. Saccharomyces cerevisiae from Brazilian kefir-fermented milk: An in vitro evaluation of probiotic properties. Microb. Pathog. 2017, 110, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Merchán, A.V.; Benito, M.J.; Galván, A.I.; Ruiz-Moyano Seco de Herrera, S. Identification and selection of yeast with functional properties for future application in soft paste cheese. LWT—Food Sci. Technol. 2020, 124, 109173. [Google Scholar] [CrossRef]
- Yousefi, M.; Khorshidian, N.; Hosseini, H. Potential application of essential oils for mitigation of Listeria monocytogenes in meat and poultry products. Front. Nutr. 2020, 7, 577287. [Google Scholar] [CrossRef]
- Oliveira, M.; Ferreira, V.; Magalhães, R.; Teixeira, P. Biocontrol strategies for Mediterranean-style fermented sausages. Food Res. Int. 2018, 103, 438–449. [Google Scholar] [CrossRef]
- Rangel-Ortega, S.d.C.; Campos-Múzquiz, L.G.; Charles-Rodriguez, A.V.; Chávez-Gonzaléz, M.L.; Palomo-Ligas, L.; Contreras-Esquivel, J.C.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Biological control of pathogens in artisanal cheeses. Int. Dairy J. 2023, 140, 105612. [Google Scholar] [CrossRef]
- Food and Drug Administration. Microorganisms and Derived Ingredients Used in Food (Partial List). Available online: https://www.fda.gov/food/generally-recognized-safe-gras/microorganisms-microbial-derived-ingredients-used-food-partial-list (accessed on 20 March 2023).
- Pavli, F.; Argyri, A.A.; Skandamis, P.; Nychas, G.J.; Tassou, C.; Chorianopoulos, N. Antimicrobial activity of oregano essential oil incorporated in sodium alginate edible films: Control of Listeria monocytogenes and spoilage in ham slices treated with high pressure processing. Materials 2019, 12, 3726. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2005, 10, 813–829. [Google Scholar] [CrossRef]
- De Billerbeck, V.G.; Roques, C.G.; Bessière, J.-M.; Fonvieille, J.-L.; Dargent, R. Effects of Cymbopogon nardus (L.) W. Watson essential oil on the growth and morphogenesis of Aspergillus niger. Can. J. Microbiol. 2001, 47, 9–17. [Google Scholar] [CrossRef]
- Castaño, H.; Ciro, G.; Zapata, J.E.; Jiménez, S. Actividad bactericida del extracto etanólico y del aceite esencial de hojas de Rosmarinus officinalis L. sobre algunas bacterias de interés alimentario. VITAE 2010, 17, 149–154. [Google Scholar]
- Jardak, M.; Elloumi-Mseddi, J.; Aifa, S.; Mnif, S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Baltar, A.; Pérez-Boto, D.; Medina, M.; Montiel, R. Genomic diversity and characterization of Listeria monocytogenes from dry-cured ham processing plants. Food Microbiol. 2021, 99, 103779. [Google Scholar] [CrossRef]
- Montiel, R.; Peirotén, Á.; Ortiz, S.; Bravo, D.; Gaya, P.; Martínez-Suárez, J.V.; Tapiador, J.; Nuñez, M.; Medina, M. Inactivation of Listeria monocytogenes during dry-cured ham processing. Int. J. Food Microbiol. 2020, 318, 108469. [Google Scholar] [CrossRef] [PubMed]
- El-Sharoud, W.M.; Belloch, C.; Peris, D.; Querol, A. Molecular identification of yeasts associated with traditional Egyptian dairy products. J. Food Sci. 2009, 74, M341–M346. [Google Scholar] [CrossRef]
- Venturini Copetti, M. Yeasts and molds in fermented food production: An ancient bioprocess. Curr. Opin. Food Sci. 2019, 25, 57–61. [Google Scholar] [CrossRef]
- Corsetti, A.; Rossi, J.; Gobbetti, M. Interactions between yeasts and bacteria in the smear surface-ripened cheeses. Int. J. Food Microbiol. 2001, 69, 1–10. [Google Scholar] [CrossRef]
- Ozturk, I.; Sagdic, O.; Yetim, H. Effects of autochthonous yeast cultures on some quality characteristics of traditional Turkish fermented sausage“Sucuk”. Food Sci. Anim. Resour. 2021, 41, 196. [Google Scholar] [CrossRef]
- Ramos-Moreno, L.; Ruiz-Pérez, F.; Rodríguez-Castro, E.; Ramos, J. Debaryomyces hansenii is a real tool to improve a diversity of characteristics in sausages and dry-meat products. Microorganisms 2021, 9, 1512. [Google Scholar] [CrossRef]
- Banjara, N.; Suhr, M.J.; Hallen-Adams, H.E. Diversity of yeast and mold species from a variety of cheese types. Curr Microbiol. 2015, 70, 792–800. [Google Scholar] [CrossRef]
- Riesute, R.; Salomskiene, J.; Moreno, D.S.; Gustiene, S. Effect of yeasts on food quality and safety and possibilities of their inhibition. Trends Food Sci. Technol. 2021, 108, 1–10. [Google Scholar] [CrossRef]
- Banjara, N.; Nickerson, K.W.; Suhr, M.J.; Hallen-Adams, H.E. Killer toxin from several food-derived Debaryomyces hansenii strains effective against pathogenic Candida yeasts. Int. J. Food Microbiol. 2016, 222, 23–29. [Google Scholar] [CrossRef]
- Jacques, N.; Casaregola, S. Safety assessment of dairy microorganisms: The hemiascomycetous yeasts. Int. J. Food Microbiol. 2008, 126, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Godič Torkar, K.; Golk Teger, S. The presence of some pathogen microorganisms, yeasts and moulds in cheese samples produced at small dairy-processing plants. Acta Agric. Slov. 2006, 88, 37–51. [Google Scholar]
- Encinas, J.P.; López-Díaz, T.M.; García-López, M.L.; Otero, A.; Moreno, B. Yeast populations on Spanish fermented sausages. Meat Sci. 2000, 54, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Núñez, F.; Rodríguez, M.M.; Bermúdez, M.E.; Córdoba, J.J.; Asensio, M.A. Composition and toxigenic potential of the mould population on dry-cured Iberian ham. Int. J. Food Microbiol. 1996, 32, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Simoncini, N.; Rotelli, D.; Virgili, R.; Quintavalla, S. Dynamics and characterization of yeasts during ripening of typical Italian dry-cured ham. Food Microbiol. 2007, 24, 577–584. [Google Scholar] [CrossRef]
- Sharaf, O.; Ibrahim, G.; Abd El-Khalik Mahmoud Effat, D.; Ibrahim, A.; Tawfek, N.F.; Effat, B.; El Shafei, K.; F El-Din, H.M.; Salem, M.M. Prevalence of some pathogenic microorganisms in factories Domiati, Feta cheeses and UHT milk in relation to public health sold under market conditions in Cairo. Artic. Int. J. Chem. Tech. Res. 2014, 6, 2807–2814. [Google Scholar]
- Singh, G.; Raksha, A.D.U. Candidal infection: Epidemiology, pathogenesis and recent advances for diagnosis. Bull Pharm. Med. Sci. 2013, 1, 1–8. [Google Scholar]
- Pfaller, M.A.; Diekema, D.J. Role of sentinel surveillance of candidemia: Trends in species distribution and antifungal susceptibility. J. Clin. Microbiol. 2002, 40, 3551–3557. [Google Scholar] [CrossRef]
- Wanderley, L.; Bianchin, A.; Arruda Teo, C.R.P.; Fuentefria, A.M. Occurrence and pathogenicity of Candida spp. in unpasteurized cheese. Braz. J. Biosci. 2013, 11, 145–148. [Google Scholar]
- Seiler, H.; Busse, M. The yeasts of cheese brines. Int. J. Food Microbiol. 1990, 11, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Tzanetakis, N.; Hatzikamari, M.; Litopoulou-Tzanetaki, E. Yeasts of the surface microflora of Feta cheese. In Proceedings of the Yeasts in the Dairy Industry: Positive and Negative Aspects, Copenhagen, Denmark, 2–3 September 1996; pp. 34–43. [Google Scholar]
- Spanamberg, A.; Pais Ramos, J.; Leoncini, O.; Hartz Alves, S.; Valente, P. High frequency of potentially pathogenic yeast species in goat’s raw milk and creamed cheese in Southern Brazil. Acta Sci. Vet. 2009, 37, 133–141. [Google Scholar] [CrossRef]
- Colombo, A.L.; Nucci, M.; Park, B.J.; Nouér, S.A.; Arthington-Skaggs, B.; da Matta, D.A.; Warnock, D.; Morgan, J. Epidemiology of candidemia in Brazil: A nationwide sentinel surveillance of candidemia in eleven medical centers. J. Clin. Microbiol. 2006, 44, 2816. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Roussos, N.; Vardakas, K.Z. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: A systematic review. Int. J. Infect. Dis. 2010, 14, e954–e966. [Google Scholar] [CrossRef]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; Van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Fröhlich-Wyder, M.T.; Arias-Roth, E.; Jakob, E. Cheese yeasts. Yeast 2019, 36, 129–141. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.; Malskær, A.H.; Jespersen, L.; Arneborg, N.; Johansen, P.G. The effects of NaCl and temperature on growth and survival of yeast strains isolated from Danish cheese brines. Curr. Microbiol. 2020, 77, 3377–3384. [Google Scholar] [CrossRef]
- Muccilli, S.; Wemhoff, S.; Restuccia, C.; Meinhardt, F. Exoglucanase-encoding genes from three Wickerhamomyces anomalus killer strains isolated from olive brine. Yeast 2013, 30, 33–43. [Google Scholar] [CrossRef]
- Buzzini, P.; Martini, A. Large-scale screening of selected Candida maltosa, Debaryomyces hansenii and Pichia anomala killer toxin activity against pathogenic yeasts. Med. Mycol. 2001, 39, 479–482. [Google Scholar] [CrossRef]
- Vadkertiová, R.; Sláviková, E. Killer activity of yeasts isolated from natural environments against some medically important Candida species. Pol. J. Microbiol. 2007, 56, 39–43. [Google Scholar] [PubMed]
- Ceugniez, A.; Drider, D.; Jacques, P.; Coucheney, F. Yeast diversity in a traditional French cheese “Tomme d’orchies” reveals infrequent and frequent species with associated benefits. Food Microbiol. 2015, 52, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Makki, G.M.; Kozak, S.M.; Jencarelli, K.G.; Alcaine, S.D. Evaluation of the efficacy of commercial protective cultures to inhibit mold and yeast in cottage cheese. Dairy Sci. 2021, 104, 2709–2718. [Google Scholar] [CrossRef]
- Ravichandran, S.; Muthuraman, S. Examining the anti-candidal activity of 10 selected Indian herbs and investigating the effect of Lawsonia inermis extract on germ tube formation, protease, phospholipase, and aspartate dehydrogenase enzyme activity in Candida albicans. Indian J. Pharmacol. 2016, 48, 47. [Google Scholar]
- Dorsaz, S.; Snäkä, T.; Favre-Godal, Q.; Maudens, P.; Boulens, N.; Furrer, P.; Ebrahimi, S.N.; Hamburger, M.; Allémann, E.; Gindro, K.; et al. Identification and mode of action of a plant natural product targeting human fungal pathogens. Antimicrob. Agents Chemother. 2017, 61, e00829-17. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Durak, A.; Pecio, Ł.; Kowalska, I. Nutraceutical potential of tinctures from fruits, green husks, and leaves of Juglans regia L. Sci. World J. 2014, 2014, 501392. [Google Scholar] [CrossRef]
- Nzeako, B.C.; N Al-Kharousi, Z.S.; Al-Mahrooqui, Z. Antimicrobial activities of clove and thyme extracts. Sultan Qaboos Univ. Med. J. 2006, 6, 33. [Google Scholar]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M. Antifungal activity of Eugenol against Penicillium, Aspergillus, and Fusarium species. J. Food Prot. 2010, 73, 1124–1128. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, W. The Candida albicans inhibitory activity of the extract from Papaya (Carica papaya L.) Seed relates to mitochondria dysfunction. Int. J. Mol. Sci. 2017, 18, 1858. [Google Scholar] [CrossRef]
- Li, W.R.; Shi, Q.S.; Dai, H.Q.; Liang, Q.; Xie, X.B.; Huang, X.M.; Zhao, G.Z.; Zhang, L.X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016, 6, 22805. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Khanniri, E.; Mortazavian, A.M. Potential application of essential oils as antimicrobial preservatives in cheese. Innov. Food Sci. Emerg. Technol. 2018, 45, 62–72. [Google Scholar] [CrossRef]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin contamination, its impact and management strategies: An updated review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Pattono, D.; Grosso, A.; Stocco, P.P.; Pazzi, M.; Zeppa, G. Survey of the presence of patulin and ochratoxin A in traditional semi-hard cheeses. Food Control 2013, 33, 54–57. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 years of research. Toxins 2016, 8, 12–15. [Google Scholar] [CrossRef] [PubMed]
- IARC Aflatoxins. A Review of Human Carcinogens. Chemical Agents and Related Occupations; IARC: Lyon, France, 2012; pp. 225–248. ISBN 978 92 832 1323 9. [Google Scholar]
- IARC; Ochratoxin, A. IARC Monogr Eval Carcinog Risk Chem to Humans; IARC: Lyon, France, 1993; pp. 489–521. [Google Scholar]
- Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; Nielsen, E.; et al. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, 6113. [Google Scholar]
- Álvarez, M.; Núñez, F.; Delgado, J.; Andrade, M.J.; Rodrigues, P. Proteomic evaluation of the effect of antifungal agents on Aspergillus westerdijkiae ochratoxin A production in a dry-cured fermented sausage-based medium. Int. J. Food Microbiol. 2022, 379, 109858. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.; Delgado, J.; Núñez, F.; Cebrián, E.; Andrade, M.J. Proteomic analyses reveal mechanisms of action of biocontrol agents on ochratoxin A repression in Penicillium nordicum. Food Control 2021, 129, 108232. [Google Scholar] [CrossRef]
- Álvarez, M.; Rodríguez, A.; Peromingo, B.; Núñez, F.; Rodríguez, M. Enterococcus faecium: A promising protective culture to control growth of ochratoxigenic moulds and mycotoxin production in dry-fermented sausages. Mycotoxin Res. 2019, 36, 137–145. [Google Scholar] [CrossRef]
- Delgado, J.; Núñez, F.; Asensio, M.A.; Owens, R.A. Quantitative proteomic profiling of ochratoxin A repression in Penicillium nordicum by protective cultures. Int. J. Food Microbiol. 2019, 305, 108243. [Google Scholar] [CrossRef]
- Meftah, S.; Abid, S.; Dias, T.; Rodrigues, P. Effect of dry-sausage starter culture and endogenous yeasts on Aspergillus westerdijkiae and Penicillium nordicum growth and OTA production. LWT—Food Sci. Technol. 2018, 87, 250–258. [Google Scholar] [CrossRef]
- Cebrián, E.; Núñez, F.; Gálvez, F.J.; Delgado, J.; Bermúdez, E.; Rodríguez, M. Selection and evaluation of Staphylococcus xylosus as a biocontrol agent against toxigenic moulds in a dry-cured ham model system. Microorganisms 2020, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Simoncini, N.; Virgili, R.; Spadola, G.; Battilani, P. Autochthonous yeasts as potential biocontrol agents in dry-cured meat products. Food Control 2014, 46, 160–167. [Google Scholar] [CrossRef]
- Nazareth, T.D.M.; Calpe, J.; Luz, C.; Mañes, J. Manufacture of a potential antifungal ingredient using lactic acid bacteria from dry-cured sausages. Foods 2023, 12, 1427. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.; Delgado, J.; Núñez, F.; Roncero, E.; Andrade, M.J. Proteomic approach to unveil the ochratoxin A repression by Debaryomyces hansenii and rosemary on Penicillium nordicum during dry-cured fermented sausages ripening. Food Control 2022, 137, 108695. [Google Scholar] [CrossRef]
- Peromingo, B.; Núñez, F.; Rodríguez, A.; Alía, A.; Andrade, M.J. Potential of yeasts isolated from dry-cured ham to control ochratoxin A production in meat models. Int. J. Food Microbiol. 2018, 268, 73–80. [Google Scholar] [CrossRef]
- Álvarez, M.; Núñez, F.; Delgado, J.; Andrade, M.J.; Rodríguez, M.; Rodríguez, A. Competitiveness of three biocontrol candidates against ochratoxigenic Penicillium nordicum under dry-cured meat environmental and nutritional conditions. Fungal. Biol. 2021, 129, 108232. [Google Scholar] [CrossRef]
- Rodríguez, A.; Bernáldez, V.; Rodríguez, M.; Andrade, M.J.; Núñez, F.; Córdoba, J.J. Effect of selected protective cultures on ochratoxin A accumulation in dry-cured Iberian ham during its ripening process. LWT—Food Sci. Technol. 2015, 60, 923–928. [Google Scholar] [CrossRef]
- Bernáldez, V.; Córdoba, J.J.; Rodríguez, M.; Cordero, M.; Polo, L.; Rodríguez, A. Effect of Penicillium nalgiovense as protective culture in processing of dry-fermented sausage “ salchichón”. Food Control 2013, 32, 69–76. [Google Scholar] [CrossRef]
- Guimarães, A.; Ramos, Ó.; Cerqueira, M.; Venâncio, A.; Abrunhosa, L. Active whey protein edible films and coatings incorporating Lactobacillus buchneri for Penicillium nordicum control in cheese. Food Bioprocess Technol. 2020, 13, 1074–1086. [Google Scholar] [CrossRef]
- Delgado, J.; Rodríguez, A.; García, A.; Núñez, F.; Asensio, M. Inhibitory effect of PgAFP and protective cultures on Aspergillus parasiticus growth and aflatoxins production on dry-fermented sausage and cheese. Microorganisms 2018, 6, 69. [Google Scholar] [CrossRef]
- Leonelli Pires de Campos, A.C.; Saldanha Nandi, R.D.; Scandorieiro, S.; Gonçalves, M.C.; Reis, G.F.; Dibo, M.; Medeiros, L.P.; Panagio, L.A.; Fagan, E.P.; Takayama Kobayashi, R.K.; et al. Antimicrobial effect of Origanum vulgare (L.) essential oil as an alternative for conventional additives in the Minas cheese manufacture. LWT—Food Sci. Technol. 2022, 157, 113063. [Google Scholar] [CrossRef]
- Vitalini, S.; Nalbone, L.; Bernardi, C.; Iriti, M.; Costa, R.; Cicero, N.; Giarratana, F.; Vallone, L. Ginger and parsley essential oils: Chemical composition, antimicrobial activity, and evaluation of their application in cheese preservation. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, B.I.; Fente, C.; Franco, C.M.; Vazquez, M.J.; Cepeda, A. Inhibitory effects of eugenol and thymol on Penicillium citrinum strains in culture media and cheese. Int. J. Food Microbiol. 2001, 67, 157–163. [Google Scholar] [CrossRef]
- Akrami, F.; Misaghi, A.; Gheisari, H.; Akhondzadeh, A.; Amiri, A.; Razi, S.; Derakhshan, Z.; Dehghani, R. The effect of Zataria multi flora Boiss essential oil on the growth and citrinin production of Penicillium citrinum in culture media and cheese. Food Chem. Toxicol. 2018, 118, 691–694. [Google Scholar]
- Noori, N.; Yahyaraeyat, R.; Khosravi, A.; Atefi, P.; Basti, A.A.; Akrami, F.; Bahonar, A.; Misaghi, A.L.I. Effect of Zataria multiflora Boiss essential oil on growth and citrinin production by Penicillium citrinum in culture media and mozzarella cheese. J. Food Sci. 2012, 32, 445–451. [Google Scholar] [CrossRef]
- Schlösser, I.; Prange, A. Effects of selected natural preservatives on the mycelial growth and ochratoxin A production of the food-related moulds Aspergillus westerdijkiae and Penicillium verrucosum. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2019, 36, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Císarová, M.; Hleba, L.; Medo, J.; Tančinová, D.; Mašková, Z.; Čuboň, J.; Kováčik, A.; Foltinová, D.; Božik, M.; Klouček, P. The in vitro and in situ effect of selected essential oils in vapour phase against bread spoilage toxicogenic aspergilli. Food Control 2020, 110, 107007. [Google Scholar] [CrossRef]
- Gützkow, K.L.; Al Ayoubi, C.; Soler Vasco, L.; Rohn, S.; Maul, R. Analysis of ochratoxin A, aflatoxin B1 and its biosynthetic precursors in cheese—Method development and market sample screening. Food Control 2022, 143, 109241. [Google Scholar] [CrossRef]
- Rodríguez-Cañás, I.; González-Jartín, J.M.; Alvariño, R.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Detection of mycotoxins in cheese using an optimized analytical method based on a QuEChERS extraction and UHPLC-MS/MS quantification. Food Chem. 2023, 408, 135182. [Google Scholar] [CrossRef]
- Mareze, J.; Ramos-Pereira, J.; Santos, J.A.; Beloti, V.; López-Díaz, T.M. Identification and characterisation of lactobacilli isolated from an artisanal cheese with antifungal and antibacterial activity against cheese spoilage and mycotoxigenic Penicillium spp. Int. Dairy J. 2022, 130, 105367. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Zadravec, M.; Brnić, D.; Perković, I.; Škrivanko, M.; Kovačević, D. Moulds and mycotoxins detected in the regional speciality fermented sausage “slavonski kulen” during a 1-year production period. Food Addit. Contam. Part A 2017, 34, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Rodriguez, A.; Magistá, D.; Magan, N. Insights into existing and future fungal and mycotoxin contamination of cured meats. Curr. Opin. Food Sci. 2019, 29, 20–27. [Google Scholar] [CrossRef]
- Zadravec, M.; Vahčić, N.; Brnić, D.; Markov, K.; Frece, J.; Beck, R.; Lešić, T.; Pleadin, J. A study of surface moulds and mycotoxins in Croatian traditional dry-cured meat products. Int. J. Food Microbiol. 2020, 317, 108459. [Google Scholar] [CrossRef]
- Carvajal-Moreno, M.; Vargas-Ortiz, M.; Hernández-Camarillo, E.; Ruiz-Velasco, S.; Rojo-Callejas, F. Presence of unreported carcinogens, Aflatoxins and their hydroxylated metabolites, in industrialized Oaxaca cheese from Mexico City. Food Chem. Toxicol. 2019, 124, 128–138. [Google Scholar] [CrossRef]
- Onmaz, N.E.; Çinicioglu, S.; Gungor, C. Moulds and aflatoxins in traditional moldy civil cheese: Presence and public health concerns. J. Hell. Vet. Med. Soc. 2020, 85, 3–11. [Google Scholar]
- Sakin, F.; Tekeli, İ.O.; Yipel, M.; Kürekci, C. Occurrence and health risk assessment of aflatoxins and ochratoxin a in Sürk, a Turkish dairy food, as studied by HPLC. Food Control 2018, 90, 317–323. [Google Scholar] [CrossRef]
- Maragos, C.M.; Probyn, C.; Proctor, R.H.; Sieve, K.K. Cyclopiazonic acid in soft-ripened and blue cheeses marketed in the USA. Food Addit. Contam. Part B 2023, 16, 14–23. [Google Scholar] [CrossRef]
- Ostry, V.; Toman, J.; Grosse, Y.; Malir, F. Cyclopiazonic acid: 50th anniversary of its discovery. World Mycotoxin J. 2018, 11, 135–148. [Google Scholar] [CrossRef]
- Díaz Nieto, C.H.; Granero, A.M.; Zon, M.A.; Fernández, H. Sterigmatocystin: A mycotoxin to be seriously considered. Food Chem. Toxicol. 2018, 118, 460–470. [Google Scholar] [CrossRef]
- IARC. Agents Classified by the IARC Monographs, Volumes 1–123; International Agency for Research on Cancer: Lyon, France, 2018; pp. 1–37.
- Pietri, A.; Leni, G.; Mulazzi, A.; Bertuzzi, T. Ochratoxin A and sterigmatocystin in long-ripened Grana cheese: Occurrence, wheel rind contamination and effectiveness of cleaning techniques on grated products. Toxins 2022, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Tebar, N.; González-Navarro, E.J.; López-Díaz, T.M.; Santos, J.A.; Ortiz de Elguea-Culebras, G.; García-Martínez, M.M.; Molina, A.; Carmona, M.; Berruga, M.I. Biological activity of extracts from aromatic plants as control agents against spoilage molds isolated from sheep cheese. Foods 2021, 10, 1576. [Google Scholar] [CrossRef]
- Coton, M.; Au, A.; Poirier, E.; Debaets, S.; Coton, E.; Dantigny, P. Production and migration of ochratoxin A and citrinin in Comté cheese by an isolate of Penicillium verrucosum selected among Penicillium spp. mycotoxin producers in YES medium. Food Microbiol. 2019, 82, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, L.; Dhivya, R.; Dhanasekaran, D.; Periasamy, V.S.; Alshatwi, A.A.; Akbarsha, M.A. Hepatotoxic effect of ochratoxin A and citrinin, alone and in combination, and protective effect of vitamin E: In vitro study in HepG2 cell. Food Chem. Toxicol. 2015, 83, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Sharma, N.; Sharma, B.; Mishra, S.; Arora, S.; Selvakumar, R.; Saurabh, V.; et al. Citrinin mycotoxin contamination in food and feed: Impact on agriculture, human health, and detection and management strategies. Toxins 2022, 14, 85. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Stoll, D.; Schütz, P.; Geisen, R. Oxidative stress induces the biosynthesis of citrinin by Penicillium verrucosum at the expense of ochratoxin. Int. J. Food Microbiol. 2015, 192, 1–6. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Graf, E.; Stoll, D.; Geisen, R. The biosynthesis of ochratoxin A by Penicillium as one mechanism for adaptation to NaCl rich foods. Food Microbiol. 2012, 29, 233–241. [Google Scholar] [CrossRef]
- Tsuda, H.; Matsumoto, T.; Ishimi, Y. Selection of lactic acid bacteria as starter cultures for fermented meat products. Food Sci. Technol. Res. 2012, 18, 713–721. [Google Scholar] [CrossRef]
- Paik, H.D.; Lee, J.Y. Investigation of reduction and tolerance capability of lactic acid bacteria isolated from kimchi against nitrate and nitrite in fermented sausage condition. Meat Sci. 2014, 97, 609–614. [Google Scholar] [CrossRef]
- Zagorec, M.; Champomier-Vergès, M.C. Lactobacillus sakei: A starter for sausage fermentation, a protective culture for meat products. Microorganisms 2017, 5, 56. [Google Scholar] [CrossRef]
- Crowther, T.W.; Boddy, L.; Maynard, D.S. The use of artificial media in fungal ecology. Fungal. Ecol. 2018, 32, 87–91. [Google Scholar] [CrossRef]
- Álvarez, M.; Andrade, M.J.; Núñez, F.; Rodríguez, M.; Delgado, J. Proteomics as a new-generation tool for studying moulds related to food safety and quality. Int. J. Mol. Sci. 2023, 24, 4709. [Google Scholar] [CrossRef] [PubMed]
- Picozzi, C.; Bonacina, G.; Vigentini, I.; Foschino, R. Genetic diversity in Italian Lactobacillus sanfranciscensis strains assessed by multilocus sequence typing and pulsed-field gel electrophoresis analyses. Microbiology 2010, 156, 2035–2045. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.J.; Rodríguez, M.; Sánchez, B.; Aranda, E.; Córdoba, J.J. DNA typing methods for differentiation of yeasts related to dry-cured meat products. Int. J. Food Microbiol. 2006, 107, 48–58. [Google Scholar] [CrossRef]
- Andrade, M.J.; Rodríguez, M.; Casado, E.; Córdoba, J.J. Efficiency of mitochondrial DNA restriction analysis and RAPD-PCR to characterize yeasts growing on dry-cured Iberian ham at the different geographic areas of ripening. Meat Sci. 2010, 84, 377–383. [Google Scholar] [CrossRef]
- Sánchez, B.; Rodríguez, M.; Casado, E.M.; Martín, A.; Córdoba, J.J. Development of an efficient fungal DNA extraction method to be used in random amplified polymorphic DNA-PCR analysis to differentiate cyclopiazonic acid mold producers. J. Food Prot. 2008, 71, 2497–2503. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).