Respiratory Distress Complicating Falciparum Malaria Imported to Berlin, Germany: Incidence, Burden, and Risk Factors
Abstract
1. Introduction
| Criterion | Definition |
|---|---|
| Jaundice | Plasma or serum bilirubin > 50 µmol/L (>3 mg/dL) with parasitemia > 100,000/µL |
| Hyper-parasitemia | >10% parasitized erythrocytes |
| Decompensated shock | Systolic blood pressure < 80 mmHg with a need for norepinephrine dosages > 0.05 µg/kg/min to maintain a mean arterial blood pressure > 65 mmHg despite adequate hydration |
| Renal impairment | Plasma or serum creatinine > 265 µmol/L (>3 mg/dL) or blood urea > 20 mmol/L (>120 mg/dL) |
| Respiratory distress (APO and ARDS) | Oxygen saturation of room air < 92%, respiratory rate > 30/min, and bilateral opacities on chest imaging |
| Acute pulmonary oedema (APO) | Oxygen saturation of room air < 92% and respiratory rate > 30/min together with bilateral opacities on chest imaging |
| Acute respiratory distress syndrome (ARDS) | Lung injury within 1 week of admission with progression of respiratory symptoms; bilateral opacities on chest imaging not explained by other lung pathologies; respiratory failure not explained by heart failure or volume overload; PaO2/FiO2 ≤ 300 mmHg under a minimum PEEP of 5 cmH2O (applied using non-invasive or invasive ventilation) |
| Significant bleeding | Including recurrent or prolonged bleeding from the nose, gums, venepuncture sites, hematemesis, or melena |
| Severe malarial anemia | Hemoglobin level < 7 g/dL and/or hematocrit < 20% with parasitemia > 0.5% |
| Impaired consciousness | Glasgow coma scale (GCS) < 11 |
| Acidosis | Base deficit > 8 mmol/L, and/or plasma bicarbonate < 15 mmol/L, and/or venous plasma lactate ≥ 5 mmol/L, or ≥45 mg/dL |
| Hypoglycemia | Blood glucose level < 2.2 mmol/L or <40 mg/dL |
| Multiple convulsions | >two convulsions within 24 h |
2. Materials and Methods
2.1. Data Collection
2.2. Clinical Management
2.3. Study Endpoints
2.4. Statistical Analysis
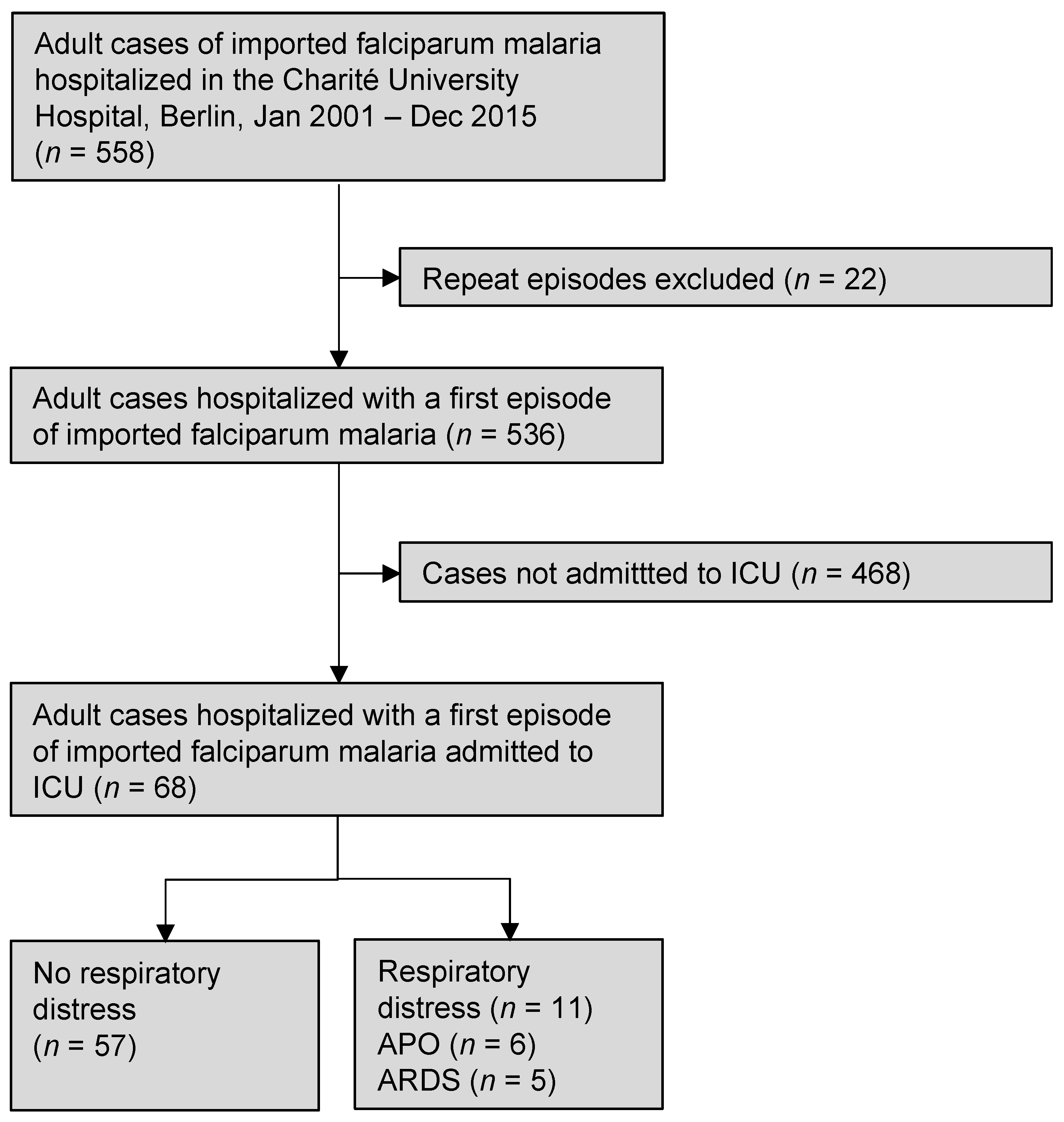
3. Results
3.1. Management of the Patients
3.2. Length of ICU Stay
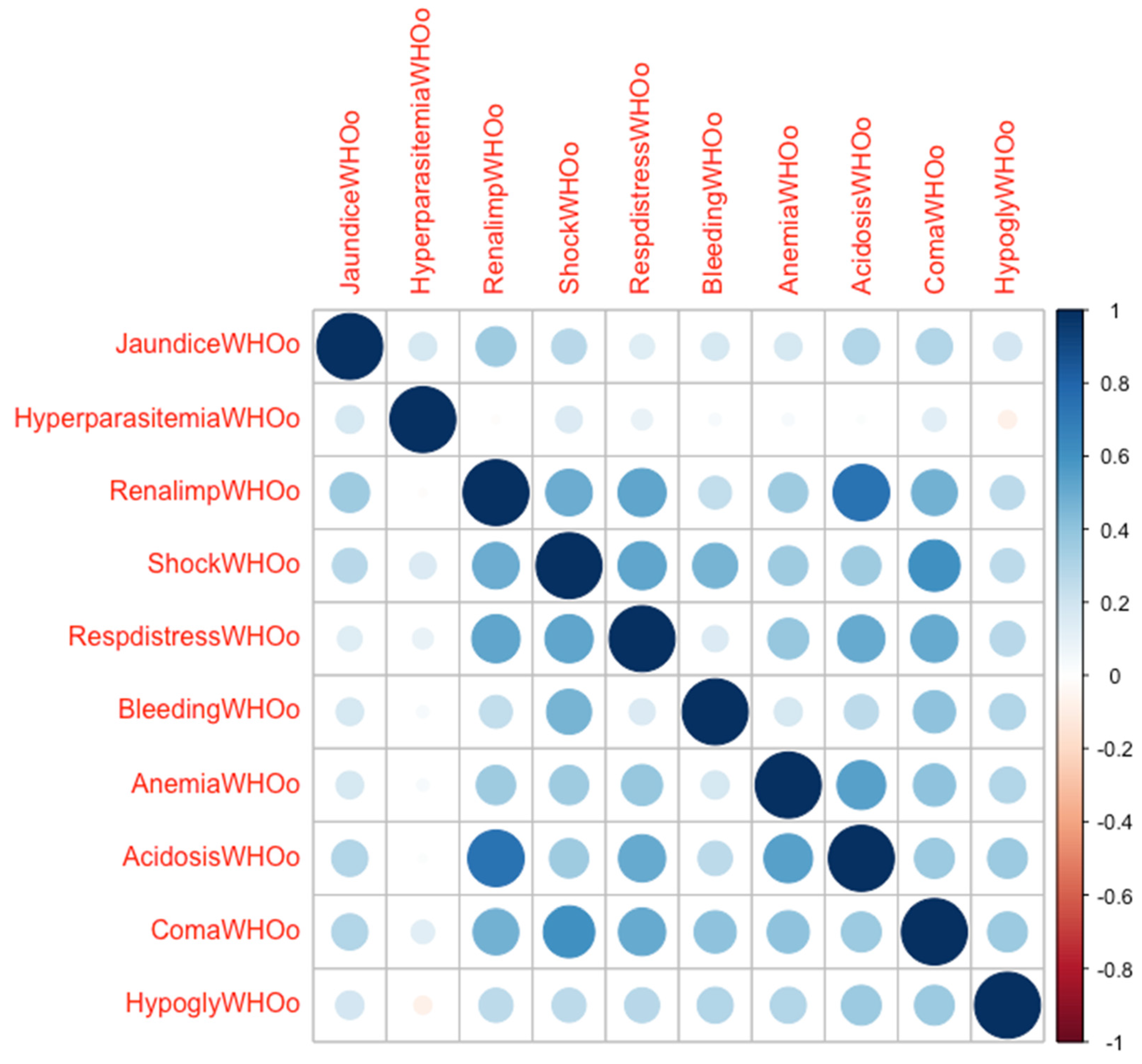
3.3. Risk Factors for Respiratory Distress
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dondorp, A.; Nosten, F.; Stepniewska, K.; Day, N.; White, N. South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group Artesunate versus Quinine for Treatment of Severe Falciparum Malaria: A Randomised Trial. Lancet 2005, 366, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Keene, C.M.; Dondorp, A.; Crawley, J.; Ohuma, E.O.; Mukaka, M. A Competing-Risk Approach for Modeling Length of Stay in Severe Malaria Patients in South-East Asia and the Implications for Planning of Hospital Services. Clin. Infect. Dis. 2018, 67, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case Characteristics, Resource Use, and Outcomes of 10 021 Patients with COVID-19 Admitted to 920 German Hospitals: An Observational Study. Lancet Respir. Med. 2020, 8, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, A.; Schranz, M.; Rexroth, U.; Hamouda, O.; Schaade, L.; Diercke, M.; Boender, T.S. Robert Koch’s Infectious Disease Surveillance Group Impact of the COVID-19 Pandemic and Associated Non-Pharmaceutical Interventions on Other Notifiable Infectious Diseases in Germany: An Analysis of National Surveillance Data during Week 1-2016-Week 32-2020. Lancet Reg. Health Eur. 2021, 6, 100103. [Google Scholar] [CrossRef]
- WHO Guidelines for Malaria. In WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2021.
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A New Simplified Acute Physiology Score (SAPS II) Based on a European/North American Multicenter Study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 Version of the Charlson Comorbidity Index Predicted in-Hospital Mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef]
- Verburg, I.W.M.; de Keizer, N.F.; de Jonge, E.; Peek, N. Comparison of Regression Methods for Modeling Intensive Care Length of Stay. PLoS ONE 2014, 9, e109684. [Google Scholar] [CrossRef]
- Thabane, L.; Mbuagbaw, L.; Zhang, S.; Samaan, Z.; Marcucci, M.; Ye, C.; Thabane, M.; Giangregorio, L.; Dennis, B.; Kosa, D.; et al. A Tutorial on Sensitivity Analyses in Clinical Trials: The What, Why, When and How. BMC Med. Res. Methodol. 2013, 13, 92. [Google Scholar] [CrossRef]
- Zhang, Z. Variable Selection with Stepwise and Best Subset Approaches. Ann. Transl. Med. 2016, 4, 136. [Google Scholar] [CrossRef]
- Bos, L.D.J.; Ware, L.B. Acute Respiratory Distress Syndrome: Causes, Pathophysiology, and Phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute Respiratory Distress Syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Odeyemi, Y.E.; Herasevich, S.; Gong, M.N.; Gajic, O.O. Clinical Strategies to Prevent Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 129–136. [Google Scholar] [CrossRef]
- Gajic, O.; Dabbagh, O.; Park, P.K.; Adesanya, A.; Chang, S.Y.; Hou, P.; Anderson, H.; Hoth, J.J.; Mikkelsen, M.E.; Gentile, N.T.; et al. Early Identification of Patients at Risk of Acute Lung Injury: Evaluation of Lung Injury Prediction Score in a Multicenter Cohort Study. Am. J. Respir. Crit. Care Med. 2011, 183, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Bice, T.; Li, G.; Malinchoc, M.; Lee, A.S.; Gajic, O. Incidence and Risk Factors of Recurrent Acute Lung Injury. Crit. Care. Med. 2011, 39, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Gosho, M.; Ohigashi, T.; Nagashima, K.; Ito, Y.; Maruo, K. Bias in Odds Ratios from Logistic Regression Methods with Sparse Data Sets. J. Epidemiol. 2021; advance online publication. [Google Scholar] [CrossRef]
- Greenland, S.; Mansournia, M.A.; Altman, D.G. Sparse Data Bias: A Problem Hiding in Plain Sight. BMJ 2016, 352, i1981. [Google Scholar] [CrossRef] [PubMed]
- Makowski, D.; Ben-Shachar, M.S.; Chen, S.H.A.; Lüdecke, D. Indices of Effect Existence and Significance in the Bayesian Framework. Front. Psychol. 2019, 10, 2767. [Google Scholar] [CrossRef]
- Makowski, D.; Ben-Shachar, M.S.; Lüdecke, D. BayestestR: Describing Effects and Their Uncertainty, Existence and Significance within the Bayesian Framework. J. Open Source Softw. 2019, 4, 1541. [Google Scholar] [CrossRef]
- Phiri, H.T.; Bridges, D.J.; Glover, S.J.; van Mourik, J.A.; de Laat, B.; M’baya, B.; Taylor, T.E.; Seydel, K.B.; Molyneux, M.E.; Faragher, E.B.; et al. Elevated Plasma von Willebrand Factor and Propeptide Levels in Malawian Children with Malaria. PLoS ONE 2011, 6, e25626. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.W.; Lampah, D.A.; Gitawati, R.; Tjitra, E.; Kenangalem, E.; Piera, K.; Price, R.N.; Duffull, S.B.; Celermajer, D.S.; Anstey, N.M. Angiopoietin-2 Is Associated with Decreased Endothelial Nitric Oxide and Poor Clinical Outcome in Severe Falciparum Malaria. Proc. Natl. Acad. Sci. USA 2008, 105, 17097–17102. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.-T.; Punsawad, C.; Glaharn, S.; De Meyer, S.F.; Viriyavejakul, P.; Van den Steen, P.E. Release of Endothelial Activation Markers in Lungs of Patients with Malaria-Associated Acute Respiratory Distress Syndrome. Malar. J. 2019, 18, 395. [Google Scholar] [CrossRef] [PubMed]
- Van den Steen, P.E.; Deroost, K.; Deckers, J.; Van Herck, E.; Struyf, S.; Opdenakker, G. Pathogenesis of Malaria-Associated Acute Respiratory Distress Syndrome. Trends Parasitol. 2013, 29, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.R.J.; Hanson, J.; Turner, G.D.H.; White, N.J.; Dondorp, A.M. Respiratory Manifestations of Malaria. Chest 2012, 142, 492–505. [Google Scholar] [CrossRef]
- Maguire, G.P.; Handojo, T.; Pain, M.C.F.; Kenangalem, E.; Price, R.N.; Tjitra, E.; Anstey, N.M. Lung Injury in Uncomplicated and Severe Falciparum Malaria: A Longitudinal Study in Papua, Indonesia. J. Infect. Dis. 2005, 192, 1966–1974. [Google Scholar] [CrossRef]
- Hanson, J.P.; Lam, S.W.K.; Mohanty, S.; Alam, S.; Pattnaik, R.; Mahanta, K.C.; Hasan, M.U.; Charunwatthana, P.; Mishra, S.K.; Day, N.P.J.; et al. Fluid Resuscitation of Adults with Severe Falciparum Malaria: Effects on Acid-Base Status, Renal Function, and Extravascular Lung Water. Crit. Care Med. 2013, 41, 972–981. [Google Scholar] [CrossRef]
- Mohan, A.; Sharma, S.K.; Bollineni, S. Acute Lung Injury and Acute Respiratory Distress Syndrome in Malaria. J. Vector Borne Dis. 2008, 45, 179–193. [Google Scholar]
- Levin, M.; Cunnington, A.J.; Wilson, C.; Nadel, S.; Lang, H.J.; Ninis, N.; McCulloch, M.; Argent, A.; Buys, H.; Moxon, C.A.; et al. Effects of Saline or Albumin Fluid Bolus in Resuscitation: Evidence from Re-Analysis of the FEAST Trial. Lancet Respir. Med. 2019, 7, 581–593. [Google Scholar] [CrossRef]
- Magazine, R.; Rao, S.; Chogtu, B.; Venkateswaran, R.; Shahul, H.A.; Goneppanavar, U. Epidemiological Profile of Acute Respiratory Distress Syndrome Patients: A Tertiary Care Experience. Lung 2017, 34, 38–42. [Google Scholar] [CrossRef]
- Mungwira, R.G.; Laurens, M.B.; Nyangulu, W.; Divala, T.H.; Nampota-Nkomba, N.; Buchwald, A.G.; Nyirenda, O.M.; Mwinjiwa, E.; Kanjala, M.; Galileya, L.T.; et al. High Burden of Malaria among Malawian Adults on Antiretroviral Therapy after Discontinuing Prophylaxis. AIDS 2022, 36, 1675–1682. [Google Scholar] [CrossRef]
- Nyein, P.P.; Aung, N.M.; Kyi, T.T.; Htet, Z.W.; Anstey, N.M.; Kyi, M.M.; Hanson, J. High Frequency of Clinically Significant Bacteremia in Adults Hospitalized With Falciparum Malaria. Open Forum. Infect. Dis. 2016, 3, ofw028. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Gupta-Wright, A.; Doherty, J.F.; Singer, M.; Walker, D. Managing Malaria in the Intensive Care Unit. Br. J. Anaesth. 2014, 113, 910–921. [Google Scholar] [CrossRef]
- Karnad, D.R.; Nor, M.B.M.; Richards, G.A.; Baker, T.; Amin, P. Council of the World Federation of Societies of Intensive and Critical Care Medicine Intensive Care in Severe Malaria: Report from the Task Force on Tropical Diseases by the World Federation of Societies of Intensive and Critical Care Medicine. J. Crit. Care 2018, 43, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Sandlund, J.; Naucler, P.; Dashti, S.; Shokri, A.; Eriksson, S.; Hjertqvist, M.; Karlsson, L.; Capraru, T.; Färnert, A. Bacterial Coinfections in Travelers with Malaria: Rationale for Antibiotic Therapy. J. Clin. Microbiol. 2013, 51, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ishioka, H.; Plewes, K.; Pattnaik, R.; Kingston, H.W.F.; Leopold, S.J.; Herdman, M.T.; Mahanta, K.; Mohanty, A.; Dey, C.; Alam, S.; et al. Associations Between Restrictive Fluid Management and Renal Function and Tissue Perfusion in Adults With Severe Falciparum Malaria: A Prospective Observational Study. J. Infect Dis. 2020, 221, 285–292. [Google Scholar] [CrossRef]
- Graça, L.; Abreu, I.G.; Santos, A.S.; Graça, L.; Dias, P.F.; Santos, M.L. Descriptive Acute Respiratory Distress Syndrome (ARDS) in Adults with Imported Severe Plasmodium Falciparum Malaria: A 10 Year-Study in a Portuguese Tertiary Care Hospital. PLoS ONE 2020, 15, e0235437. [Google Scholar] [CrossRef]
- Marrelli, M.T.; Brotto, M. The Effect of Malaria and Anti-Malarial Drugs on Skeletal and Cardiac Muscles. Malar. J. 2016, 15, 524. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Litell, J.M.; Malinchoc, M.; Kashyap, R.; Schiller, H.J.; Pannu, S.R.; Singh, B.; Li, G.; Gajic, O. The Role of Potentially Preventable Hospital Exposures in the Development of Acute Respiratory Distress Syndrome: A Population-Based Study. Crit. Care Med. 2014, 42, 31–39. [Google Scholar] [CrossRef]
- Litell, J.M.; Gong, M.N.; Talmor, D.; Gajic, O. Acute Lung Injury: Prevention May Be the Best Medicine. Respir. Care 2011, 56, 1546–1554. [Google Scholar] [CrossRef]
- McHugh, M.D.; Aiken, L.H.; Sloane, D.M.; Windsor, C.; Douglas, C.; Yates, P. Effects of Nurse-to-Patient Ratio Legislation on Nurse Staffing and Patient Mortality, Readmissions, and Length of Stay: A Prospective Study in a Panel of Hospitals. Lancet 2021, 397, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Aiken, L.H.; Sloane, D.M.; Bruyneel, L.; Van den Heede, K.; Griffiths, P.; Busse, R.; Diomidous, M.; Kinnunen, J.; Kózka, M.; Lesaffre, E.; et al. Nurse Staffing and Education and Hospital Mortality in Nine European Countries: A Retrospective Observational Study. Lancet 2014, 383, 1824–1830. [Google Scholar] [CrossRef] [PubMed]

| Substance | Dosage | Duration | Median Parasitemia (IQR) | Number (%) of Cases Treated |
|---|---|---|---|---|
| Artemisinin derivates | 8.0 (2.5; 10.3) | 33 (48.5) | ||
| Artesunate | Loading dose of 2.4 mg/kg IBW intravenously | 2.4 mg/kg IBW 12, 24, 48, and 72 h after loading dose. When able to take oral medication, the patients received a full course of oral artemether/lumefantrine or dihydroartemisinin/piperaquine | 9.5 (5.1; 11.3) | 25 (36.8) |
| Artemether/Lumefantrine | Four tablets of 20/120 mg orally | 0, 8, 24, 36, 48, and 60 h after diagnosis | 1.9 (0.7; 2.5) | 6 (8.8) |
| Dihydroartemisinin/Piperaquine | Four tablets of 320/40 mg orally | 0, 24, and 48 h after diagnosis | - | 2 (2.9) |
| Quinine-based regimens (n = 35) | 8.0 (4.3; 9.6) | 35 (51.5) | ||
| Quinine/Doxycyline | 20 mg quinine hydrochloride (16.4 mg base)/kg IBW loading dose intravenously. Doxycycline 100 mg intravenously twice daily | 10 mg quinine hydrochloride/kg IBW thrice daily starting 8 h after loading dose. After 48 h, 10 mg/kg IBW twice daily | 7.3 (3.4; 9.1) | 31 (45.6) |
| Quinine/Clindamycin | 20 mg quinine hydrochloride (16.4 mg base)/kg IBW loading dose intravenously. Clindamycin: 600–900 mg IV thrice daily intravenously | 10 mg quinine hydrochloride/kg IBW thrice daily starting 8 h after loading dose. After 48 h, 10 mg/kg IBW twice daily | 11.3 (6.5; 18.6) | 4 (5.9) |
| Type of Co-Infection | Isolated Pathogen | Patients Affected, n |
|---|---|---|
| Community-acquired co-infections (n = 13) | ||
| Chronic viral co-infection | HIV HCV | 7 1 |
| Mixed malaria | P. malariae + P. falciparum | 2 |
| Other travel-associated co-infection | L. interrogans Dengue virus | 1 1 |
| ≥1 Co-infection | HBV + P. vivax + P. falciparum | 1 |
| Healthcare-associated co-infections (n = 8) | ||
| Central-line-associated bloodstream infection (CLABSI) | S. epidermidis | 3 |
| Aspiration/pneumonia | S. aureus (MSSA) S. aureus (MSSA) and P. aeruginosa | 1 1 |
| Catheter-associated urinary tract infection (CAUTI) | E. coli | 3 |
| Criterion | Patients Affected, n (%) | Number of Total Malaria-Specific Complications on Admission, Median (IQR) | Median LOS-ICU (IQR) | Hazard Ratio (95%CI) for ICU Discharge by 38 h | p-Value | Hazard Ratio (95%CI) for ICU Discharge by 61 h | p-Value | Hazard Ratio (95%CI) for ICU Discharge by 91 h | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Jaundice | 21 (30.9) | 2 (1; 5) | 64 (48; 122) | 0.78 (0.44–1.39) | 0.405 | 0.68 (0.33–1.38) | 0.283 | 1.00 (0.37–2.67) | 0.993 |
| Hyper-parasitemia | 18 (26.5) | 2 (1; 4) | 66 (40; 109) | 0.84 (0.45–1.57) | 0.586 | 0.77 (0.36–1.67) | 0.515 | 0.98 (0.34–2.85) | 0.968 |
| Decompensated shock | 12 (17.7) | 4 (4; 6) | 160(82; 332) | 0.28 (0.13–0.60) | 0.001 | 0.31 (0.13–0.73) | 0.008 | 0.29 (0.09–0.98) | 0.045 |
| Renal impairment | 12 (17.7) | 5 (3; 6) | 188 (109; 258) | 0.288 (0.14–0.58) | <0.001 | 0.38 (0.18–0.80) | 0.011 | 0.60 (0.22–1.64) | 0.319 |
| Respiratory distress (APO + ARDS) | 11 (16.2) | 4 (3; 6) | 200 (146; 390) | 0.17 (0.07–0.42) | <0.001 | 0.17 (0.06–0.44) | <0.001 | 0.21 (0.06–0.67) | 0.009 |
| APO | 6 (8.8) | 5 (3; 7) | 146 (77; 192) | 0.47 (0.19–1.21) | 0.118 | 0.48 (0.17–1.38) | 0.175 | 0.63 (0.18–2.23) | 0.47 |
| ARDS | 5 (7.4) | 4 (3; 4) | 275 (238; 504) | 0.24 (0.09–0.65) | 0.005 | 0.28 (0.10–0.77) | 0.013 | 0.41 (0.14–1.23) | 0.111 |
| Significant bleeding | 10 (14.7) | 4 (2; 6) | 115 (67; 134) | 0.52 (0.25–1.10) | 0.084 | 0.63 (0.28–1.41) | 0.262 | 0.91 (0.33–2.55) | 0.858 |
| Severe malarial anemia | 10 (14.7) | 4 (2; 6) | 139 (64; 428) | 0.30 (0.12–0.73) | 0.008 | 0.32 (0.12–0.85) | 0.023 | 0.17 (0.04–0.77) | 0.021 |
| Coma | 7 (10.3) | 6 (4; 7) | 195 (109; 390) | 0.36 (0.16–0.82) | 0.016 | 0.45 (0.19–1.08) | 0.073 | 0.70 (0.25–2.00) | 0.51 |
| Metabolic acidosis | 7 (10.3) | 6 (4; 7) | 195 (124; 352) | 0.32 (0.13–0.77) | 0.011 | 0.40 (0.16–1.00) | 0.050 | 0.58 (0.20–1.69) | 0.317 |
| Hypoglycemia | 1 (1.5) | 9 (9; 9) | 195 (195; 195) | 0.47 (0.19–1.21) | 0.118 | 0.48 (0.17–1.38) | 0.175 | 1.20 (0.15–9.56) | 0.862 |
| Convulsions | 0 (0.0) | - | - | - | - | - | - | - | - |
| Covariate | Coefficient | Exponent of Coefficient (95%CI) | p-Value |
|---|---|---|---|
| Age | −0.011 | 0.989 (0.954; 1.026) | 0.563 |
| Origin from malaria-endemic country | −0.087 | 0.917 (0.307; 2.736) | 0.876 |
| Artemisinin therapy | 0.862 | 2.368 (1.013; 5.538) | 0.047 |
| Severe malarial anemia | −0.169 | 0.845 (0.236; 3.023) | 0.795 |
| Shock | −0.737 | 0.479 (0.185; 1.239) | 0.129 |
| Respiratory distress | −1.427 | 0.240 (0.077; 0.751) | 0.014 |
| Bayesian Logistic Regression | Firth’s Logistic Regression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Covariate | Total (n = 68) | No Respiratory Complications (n = 57) | Respiratory Distress (n = 11) | Median of Posterior Distribution | 95% Credibility Interval | Pd 1 (%) | % in ROPE 2 | p-Value | Coefficient | Unadjusted OR (95% Confidence Interval) |
| Socio-demographic parameters | ||||||||||
| Age, median (IQR), y | 40 (31–52) | 39 (31–53) | 47 (37–51) | 0.02 | −0.03, 0.06 | 73.2 | 100 | 0.51 | 0.015 | 1.02 (0.97–1.06) |
| Male gender, n (%) | 43 (63.2) | 36 (63.2) | 7 (63.6) | 0.03 | −1.27, 1.58 | 51.9 | 20.61 | 0.98 | −0.018 | 0.98 (0.28–3.82) |
| Origin from malaria-endemic country, n (%) | 35 (51.5) | 29 (50.9) | 6 (54.5) | 0.16 | −1.16, 1.44 | 59.60 | 22.37 | 0.84 | 0.133 | 1.14 (0.33–4.13) |
| History of ≥ 1 previous malaria episode, n (%) | 9 (13.2) | 8 (14.0) | 1 (9.1) | −0.81 | −3.78, 1.22 | 76.48 | 11.74 | 0.84 | −0.184 | 0.83 (0.08–4.36) |
| Traveling as a tourist, n (%) | 20 (29.4) | 16 (28.1) | 4 (36.4) | 0.36 | −1.14, 1.36 | 68.80 | 11.74 | 0.54 | 0.411 | 1.51 (0.38–5.45) |
| Duration from symptom onset to diagnosis 3, median (IQR), d | 5 (3–7) | 5 (3–7) | 5 (3–5) | −0.18 | −0.54, 0.14 | 85.60 | 50.82 | 0.34 | −0.145 | 0.87 (0.61–1.15) |
| Co-morbidity | ||||||||||
| No. of chronic co-morbidities, median (IQR) | 0 (0–1) | 0 (0–1) | 1 (0–2) | 0.46 | −0.34, 1.22 | 88.45 | 18.55 | 0.19 | 0.483 | 1.62 (0.78–3.30) |
| CA-CCI, median (IQR) | 0 (0–2) | 0 (0–2) | 2 (0–5) | 0.44 | 0.11, 0.79 | 99.52 | 4.55 | 0.01 | 0.412 | 1.51 (1.10–2.13) |
| Diabetes (total n) n (%) | 3 (4.4) | 3 (5.3) | 0 (0) | −6.61 | −25.74, 0.67 | 94.83 | 1.47 | 0.79 | −0.390 | 0.68 (0.01–7.72) |
| History of or active malignancy, n (%) | 3 (4.4) | 1 (1.8) | 2 (18.2) | 2.70 | 0.15, 6.08 | 97.97 | 0.13 | 0.04 | 2.294 | 9.91 (1.19–117.58) |
| Chronic pulmonary disease, n (%) | 1 (1.5) | 0 (0) | 1 (9.1) | 15.76 | 1.78, 49.32 | 99.25 | 0.0 | 0.07 | 2.80 | 16.43 (0.82–2465.97) |
| Chronic alcohol use, n (%) | 4 (5.9) | 4 (7.0) | 0 (0) | −6.86 | −23.25, 0.40 | 96.30 | 1.42 | 0.64 | 0.700 | 0.52 (0.00–5.40) |
| Obesity, n 4 (%) | 8 (13.3) | 7 (13.7) | 1 (11.1) | −0.62 | −3.91, 1.38 | 71.40 | 12.24 | 0.95 | −0.065 | 0.94 (0.09–5.12) |
| BMI 4, kg/m2, median (IQR) | 24.5 (23.0–26.8) | 24.5 (23.2–27.1) | 24.1 (22.7–24.5) | −0.08 | −0.29, 0.11 | 76.80 | 85.13 | 0.52 | −0.062 | 0.94 (0.76–1.13) |
| Any type of co-infection, n (%) | 19 (27.9) | 10 (17.5) | 9 (81.8) | 3.02 | 1.55, 4.77 | 100 | 0.0 | <.001 | 2.844 | 17.2 (4.13–100.87) |
| HIV co-infection, n (%) | 7 (10.3) | 5 (8.8) | 2 (18.2) | 0.75 | −1.35, 2.44 | 77.20 | 11.29 | 0.30 | 0.921 | 2.51 (0.40–12.38) |
| Malaria-specific complications | ||||||||||
| Jaundice, n (%) | 21 (30.9) | 16 (28.1) | 5 (45.5) | 0.71 | −0.64, 2.08 | 85.65 | 11.95 | 0.25 | 0.755 | 2.13 (0.58–7.68) |
| Hyper-parasitemia, n (%) | 18 (26.5) | 14 (24.6) | 4 (36.4) | 0.50 | −0.97, 1.87 | 74.60 | 16.42 | 0.39 | 0.588 | 1.80 (0.45–6.58) |
| Decompensated shock, n (%) | 12 (17.7) | 5 (8.8) | 7 (63.3) | 2.95 | 1.45, 4.62 | 100 | 0.0 | <0.001 | 2.767 | 15.90 (3.89–75.6) |
| Renal impairment, n (%) | 12 (17.7) | 5 (8.8) | 7 (63.3) | 2.89 | 1.33, 4.62 | 100 | 0.0 | <0.001 | 2.767 | 15.90 (3.89–75.6) |
| Significant bleeding, n (%) | 10 (14.7) | 7 (12.3) | 3 (27.3) | 0.95 | −0.76, 2.53 | 87.33 | 10.29 | 0.19 | 1.020 | 2.77 (0.58–11.66) |
| Severe malarial anemia, n (%) | 10 (14.7) | 5 (8.8) | 5 (45.5) | 2.20 | 0.66, 3.79 | 99.78 | 0 | 0.005 | 2.089 | 8.70 (1.93–35.71) |
| Coma, n (%) | 7 (10.3) | 2 (3.5) | 5 (45.5) | 3.27 | 1.46, 5.56 | 100 | 0.0 | <0.001 | 2.933 | 18.79 (3.71–124.30) |
| Laboratory parameters | ||||||||||
| Minimum base excess on admission 5, mmol/L, median (IQR) | −0.7 (−2.8–1.0) | −0.5 (−2.2–1.3) | −5.3 (−9.75– −3.6) | −0.35 | −0.57, −0.16 | 100 | 2.5 | <0.001 | −0.305 | 0.74 (0.58–0.88) |
| Hypoglycemia, n (%) | 1 (1.5) | 0 (0) | 1 (9.1) | 16.27 | 1.60, 48.69 | 99.42 | 0.0 | 0.07 | 2.799 | 16.43 (0.82–2465.07) |
| Parasitemia, %, median (IQR) | 8.0 (2.5–10.1) | 8.0 (2.5–10.0) | 8.0 (4.0–16.0) | 0.06 | −0.02, 0.14 | 92.90 | 100 | 0.12 | 0.058 | 1.06 (0.98–1.14) |
| Hemoglobin on admission, g/dL, median (IQR) | 12.8 (11.1–13.9) | 12.9 (11.4–13.7) | 11.3 (8.7–14.2) | −0.15 | −0.38, 0.09 | 89.22 | 62.37 | 0.22 | −0.143 | 0.87 (0.69–1.09) |
| Blood urea on admission 6, mg/dL, median (IQR) | 44 (28, 73) | 41 (28–68) | 66 (48–141) | 0.02 | 0.0, 0.03 | 99.55 | 100 | 0.015 | 0.015 | 1.02 (1.00–1.03) |
| Hypoalbuminemia 7 (< 35 g/L), n (%) | 25 (38.5) | 17 (30.9) | 8 (80.0) | 2.15 | 0.69, 4.04 | 99.85 | 0.0 | 0.004 | 2.012 | 7.48 (1.83–42.82) |
| Disease severity on admission | ||||||||||
| SAPS II score on admission 8, median (IQR) | 32 (24–40) | 26 (21–36) | 42 (36–54) | 0.09 | 0.04, 0.16 | 99.98 | 100 | <0.001 | 0.08 | 1.09 (1.03–1.16) |
| Management | ||||||||||
| Fluid intake day 1 9, mL/kg/h, median (IQR) | 2.2 (1.6–3.1) | 2.1 (1.6–2.8) | 2.7 (2.3–4.8) | 0.80 | 0.26, 1.42 | 99.78 | 0.0 | <0.001 | 0.720 | 2.04 (1.26–3.64) |
| Fluid intake day 2, mL/kg/h, median (IQR) | 1.4 (.9–2.3) | 1.1 (.8–1.8) | 2.4 (2.1–3.6) | 0.85 | 0.24, 1.60 | 99.78 | 0.0 | 0.006 | 0.750 | 2.11 (1.23–4.21) |
| Transfusion required, n (%) | 11 (16.2) | 5 (8.8) | 6 (54.5) | 2.57 | 1.13, 4.16 | 100 | 0.0 | <0.001 | 2.423 | 11.3 (2.76–50.91) |
| RRT required, n (%) | 7 (10.3) | 2 (3.5) | 5 (45.5) | 3.21 | 1.49, 5.47 | 100 | 0.0 | <0.001 | 2.933 | 18.79 (3.71–124.30) |
| Artemisinin-based regimen, n (%) | 33 (48.5) | 27 (47.4) | 6 (54.5) | 0.29 | −0.99, 1.62 | 67.00 | 21.79 | 0.67 | 0.271 | 1.31 (0.37–4.75) |
| Parameter | Median, (95% CI) | OR (95% CI) | Pd (%) | % in ROPE (%) |
|---|---|---|---|---|
| Intercept | −5.48 (−8.87, −3.15) | - | 100 | 0 |
| Decompensated shock | 2.44 (0.42, 4.73) | 11.47 (1.52, 113.30) | 99.10 | 0 |
| Any type of co-infection | 2.01 (0.15, 4.14) | 7.46 (1.16, 62.80) | 98.35 | 0 |
| Fluid intake on day 1 (mL/kg/h) | 0.77 (0.11, 1.63) | 2.16 (1.12, 5.10) | 98.72 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmeister, B. Respiratory Distress Complicating Falciparum Malaria Imported to Berlin, Germany: Incidence, Burden, and Risk Factors. Microorganisms 2023, 11, 1579. https://doi.org/10.3390/microorganisms11061579
Hoffmeister B. Respiratory Distress Complicating Falciparum Malaria Imported to Berlin, Germany: Incidence, Burden, and Risk Factors. Microorganisms. 2023; 11(6):1579. https://doi.org/10.3390/microorganisms11061579
Chicago/Turabian StyleHoffmeister, Bodo. 2023. "Respiratory Distress Complicating Falciparum Malaria Imported to Berlin, Germany: Incidence, Burden, and Risk Factors" Microorganisms 11, no. 6: 1579. https://doi.org/10.3390/microorganisms11061579
APA StyleHoffmeister, B. (2023). Respiratory Distress Complicating Falciparum Malaria Imported to Berlin, Germany: Incidence, Burden, and Risk Factors. Microorganisms, 11(6), 1579. https://doi.org/10.3390/microorganisms11061579






