Genome Analysis of Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus ST398 Strains Isolated from Patients with Invasive Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolates Included
2.2. Genome Sequencing and Sequence Analysis
2.3. Molecular Typing Methods
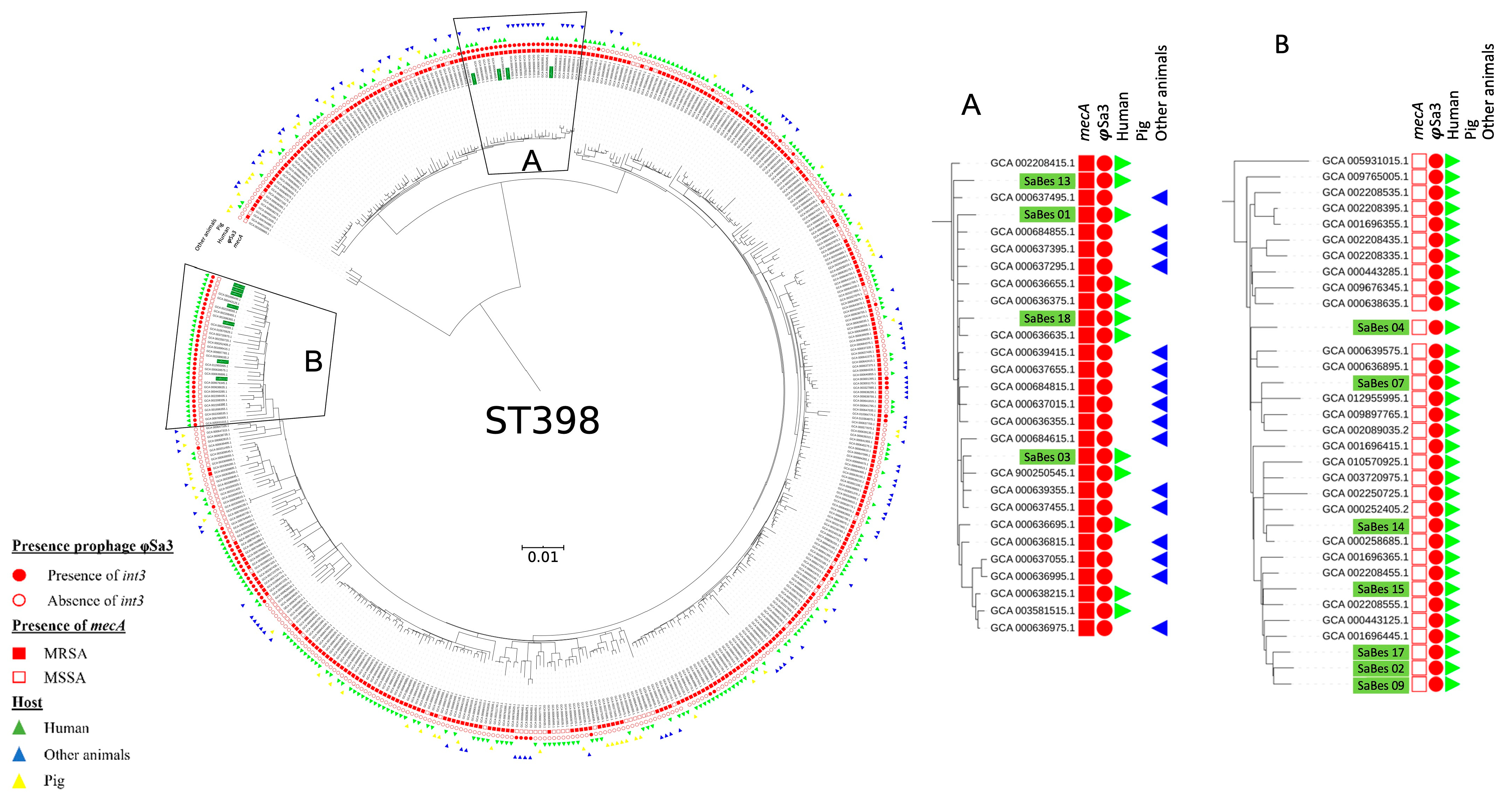
2.4. Phylogeny of S. aureus ST398
2.5. Ethics Approval and Consent to Participate
3. Results
3.1. Genome Content and Typing of S. aureus ST398
3.2. Spa Typing and Phylogeny of ST398 S. Aureus Isolates
3.3. Prophage Content of S. aureus ST398 Isolates
3.4. Characteristics of Patients and Types of Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouiller, K.; Gbaguidi-Haore, H.; Hocquet, D.; Cholley, P.; Bertrand, X.; Chirouze, C. Clonal Complex 398 Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections Are Associated with High Mortality. Clin. Microbiol. Infect. 2016, 22, 451–455. [Google Scholar] [CrossRef]
- Price, L.B.; Stegger, M.; Hasman, H.; Aziz, M.; Larsen, J.; Andersen, P.S.; Pearson, T.; Waters, A.E.; Foster, J.T.; Schupp, J.; et al. Staphylococcus aureus CC398: Host Adaptation and Emergence of Methicillin Resistance in Livestock. MBio 2012, 3, e00305-11. [Google Scholar] [CrossRef] [PubMed]
- Uhlemann, A.-C.; Porcella, S.F.; Trivedi, S.; Sullivan, S.B.; Hafer, C.; Kennedy, A.D.; Barbian, K.D.; McCarthy, A.J.; Street, C.; Hirschberg, D.L.; et al. Identification of a Highly Transmissible Animal-Independent Staphylococcus aureus ST398 Clone with Distinct Genomic and Cell Adhesion Properties. MBio 2012, 3, e00027-12. [Google Scholar] [CrossRef] [PubMed]
- Chroboczek, T.; Boisset, S.; Rasigade, J.-P.; Tristan, A.; Bes, M.; Meugnier, H.; Vandenesch, F.; Etienne, J.; Laurent, F. Clonal Complex 398 Methicillin Susceptible Staphylococcus aureus: A Frequent Unspecialized Human Pathogen with Specific Phenotypic and Genotypic Characteristics. PLoS ONE 2013, 8, e68462. [Google Scholar] [CrossRef]
- Diene, S.M.; Corvaglia, A.R.; François, P.; van der Mee-Marquet, N. Regional Infection Control Group of the Centre Region Prophages and Adaptation of Staphylococcus aureus ST398 to the Human Clinic. BMC Genom. 2017, 18, 133. [Google Scholar] [CrossRef] [PubMed]
- Laumay, F.; Benchetrit, H.; Corvaglia, A.-R.; van der Mee-Marquet, N.; François, P. The Staphylococcus aureus CC398 Lineage: An Evolution Driven by the Acquisition of Prophages and Other Mobile Genetic Elements. Genes 2021, 12, 1752. [Google Scholar] [CrossRef]
- van der Mee-Marquet, N.L.; Corvaglia, A.; Haenni, M.; Bertrand, X.; Franck, J.-B.; Kluytmans, J.; Girard, M.; Quentin, R.; François, P. Emergence of a Novel Subpopulation of CC398 Staphylococcus aureus Infecting Animals Is a Serious Hazard for Humans. Front. Microbiol. 2014, 5, 652. [Google Scholar] [CrossRef]
- Ward, M.J.; Gibbons, C.L.; McAdam, P.R.; van Bunnik, B.A.D.; Girvan, E.K.; Edwards, G.F.; Fitzgerald, J.R.; Woolhouse, M.E.J. Time-Scaled Evolutionary Analysis of the Transmission and Antibiotic Resistance Dynamics of Staphylococcus aureus Clonal Complex 398. Appl. Environ. Microbiol. 2014, 80, 7275–7282. [Google Scholar] [CrossRef]
- Bouiller, K.; Bertrand, X.; Hocquet, D.; Chirouze, C. Human Infection of Methicillin-Susceptible Staphylococcus aureus CC398: A Review. Microorganisms 2020, 8, 1737. [Google Scholar] [CrossRef]
- Valour, F.; Tasse, J.; Trouillet-Assant, S.; Rasigade, J.-P.; Lamy, B.; Chanard, E.; Verhoeven, P.; Decousser, J.-W.; Marchandin, H.; Bés, M.; et al. Methicillin-Susceptible Staphylococcus aureus Clonal Complex 398: High Prevalence and Geographical Heterogeneity in Bone and Joint Infection and Nasal Carriage. Clin. Microbiol. Infect. 2014, 20, O772–O775. [Google Scholar] [CrossRef]
- Chen, H.; Yin, Y.; Li, X.; Li, S.; Gao, H.; Wang, X.; Zhang, Y.; Liu, Y.; Wang, H. Whole-Genome Analysis of Livestock-Associated Methicillin-Resistant Staphylococcus aureus Sequence Type 398 Strains Isolated from Patients with Bacteremia in China. J. Infect. Dis. 2020, 221, S220–S228. [Google Scholar] [CrossRef] [PubMed]
- Sauget, M.; Bouiller, K.; Richard, M.; Chagrot, J.; Cholley, P.; Hocquet, D.; Bertrand, X. Increasing Incidence of Bloodstream Infections Due to Staphylococcus aureus Clonal Complex 398 in a French Hospital between 2010 and 2017. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- Bouiller, K.; Hocquet, D.; Sauget, M.; Bertrand, X.; Chirouze, C. Epidemiology and Risk Factors of Staphylococcus aureus CC398 Bone and Joint Infections. BMC Infect. Dis. 2020, 20, 384. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of Acquired Antimicrobial Resistance Genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic Resistome Surveillance with the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef]
- Goerke, C.; Pantucek, R.; Holtfreter, S.; Schulte, B.; Zink, M.; Grumann, D.; Bröker, B.M.; Doskar, J.; Wolz, C. Diversity of Prophages in Dominant Staphylococcus aureus Clonal Lineages. J. Bacteriol. 2009, 191, 3462–3468. [Google Scholar] [CrossRef]
- van Wamel, W.J.B.; Rooijakkers, S.H.M.; Ruyken, M.; van Kessel, K.P.M.; van Strijp, J.A.G. The Innate Immune Modulators Staphylococcal Complement Inhibitor and Chemotaxis Inhibitory Protein of Staphylococcus aureus Are Located on Beta-Hemolysin-Converting Bacteriophages. J. Bacteriol. 2006, 188, 1310–1315. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A Genome Comparison Visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Benito, D.; Lozano, C.; Rezusta, A.; Ferrer, I.; Vasquez, M.A.; Ceballos, S.; Zarazaga, M.; Revillo, M.J.; Torres, C. Characterization of Tetracycline and Methicillin Resistant Staphylococcus aureus Strains in a Spanish Hospital: Is Livestock-Contact a Risk Factor in Infections Caused by MRSA CC398? Int. J. Med. Microbiol. 2014, 304, 1226–1232. [Google Scholar] [CrossRef]
- Valentin-Domelier, A.-S.; Girard, M.; Bertrand, X.; Violette, J.; François, P.; Donnio, P.-Y.; Talon, D.; Quentin, R.; Schrenzel, J.; van der Mee-Marquet, N.; et al. Methicillin-Susceptible ST398 Staphylococcus aureus Responsible for Bloodstream Infections: An Emerging Human-Adapted Subclone? PLoS ONE 2011, 6, e28369. [Google Scholar] [CrossRef]
- Larsen, J.; Petersen, A.; Sørum, M.; Stegger, M.; van Alphen, L.; Valentiner-Branth, P.; Knudsen, L.K.; Larsen, L.S.; Feingold, B.; Price, L.B.; et al. Meticillin-Resistant Staphylococcus aureus CC398 Is an Increasing Cause of Disease in People with No Livestock Contact in Denmark, 1999 to 2011. Eurosurveillance 2015, 20, 30021. [Google Scholar] [CrossRef] [PubMed]
- Verkade, E.; Kluytmans, J. Livestock-Associated Staphylococcus aureus CC398: Animal Reservoirs and Human Infections. Infect. Genet. Evol. 2014, 21, 523–530. [Google Scholar] [CrossRef]
- Ramos, B.; Rosalino, L.M.; Palmeira, J.D.; Torres, R.T.; Cunha, M.V. Antimicrobial Resistance in Commensal Staphylococcus aureus from Wild Ungulates Is Driven by Agricultural Land Cover and Livestock Farming. Environ. Pollut. 2022, 303, 119116. [Google Scholar] [CrossRef]
- Sieber, R.N.; Skov, R.L.; Nielsen, J.; Schulz, J.; Price, L.B.; Aarestrup, F.M.; Larsen, A.R.; Stegger, M.; Larsen, J. Drivers and Dynamics of Methicillin-Resistant Livestock-Associated Staphylococcus aureus CC398 in Pigs and Humans in Denmark. MBio 2018, 9, e02142-18. [Google Scholar] [CrossRef]
- van Alen, S.; Ballhausen, B.; Kaspar, U.; Köck, R.; Becker, K. Prevalence and Genomic Structure of Bacteriophage Phi3 in Human-Derived Livestock-Associated Methicillin-Resistant Staphylococcus aureus Isolates from 2000 to 2015. J. Clin. Microbiol. 2018, 56, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Cuny, C.; Abdelbary, M.; Layer, F.; Werner, G.; Witte, W. Prevalence of the Immune Evasion Gene Cluster in Staphylococcus aureus CC398. Vet. Microbiol. 2015, 177, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Salmenlinna, S.; Lyytikäinen, O.; Vainio, A.; Myllyniemi, A.L.; Raulo, S.; Kanerva, M.; Rantala, M.; Thomson, K.; Seppänen, J.; Vuopio, J. Human Cases of Methicillin-Resistant Staphylococcus aureus CC398, Finland. Emerg. Infect. Dis. 2010, 16, 1626–1629. [Google Scholar] [CrossRef]
- Larsen, J.; Petersen, A.; Larsen, A.R.; Sieber, R.N.; Stegger, M.; Koch, A.; Aarestrup, F.M.; Price, L.B.; Skov, R.L. Danish MRSA Study Group Emergence of Livestock-Associated Methicillin-Resistant Staphylococcus aureus Bloodstream Infections in Denmark. Clin. Infect. Dis. 2017, 65, 1072–1076. [Google Scholar] [CrossRef]
- Tegegne, H.A.; Koláčková, I.; Florianová, M.; Wattiau, P.; Gelbíčová, T.; Boland, C.; Madec, J.-Y.; Haenni, M.; Karpíšková, R. Genomic Insights into Methicillin-Resistant Staphylococcus aureus Spa Type T899 Isolates Belonging to Different Sequence Types. Appl. Environ. Microbiol. 2021, 87, e01994-20. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Clasen, J.; Hansen, J.E.; Paulander, W.; Petersen, A.; Larsen, A.R.; Frees, D. Copresence of Tet(K) and Tet(M) in Livestock-Associated Methicillin-Resistant Staphylococcus aureus Clonal Complex 398 Is Associated with Increased Fitness during Exposure to Sublethal Concentrations of Tetracycline. Antimicrob. Agents Chemother. 2016, 60, 4401–4403. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Espinosa-Gongora, C.; Guardabassi, L. Human Health Risks Associated with Antimicrobial-Resistant Enterococci and Staphylococcus aureus on Poultry Meat. Clin. Microbiol. Infect. 2016, 22, 130–140. [Google Scholar] [CrossRef] [PubMed]
- van der Mee-Marquet, N.; Corvaglia, A.-R.; Valentin, A.-S.; Hernandez, D.; Bertrand, X.; Girard, M.; Kluytmans, J.; Donnio, P.-Y.; Quentin, R.; François, P. Analysis of Prophages Harbored by the Human-Adapted Subpopulation of Staphylococcus aureus CC398. Infect. Genet. Evol. 2013, 18, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.; van Luit, M.; Pluister, G.N.; Frentz, D.; Haenen, A.; Landman, F.; Witteveen, S.; van Marm-Wattimena, N.; van der Heide, H.G.; Schouls, L.M. Changing Characteristics of Livestock-Associated Meticillin-Resistant Staphylococcus aureus Isolated from Humans—Emergence of a Subclade Transmitted without Livestock Exposure, The Netherlands, 2003 to 2014. Eurosurveillance 2016, 21, 30236. [Google Scholar] [CrossRef]
- Coombs, G.W.; Daley, D.; Shoby, P.; Yee, N.W.T.; Robinson, J.O.; Murray, R.; Korman, T.M.; Warner, M.S.; Papanaoum, K.; Derrington, P.; et al. Genomic Characterisation of CC398 MRSA Causing Severe Disease in Australia. Int. J. Antimicrob. Agents 2022, 59, 106577. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Z.; Liu, C.; Zhang, X.; Lin, X.; Chi, S.; Zhou, T.; Chen, Z.; Chen, X. Prevalence of Staphylococcus aureus Carrying Panton-Valentine Leukocidin Genes among Isolates from Hospitalised Patients in China. Clin. Microbiol. Infect. 2008, 14, 381–384. [Google Scholar] [CrossRef]
- Li, X.; Huang, T.; Xu, K.; Li, C.; Li, Y. Molecular Characteristics and Virulence Gene Profiles of Staphylococcus aureus Isolates in Hainan, China. BMC Infect. Dis. 2019, 19, 873. [Google Scholar] [CrossRef]
- Welinder-Olsson, C.; Florén-Johansson, K.; Larsson, L.; Öberg, S.; Karlsson, L.; Åhrén, C. Infection with Panton-Valentine Leukocidin–Positive Methicillin-Resistant Staphylococcus aureus T034. Emerg. Infect. Dis. 2008, 14, 1271–1272. [Google Scholar] [CrossRef]

| Isolate | spa Type | SCCmec | Resistance Genes | Prophages | IEC Genes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mecA | blaZ | erm (T) | tet (M) | Prophagic elements (n) | φSa3 | scn | sak | chp | |||
| SaBes 01 | t4132 | IVa (2B) | + | + | − | + | 3 | + | + | + | + |
| SaBes 02 | t1451 | NA | − | + | + | − | 2 | + | + | − | + |
| SaBes 03 | t899 | IVa (2B) | + | + | − | + | 2 | + | + | + | + |
| SaBes 04 | t1451 | NA | − | − | + | − | 2 | + | + | − | + |
| SaBes 07 | t1451 | NA | − | + | + | − | 3 | + | + | − | + |
| SaBes 09 | t1451 | NA | − | + | + | − | 5 | + | + | − | + |
| SaBes 13 | t1939 | IVa (2B) | + | + | − | + | 3 | + | + | + | + |
| SaBes 14 | t1451 | NA | − | + | + | − | 3 | + | + | − | + |
| SaBes 15 | t1451 | NA | − | + | + | − | 5 | + | + | − | + |
| SaBes 17 | t1451 | NA | − | + | + | − | 5 | + | + | − | + |
| SaBes 18 | t2922 | IVa (2B) | + | + | − | + | 2 | + | + | + | + |
| Patient | Isolate | mecA | Age (y) | Sex | CCI | Sampling Date | Type of Infection | HAI | Source of Infection | Complications | Setting | Profession | Hosp. in the Last 3 Months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | SaBes 01 | Yes | 21 | M | 0 | 2013 | OM | Yes | Skin | - | Rural | NA | Yes |
| Patient 2 | SaBes 02 | No | 60 | M | 4 | 2013 | Pressure ulcer stage IV | Yes | Skin | - | Rural | retired | Yes |
| Patient 3 | SaBes 03 | Yes | 77 | F | 5 | 2015 | OM | Yes | Skin | Multiple surgeries | Urban | retired | Yes |
| Patient 4 | SaBes 04 | No | 58 | M | 7 | 2015 | OM | Yes | Skin | - | Rural | NA | Yes |
| Patient 5 | SaBes 07 | No | 74 | F | 4 | 2017 | OM | Yes | Skin | Relapse | Urban | retired | No |
| Patient 6 | SaBes 09 | No | 48 | F | 3 | 2017 | BSI | No | Skin | Endocarditis, arthritis | Urban | NA | No |
| Patient 7 | SaBes 13 | Yes | 49 | F | 0 | 2016 | BSI | NA | Unknown | - | Rural | farmer | No |
| Patient 8 | SaBes 14 | No | 83 | F | 5 | 2016 | BSI | No | Unknown | - | Rural | retired | No |
| Patient 9 | SaBes 15 | No | 83 | M | 7 | 2016 | BSI | No | Skin | - | Urban | retired | No |
| Patient 10 | SaBes 17 | No | 71 | M | 7 | 2015 | BSI | Yes | Skin | - | Urban | retired | Yes |
| Patient 11 | SaBes 18 | Yes | 86 | M | 6 | 2015 | BSI | No | Unknown | Death | Rural | retired | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeggay, A.; Atchon, A.; Valot, B.; Hocquet, D.; Bertrand, X.; Bouiller, K. Genome Analysis of Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus ST398 Strains Isolated from Patients with Invasive Infection. Microorganisms 2023, 11, 1446. https://doi.org/10.3390/microorganisms11061446
Zeggay A, Atchon A, Valot B, Hocquet D, Bertrand X, Bouiller K. Genome Analysis of Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus ST398 Strains Isolated from Patients with Invasive Infection. Microorganisms. 2023; 11(6):1446. https://doi.org/10.3390/microorganisms11061446
Chicago/Turabian StyleZeggay, Abdeljallil, Alban Atchon, Benoit Valot, Didier Hocquet, Xavier Bertrand, and Kevin Bouiller. 2023. "Genome Analysis of Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus ST398 Strains Isolated from Patients with Invasive Infection" Microorganisms 11, no. 6: 1446. https://doi.org/10.3390/microorganisms11061446
APA StyleZeggay, A., Atchon, A., Valot, B., Hocquet, D., Bertrand, X., & Bouiller, K. (2023). Genome Analysis of Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus ST398 Strains Isolated from Patients with Invasive Infection. Microorganisms, 11(6), 1446. https://doi.org/10.3390/microorganisms11061446





