Abstract
Parasitic diseases are responsible for substantial losses in reproduction and productivity in swine, creating a major impairment to efficient and profitable livestock management. The use of phytotherapeutic remedies has notably increased over the past decade due to their bioavailability, decreased toxicity, non-polluting nature, and to some extent due to their antiparasitic effect. The aim of this study was to evaluate the antiparasitic potential of Cucurbita pepo L. and Coriandrum sativum L. against protozoa and nematodes found in swine. The samples were collected from weaners, fatteners, and sows and examined via flotation (Willis and McMaster), active sedimentation, Ziehl-Neelsen staining as modified by Henricksen, a modified Blagg method, and eggs/oocyst culture. The parasite species detected were Ascaris suum, Trichuris suis, Oesophagostomum spp., Balantioides coli (syn. Balantidium coli), Eimeria spp., and Cryptosporidium spp., depending on age category. A dose of 500 mg/kg bw/day of C. pepo and 170 mg/kg bw/day of C. sativum powders, administered for ten consecutive days, demonstrated a pronounced anthelmintic (pumpkin) and antiprotozoal (coriander) effect against the aforementioned parasites. Future studies are required to ascertain the optimal dose that maximizes their antiparasitic effectiveness. The current study represents the first Romanian report on the in vivo antiparasitic activity of these two plants tested on digestive parasites in swine.
1. Introduction
Low-input farming systems can be defined as extensive technological structures that maximize economic and environmental sustainability [1]. In Romania, farmers are raising local breeds (Bazna, Mangalitza, Black of Strei, White of Banat) that are better adapted to processing fibrous feeds, possess a higher tolerance to endemic parasites, have meat with superior organoleptic properties, and are more suitable for organic production [2,3]. Consumers are becoming increasingly interested in buying animal-derived products from free-range farms that are concerned about the wellbeing of their animals [4]. Parasitic infections, for which gastrointestinal protozoa and nematodes are widely considered responsible, are one of the main issues affecting the health and welfare of pigs, decreasing their reproductive performance and productivity [5]. The most important parasites diagnosed in low-input (free-range) farms are Balantioides coli (syn. Balantidium coli), Cryptosporidium spp., Eimeria spp./Cystoisospora suis, Ascaris suum, Trichuris suis, Strongyloides ransomi, and Oesophagostomum spp. [6,7].
Classic antiparasitic drugs (triazine, avermectins, benzimidazole, and imidazothiazoles) are used to treat these parasitic infections in swine [8]. The main drawback of their use is the emergence of antiparasitic resistance to most molecules, along with their residues in animal products [5,9]. The use of traditional herbal medicines is increasing, as medicinal plants have fewer adverse effects than allopathic antiparasitic treatments. Thus, traditional herbal medicine has garnered the attention of numerous researchers, encouraging the screening of certain plants with therapeutic properties in order to assess the effects of their bioactive compounds [10]. Plants generally produce a significant number of secondary metabolites derived from primary ones through biosynthesis, constituting an important resource for several pharmaceutical drugs [11]. Secondary metabolites are divided into three main groups: terpenes (mono- and sesquiterpenes, saponins, and glycosides), phenolic compounds (tannins and flavonoids) and nitrogen-containing compounds (alkaloids and non-protein amino acids), which are believed to represent the main sources of the antiparasitic effect [9]. Phytotherapy is currently viewed as an alternate solution in controlling gastrointestinal parasites in both humans and animals [12]. Phytobiotics are a new class of natural plant-based additives that are highly acceptable among consumers. They can enhance animal productivity along with nutrient absorption, growth performance, and improved digestibility [13].
Coriandrum sativum L. is an aromatic and medicinal plant that is widespread throughout the world as a result of being cultivated for its aromatic seeds. It is an annual herb belonging to the Apiaceae family [12,14]. Phytochemical screening of C. sativum showed that it contains essential oils, tannins, terpenoids, alkaloids, phenols, flavonoids, fatty acids, sterols, and glycosides. It also contains high levels of proteins, carbohydrates, fibres, and a wide range of minerals and vitamins [15]. Previous pharmacological studies revealed that C. sativum plays several biological roles, including antibacterial, antifungal, antiprotozoal, anthelmintic, insecticidal, neuroprotective, antioxidant, cardioprotective, anti-inflammatory, analgesic, antidiabetic, gastroprotective, diuretic, and hepatoprotective effects [10,12,14,15,16,17,18,19]. The observed biological roles can be attributed to the main active compounds in Coriandrum sativum, i.e., in its essential oils (linalool, camphor, geranyl acetate, graniol, pinene, and terpine) and oils (petroselinic acid, linoleic acid, and oleic acid) [10,12,14,18,19].
Pumpkins, including Cucurbita pepo, Cucurbita maxima, and Cucurbita moschata, are gourd squashes belonging to the genus Cucurbita and the family Cucurbitaceae. C. pepo, the summer squash, is cultivated worldwide as a vegetable [20,21,22]. Pumpkin seeds contain 41.59% oil, 25.4% protein, 5.2% moisture, 25.19% carbohydrates, 5.34% fibre, and 2.49% total ash [21]. Moreover, pumpkin seed oil is used as a nutritional supplement, as it is a natural source of unsaturated fatty acids (omega 9, 6, and 3), lutein, carotenoids, phytosterols, tocopherols, chlorophyll, and trace elements, including selenium and zinc [20,21,23,24,25]. Pumpkin seed extracts are a valuable source of protein and bioactive phytochemicals with positive effects on the general wellbeing, immunity, weight gain, and appetite of chickens [26,27]. Traditionally, this plant is used to treat different medical conditions, including whooping cough, urinary problems, scurvy, rheumatism, haemorrhoids, miscarriage, prostate cancer, constipation, and blindness [21,22,28]. Other medicinal and pharmacological benefits of C. pepo seeds include anti-inflammatory, antioxidant, antimicrobial, and antiparasitic effects [22,24,28,29]. These were attributed to the presence of certain classes of compounds, including flavonoids, terpenoids, cardiac glycosides, and cucurbitacin glycosides [22,25,28].
The aim of the present study, carried out on two free-range (low-input) Transylvanian farms, was to assess the antiparasitic potential of C. sativum and C. pepo, present in the Romanian flora, against naturally occurring gastrointestinal parasites in swine.
2. Materials and Methods
2.1. Biochemical Analyses of Coriandrum sativum and Cucurbita pepo
C. pepo (pumpkin) seeds and C. sativum (coriander) fruits were used for analysis. Bǎieş et al. [30] described in detail the materials and methods used to analyse the chemical composition of the alcoholic extracts of C. sativum fruits and C. pepo seeds. The biologically active compounds in these alcoholic plant extracts were analysed by means of high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS). All steps of the procedure were carried out at the Iuliu Haţieganu University of Medicine and Pharmacy in Cluj-Napoca.
2.2. Swine Husbandry
The farms subject to this study were located in Transylvania, a temperate continental climate region positioned in the hills, covered fields, and forests. The samples originated from two low-input swine farms (Farm 1—F1; Farm 2—F2), both raising local Mangalitza and Bazna breeds. In September 2021, when the study was initiated, F1 had a herd of 380 pigs, while F2 had 290 animals. The main water supply was the publicly available drinking water. The barns were cleaned daily throughout the year. Furthermore, before starting the experiment, a rigorous mechanical cleaning was carried out, followed by the disinfection of shelters and paddocks. The outdoor environment was accessible to the pigs at all times. The animals had access to pasture and enrichments (mud bath, straw, roughage, and toys such as chains, bricks, etc.), and the housing area was bordered by an electric fence [6].
2.3. Experimental Design and Sampling Procedures
Before starting the experiment, a pilot study was conducted on a small number of pigs, through which different doses (according to the literature) of C. pepo and C. sativum were tested. The feeding behaviour of the animals, antiparasitic efficacy of the plants, and potential side effects were observed.
Each study herd was divided into three age groups: weaners (aged 10 to 11 weeks and weighing 10 to 12 kg), fatteners (aged five to six months and weighing 45 to 50 kg), and sows (aged one to three years and weighing 140 to 150 kg). Each experimental group on which each plant was tested included 10 weaners, 10 fatteners, and 10 sows (n = 30 animals), similar to the untreated control groups (n = 30 animals) on each farm. In F1, the experiment was performed on Mangalitza pigs, while in F2, Bazna pigs were used.
C. pepo and C. sativum were cultivated in Romania and licensed companies provided the plant samples. The seeds of C. pepo and fruits of C. sativum were ground to a fine powder, resulting in a diet with either pumpkin (1%, 1.35%, or 2.5%) or coriander (0.34%, 0.45%, or 0.85%), along with cereal flour. The feed was provided to the animals according to their age, at a final average concentration calculated by the average body weight of each group (Table 1). The total amount of feed received by each pig was as follows: 0.6 kg, 2 kg, and 3 kg/day per weaner, fattener, and sow, respectively. Thus, pigs belonging to the respective experimental group were administered either 500 mg/kg bw/day of C. pepo or 170 mg/kg bw/day of C. sativum, divided into two portions, for a period of 10 consecutive days (where bw = body weight). Prior to the beginning of the experiment (day 0), a coproparasitological examination was carried out in order to assess the presence of parasite species. Subsequently, two more coproparasitological examinations were performed, on days 14 and 28 of the study. This procedure was applied on each farm for each plant and each experimental group.

Table 1.
Composition of pig diets by experimental group and age category.
Faecal samples weighing approximately 20 g each were harvested individually from the rectum of the animals and stored in sterile containers at a temperature of 2–8 °C for 24 to 48 h until testing. The samples were also subjected to macroscopic examination, aiming to detect any visible parasites, before being labelled and stored. Different coproparasitological methods, including flotation (Willis method, McMaster method), centrifugal sedimentation, Ziehl-Neelsen staining as modified by Henricksen, a modified Blagg technique, and faecal cultures (nematode larvae/protozoan oocyst cultures) were involved during testing. The McMaster quantitative method was used to establish the individual intensity of parasitism [6,31,32].
2.4. Evaluation of Antiparasitic Efficacy
A faecal egg count reduction test (FECRT) was performed to evaluate the antiparasitic efficacy of C. sativum and C. pepo using the following formula: FECR (%) = 100 × (1–[T2/T1] × [C1/C2]), with T1 and T2 representing the mean pre- and post-treatment faecal egg counts (FEC) of a treated experimental group, and C1 and C2 representing the mean FEC in the untreated control group before (C1) and after (C2) therapy [33,34]. The same formula was used for both oocysts and cysts.
2.5. Ontologies and Ethics Statement
Table S1 details the ontological analyses of all pathogens, diseases, medicinal plants, and chemical compounds used in the above study, according to the data management plan of the PPILOW (Poultry and Pig Low-input and Organic production systems’ Welfare) project.
The behaviour and clinical condition of the pigs involved were assessed both prior to and throughout the experiment. The bioethical rules for experimentation on animals in both national (law no. 43, 2014) and European (EU Directive 63/2010) legislation were followed.
2.6. Statistical Analysis
The prevalence of each parasite was reported by age group, plant, and farm. We used column graphs to represent the prevalence (Excel ®, Microsoft Office 365). A positive test result for Cryptosporidium spp. infection was reported as absolute frequency per age group, plant, and farm. The association between a positive result for Cryptosporidium spp. and the group (EG vs. CG) was tested with Fisher’s exact test, considering the theoretical frequencies (theoretical frequencies less than 5 in more than 20% of cells).
The distribution of measurements was visually checked with histograms (bell-shaped and symmetrical distribution indicates no deviation from the normal distribution) and box-and-whisker plots (symmetrical box centred around the median, a similar length of the whiskers, and no outliers indicate no deviation from the normal distribution). The graphical method was chosen to investigate the distribution of raw data because the sample size per age group in each farm was small (n = 10). According to the distribution, comparisons between cases (experimental group—EG) and controls (CG) by farm and each investigated day (0, 14, and 28) was made with the two-sided Mann–Whitney test at a significance level (α) of 5%. The effectiveness of the investigated plants (day 0, day 14, and day 28) was tested on each group and each farm with the Friedman test, considering a two-sided test and an adjusted α. The maximum possible number of parasites (intensity) per farm and age group (five in our case) was used to adjust the significance level, so the results were considered statistically significant whenever the Friedman test p-value was less than 0.01. No post hoc analysis followed the Friedman test due to the limited measurements per investigated day.
The Statistica program (v. 13.5, TIBCO, Tusla, OK, USA) was used to analyse the raw data.
3. Results
3.1. Analysis of Plant Extracts
Following the chemical analysis of C. pepo and C. sativum alcoholic extracts, the bioactive compounds identified were polyphenols (chlorogenic acid, p-coumaric acid, ferulic acid, rutoside, syringic acid, and vanillic acid) and sterols (ergosterol, stigmasterol, β-sitosterol, and campesterol) for coriander and tocopherols (γ-tocopherol and Δ-tocopherol) and sterols (stigmasterol, β-sitosterol, and campesterol) for pumpkin.
3.2. Analysis of Antiparasitic Activity of Plants
The coproparasitological examination revealed co-infections of up to five species of gastrointestinal parasites, namely Balantioides coli, Eimeria spp., Cryptosporidium spp, Trichuris suis, Ascaris suum, and Oesophagostomum spp. An examination of oocyst/egg cultures revealed that all belonged to the Eimeria genus, whereas all L3 larvae (contained within strongylid eggs) belonged to the Oesophagostomum genus. Neither centrifugal sedimentation nor the Blagg method gave positive results. The flotation, oocyst/egg culture, and McMaster methods indicated the infection’s prevalence and mean intensity, depending on the age group, farm, and administered plant.
No toxic reactions were recorded in any animal involved in this study. Although this did not represent the objective of the study, clinical observation indicated that animals in both experimental groups consumed their feed better and had a higher growth rate than those in the control groups. This was more obvious in weaners and fatteners.
On both farms and in all three age groups (weaners, fatteners, and sows), identical parasite species and similar co-infection patterns by protozoa and nematodes were found. Eimeria spp., B. coli, Cryptosporidium spp., A. suum, and T. suis were observed in weaners on both farms. In fatteners from both farms, only Eimeria spp., B. coli, A. suum, and T. suis were detected. Lastly, in sows, Eimeria spp., B. coli, Cryptosporidium spp., Oesophagostomum, and A. suum were identified on both farms.
C. pepo generally demonstrated a strong anthelmintic effect against A. suum and T. suis, while C. sativum had a good antiprotozoal activity against Eimeria and B. coli, with efficacy according with the breed of pigs and age group (Figure 1, Figure 2 and Figure 3, and Table 2, Table 3, Table 4 and Table 5).
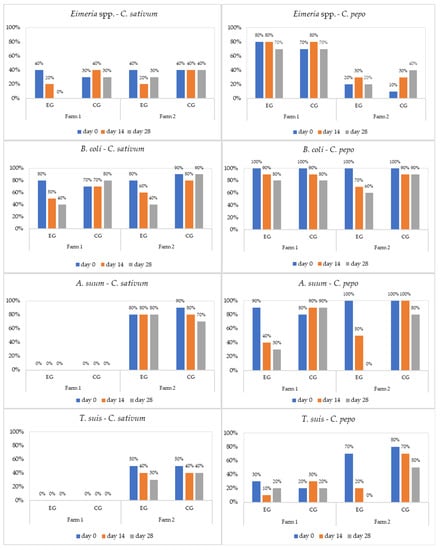
Figure 1.
Prevalence (%) of parasites in weaners by treatment (EG = experimental group; CG = control group).
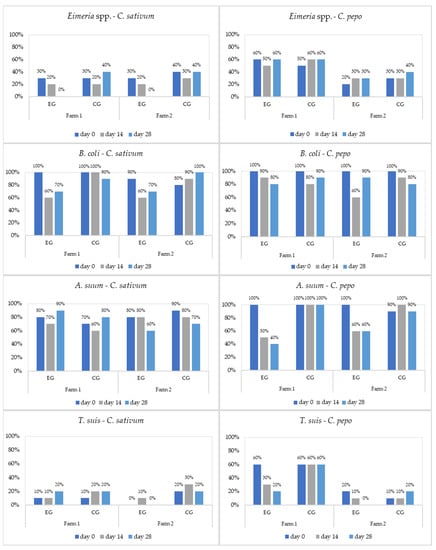
Figure 2.
Prevalence (%) of parasites in fatteners by treatment (EG = experimental group; CG = control group).
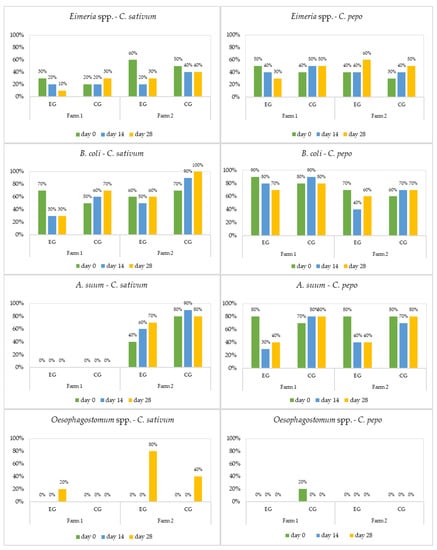
Figure 3.
Prevalence (%) of parasites in sows by treatment (EG = experimental group; CG = control group).

Table 2.
Antiparasitic effects of C. sativum and C. pepo in weaners.

Table 3.
Antiparasitic effects of C. sativum and C. pepo in fatteners.

Table 4.
Antiparasitic effects of C. sativum and C. pepo in sows.

Table 5.
Percentage of faecal egg/oocyst/cyst count reduction (%) recorded on days 14 and 28 post-treatment (using FECR formula).
Statistical significances were found between experimental groups (EGs) and controls (EG 14/ CG 14, EG 28/CG 28) and between EGs (EG 0/EG 14/EG 28) for different plants and farms (pig breeds).
The C. sativum group had statistically significant values (SSVs) only for B. coli, in both farms and in all age groups (Table 2, Table 3 and Table 4). The C. pepo group showed SSVs for B. coli in weaners and fatteners from both farms, for T. suis in weaners from F2, and for A. suum in all age groups from both farms (Table 2, Table 3 and Table 4).
The studied plants showed limited antiparasitic effects against Cryptosporidium spp. and no effects on Oesophagostomum spp. Overall, the antiparasitic effects of C. pepo and C. sativum increased at day 14, with a maximum therapeutic activity at the end of the experiment (day 28).
The therapeutic efficacy (reduction in parasitic intensity, %) of C. sativum (CS) and C. pepo (CP) against diagnosed parasites in all age groups was as follows: CS = 23.2–79.5%, CP = 2.3–59.5% for B. coli; CS = 25.4–100%, CP = 11.6–99.6% for Eimeria spp.; CS = 0–0.3%, CP = 50.1–100% for T. suis; and CS = 7.2–30.3%, CP = 70.3–100% for A. suum (Table 5).
4. Discussion
The antiparasitic effects of two aromatic and medicinal plants commonly found in Romania’s flora, namely coriander and pumpkin, were successfully evaluated. We found a higher efficacy of coriander fruits for protozoa and better efficacy of pumpkin seeds for helminths.
The therapeutic potential of medicinal plants renders phytotherapy an alternative to synthetic drugs [11,35]. C. pepo and C. sativum were found to be effective against several gastrointestinal parasites in swine [11,30,36]. The mechanism of antiparasitic action of C. sativum can be attributed to the main bioactive compounds in coriander, i.e., terpenoids, which are components of its essential oils [10,11,12,19], while for pumpkin it was assigned to the presence of cucurbitacins [20,22,25]. Biological compounds such as polyphenols, tocopherols, and sterols present in the studied plants demonstrated effective antiparasitic properties, both in vitro and in vivo [10,11,22,35]. An in-depth analysis of the compounds identified in C. pepo and C. sativum alcoholic extracts has already been detailed in a previous article [30].
Coriander is a plant with very low toxicity for both humans and animals, even when consumed in large quantities [37]. No information is available on the therapeutic dose of coriander fruits in pigs. We therefore extrapolated the dosage in our experiments based on human reports (100–500 mg/kg/day), along with those for other animal species, namely rats (100–12,000 mg/kg/day) and mice (500–5000 mg/kg/day) [38,39,40,41]. In the present study, a dose of 170 mg/kg/day (divided into two portions) for 10 consecutive days was used. Coriander oil and its major constituent, linalool, have negligible reproductive, neurological, and dermal toxicity in laboratory animals [38,39,40,41]. Limited information exists regarding the safety of orally administered coriander for traditional medicine purposes. Adverse effects associated with the medicinal use of coriander seeds and leaves as empirical treatments have not been documented, although a case report from Iran described endocrine toxicity in a female patient [37,42].
The reported therapeutic dose of Cucurbita seeds varies with the animal species, as follows: pigs 5000 mg/kg/day, rats 200–2000 mg/kg/day, mice 300–7600 mg/kg/day, and sheep 5000 mg/kg/day, while in humans it is 100–1000 mg/kg/day [43,44,45,46,47,48,49]. In the current study, a dose of 500 mg/kg/day of pumpkin seeds was administered according to the same therapeutic protocol as that of coriander. C. pepo is generally considered safe and non-toxic, and reports of toxicity are scarce [50,51]. Among the only reported side effects—oral allergic syndromes, nausea, diarrhoea, or pruritus in humans and oligospermia and androgen insufficiency in rats—were listed [52,53].
C. sativum showed statistically significant efficacy for B. coli on all age groups regardless of pig breed. C. pepo showed efficacy supported by statistical significance on A. suum for both pig breeds and age groups, limited efficacy for B. coli on any-breed fatteners and Bazna pig weaners, and for T. suis on Bazna pig weaners. The absence of significant differences between EG and CG on day 0 for each farm, plant, age category, and diagnosed parasite showed the appropriateness of the study design (Table 2, Table 3 and Table 4).
Eimeriosis is a common parasitic infection in swine that is sporadically related to clinical disease and occasionally linked to diarrhoea and weight loss [54]. Coccidiosis was diagnosed on both farms and in all age groups. C. sativum demonstrated a stronger anticoccidial (25.4–100%) effect than C. pepo (11.6–96.6%) (Table 5). A commercial herbal formula containing several medicinal plants, including C. sativum, effectively controlled experimental coccidiosis in chickens and can potentially be used successfully as a natural anticoccidial drug [55]. Junkuszew et al. [56] established that treating lambs diagnosed with a low intensity of parasitic infection with a mixture of medicinal plants containing C. pepo effectively reduced the number of coccidia, also showing a beneficial effect on the growth and body development of the animals. In an in vitro study, C. pepo and C. sativum alcoholic extracts demonstrated strong anticoccidial effects against Eimeria spp. oocysts isolated from swine [57]. Several studies have shown that coriander seed supplements used in poultry feed acted as an alternative to antibiotics and antiparasitics, reducing cholesterol levels and improving both blood parameters and growth performance [58,59].
Cryptosporidiosis represents a public health issue. The infection has been reported worldwide as a frequent cause of diarrhoea in both humans and animals [60,61]. In the present study, Cryptosporidium spp. was diagnosed in weaners and sows from both farms (Figure 1 and Figure 3). In F2, the plants used were completely inefficacious against these protozoa, while in F1 they showed a weak antiprotozoal efficacy. For Cryptosporidium, the results just indicated its presence, whereas the average intensity was not detected, because the quantitative sensitivity of the usual coproparasitological methods when counting oocysts is absent. Both aqueous and alcoholic C. sativum extracts had a weak effect on Cryptosporidium oocyst shedding, in laboratory infected Balb/c mice in vitro and in vivo [60,61].
Balantioides coli (previously known as Balantidium coli) is considered the largest protozoan and the only parasitic ciliate known to infect humans. This parasite is often found as a commensal in the lumen of the cecum and large intestine of swine, nonhuman primates, and humans [62]. In one study, the prevalence of the B. coli infection on a Danish swine farm increased from 57% in suckling piglets to 100% in most groups of pigs that were several weeks old [63]. In our study, B. coli was identified in all age groups, with varying prevalence (30–100%) and intensity. Both plants were efficient against B. coli; however, coriander (23.2–79.5%) was superior to pumpkin (2.3–59.5%) (Table 5). The current study serves as a first report on the antiprotozoal effects of C. pepo and C. sativum against balantidiasis.
Ascaris suum is a highly prevalent intestinal nematode in swine worldwide, the infection having a strong negative effect on productivity and health [64]. A. suum was only identified in weaners and fatteners from both farms. C. pepo (70.3–100%) was very effective against A. suum, while C. sativum (7.2–30.3%) had a weak anthelmintic effect (Table 5). A previous study established the in vitro anthelmintic effects of C. sativum and C. pepo ethanolic extracts in different concentrations (0.312–5%) against A. suum [30]. C. sativum hydroalcoholic extract (4 mg/mL) led to a 45% A. suum L2 development inhibition after 20 days of incubation [64]. Pumpkin seed extract used in vitro at a concentration of 54.5% against A. suum adults exerted a lethal effect after 11 h 48 min, while at a concentration of 70.5% the time was shortened to 7 h 48 min [65]. C. pepo seed oil had a strong anthelmintic efficacy against Toxocara cati, inhibiting embryonic development and killing the second-stage larvae, and could be used as an effective alternative for the treatment of toxocarosis [20]. C. pepo ethanolic extract demonstrated a proficient anthelmintic effect on embryonating A. suum eggs at all tested concentrations (62.5, 125, 250, 500, 1000, and 2000 μg/mL) [66]. In vivo trials showed that pumpkin, used in a dose of 100 mg/chicken, killed 45 ± 2.3 % of A. galli worms in the intestinal tract [67]. Aziz et al. [68] demonstrated that treating chickens with a dose of 2000 mg/kg of pumpkin seeds led to a mortality rate in A. galli adults similar to that achieved with fenbendazole. Hellawi and Ibrahim [69] showed that pumpkin seed extract had an inhibitory effect on the larval development of A. galli and was more effective and safer than levamisole.
Trichuris suis, the porcine whipworm, is genetically related to Trichuris trichiura, the human whipworm [70]. T. suis has shown substantial potential as a treatment for human autoimmune disorders, including inflammatory bowel disease and multiple sclerosis [71]. T. suis was diagnosed in weaners and fatteners from F1 and F2. C. pepo (50.1–100%) showed a strong anthelmintic effect against this nematode, while C. sativum (0–3.3%) had almost no effect (Table 5). No studies on the efficacy of C. pepo and C. sativum against T. suis have been reported before.
Oesophagostomum spp., the pig nodular worm, has a worldwide distribution, representing a severe problem for swine health and productivity [72]. Oesophagostomum was the last parasite identified. Although this strongyle was diagnosed on both farms, it only infected sows (Figure 3). Because of the infrequent occurrence (low intensity and prevalence) of Oesophagostomum spp., it was impossible to come to any reliable statistical interpretation regarding the efficacy of C. pepo and C. sativum against it (Table 4). Other reports looking at different strongyles outlined the effect of crude aqueous C. sativum extract (0.45 and 0.9 g/kg) against H. contortus artificial infection in sheep. This extract led to the quantitative reduction of H. contortus eggs and adults, comparable to albendazol [73]. Coriander essential oil showed a strong anthelmintic efficacy against ovine gastrointestinal nematodes in vitro by inhibiting egg hatching, larval development, and motility [74,75]. In swine treated with pumpkin seeds at a dose of 5 g/kg repeated three times during one week, the therapeutic efficacy of the plant against Oesophagostomum spp. larvae was similar to that of ivermectin [49]. Strickland et al. [76] reported a 65.5% decrease in faecal H. contortus egg counts during treatment of sheep with pumpkin seeds, but these increased back to the initial levels as soon as the sheep finished the treatment. Pumpkin seed supplements given at a dose of 5 g/kg/day for 42 consecutive days were not an effective treatment for gastrointestinal nematode infection in kids and lambs [77].
The antiprotozoal effect of the Cucurbita genus against numerous parasite species has been documented, including Entamoeba histolytica, Blastocystis spp., Dientamoeba fragilis, Histomonas meleagridis, Tetratrichomonas gallinarum, Plasmodium falciparum, and Giardia lamblia [78,79,80,81,82,83]. Furthermore, C. pepo extracts showed anthelmintic effects against Raillietina spp., Heterakis spp., Heligmosoides bakeri, Hymenolepis nana, Taenia solium, and Aspiculuris tetraptera [35,84,85,86,87]. Boros et al. [36] showed that C. sativum and C. pepo alcoholic extracts completely inhibited the mobility of T. spiralis and T. britovi larvae.
Several studies have shown the antiparasitic effects of C. sativum. Coriander essential oil demonstrated significant antiprotozoal effects against Leishmania amazonensis and Leismania infantum amastigotes and promastigotes, but was not effective against Leismania chagasi promastigote [88,89,90]. C. sativum presented an anticestodal effect against Echinococcus granulosus and Hymenolepis nana [12,91].
5. Conclusions
The present study showed that the use of powdered C. sativum fruit and C. pepo seed, given at a dose of 170 mg/kg/day and 500 mg/kg/day, respectively, for 10 consecutive days, was efficient against gastrointestinal parasites in swine. Statistical analysis showed that coriander was more effective against protozoa while pumpkin exhibited better efficacy against helminths. Considering all the constraints of Romanian livestock farming, these results are a beacon of hope for better management and welfare practices in low-input swine farms.
The lack of toxicity for C. pepo and C. sativum, along with our results, allow us to suggest that these medicinal plants could provide the basis for developing a new line of antiparasitic herbal medication, eventually including other plants. Consequently, health and welfare conditions could be greatly improved through the use of these novel therapeutic alternatives. However, additional studies are required to establish the types of bioactive compounds responsible for the antiparasitic properties of these plants and further evaluate the minimum effective dose and the therapeutic protocol tailored to each age category of swine.
In addition, to the best of our knowledge, this is the first ethnopharmacological report on the antiparasitic effects of C. pepo and C. sativum traditionally used as a novel treatment option in Romania against digestive protozoan and nematode infections in swine.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11051230/s1, Table S1. Ontologies/pathogens, diseases, medicinal plants, and chemical compounds used in experiment.
Author Contributions
Conceptualization, M.-H.B., M.S. and V.C.; methodology, M.-H.B., V.-D.C. and A.M.; software, M.-H.B. and S.D.B.; validation, M.-H.B., M.S., A.C.-P., D.M. and S.D.B.; formal analysis, M.S., S.D.B., A.C.-P. and D.M.; investigation, M.-H.B., V.-D.C. and A.M.; resources, M.S., A.M. and V.C; data curation, M.-H.B. and V.-D.C.; writing—original draft preparation, M.-H.B., V.-D.C., A.C.-P. and M.S.; writing—review and editing, M.-H.B., S.D.B., V.-D.C., A.C.-P., D.M., M.S. and V.C.; visualization, M.-H.B., S.D.B. and M.S.; supervision, M.S., D.M. and V.C.; project administration, M.S. and V.C.; funding acquisition, M.S. and V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca (USAMV Cluj-Napoca) and by the PPILOW project. The PPILOW project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement N°816172.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania (permit no. 231/02.11.2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to acknowledge the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca and the Iuliu Haţieganu University of Medicine and Pharmacy of Cluj-Napoca for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Poux, X. Low input farming systems in Europe: What is at stake. In Low Input Farming Systems: An Opportunity to Develop Sustainable Agriculture, Proceedings of the JRC Summer University Ranco, 2–5 July 2007; Office for Official Publications of the European Communities: Luxembourg, 2008; pp. 1–11. [Google Scholar]
- Botha, M.; Petrescu-Mag, I.V.; Gavriloaie, C. Rustic gene reserves for the future of breed improvement technologies: Old swine (Sus scrofa domesticus) strains and their perspectives. Porc. Res. 2016, 6, 37–56. [Google Scholar]
- Matiuti, M.H.; Hutu, I.; Matiuti, C.L. A possible variant of preserving in vivo the local pigs breeds in Romania. Danub. Anim. Genet. Resour. 2017, 58–61. [Google Scholar]
- Miao, Z.H.; Glatz, P.C.; Ru, Y.J. Review of production, husbandry and sustainability of free-range pig production systems. Asian-Australas. J. Anim. Sci. 2004, 17, 1615–1634. [Google Scholar] [CrossRef]
- Terán Ramírez, V. Efecto del Extracto Hidroalcohólico de Semillas de Coriandrum sativum Sobre la Formación de Larva 2 de Ascaris suum y Trichuris ovis, en Condiciones de Laboratorio. Ph.D. Thesis, Universidad Nacional de Trujillo, Facultad de Ciencias Biológicas Escuela Académico Profesional de Microbiología y Parasitología, Trujillo, Peru, 2015. [Google Scholar]
- Băieş, M.H.; Boros, Z.; Gherman, C.M.; Spînu, M.; Mathe, A.; Pataky, S.; Lefkaditis, M.; Cozma, V. Prevalence of swine gastrointestinal parasites in two free-range farms from nord-west region of Romania. Pathogens 2022, 11, 954. [Google Scholar] [CrossRef]
- Eijck, I.A.J.M.; Borgsteede, F.H.M. A survey of gastrointestinal pig parasites on free-range, organic and conventional pig farms in the Netherlands. Vet. Res. Commun. 2005, 29, 407–414. [Google Scholar] [CrossRef]
- Pettersson, E.; Sjölund, M.; Wallgren, T.; Lind, E.O.; Höglund, J.; Wallgren, P. Management practices related to the control of gastrointestinal parasites on Swedish pig farms. Porc Health Manag. 2021, 7, 12. [Google Scholar] [CrossRef]
- Zajíčková, M.; Nguyen, L.T.; Skálová, L.; Stuchlíková, L.R.; Matoušková, P. Anthelmintics in the future: Current trends in the discovery and development of new drugs against gastrointestinal nematodes. Drug Discov. Today 2020, 25, 430–437. [Google Scholar] [CrossRef]
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Antiparasitic, antiprotozoal, molluscicidal and insecticidal activity of medicinal plants (part 2)–plant based review. Sch. Acad. J. Pharm. 2016, 5, 194–207. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Ghalesefidi, M.J.; Azami, M.; Mohaghegh, M.A.; Hejazi, S.H.; Ghomashlooyan, M. In vitro and in vivo anthelmintic activity of seed extract of Coriandrum sativum compared to Niclosamid against Hymenolepis nana infection. J. Parasit. Dis. 2016, 40, 1307–1310. [Google Scholar] [CrossRef]
- Kalkal, H.; Kumar, P.; Vohra, S. Advances in control strategies and vaccine development of coccidiosis in poultry. Pharma. Innov. 2021, 10, 1084–1090. [Google Scholar]
- Momin, A.H.; Acharya, S.S.; Gajjar, A.V. Coriandrum sativum-review of advances in phytopharmacology. Int. J. Pharm. Sci. 2012, 3, 1233. [Google Scholar]
- Al-Snafi, A.E. A review on chemical constituents and pharmacological activities of Coriandrum sativum. IOSR J. Pharm. 2016, 6, 17–42. [Google Scholar] [CrossRef]
- Paarakh, P.M. Coriandrum sativum Linn. Review. Pharmacologyonline 2009, 3, 561–573. [Google Scholar]
- Pathak Nimish, L.; Kasture Sanjay, B.; Bhatt Nayna, M.; Rathod Jaimik, D. Phytopharmacological properties of Coriander sativum as a potential medicinal tree: An overview. J. Appl. Pharm. Sci. 2011, 1, 20–25. [Google Scholar]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef]
- Kumar, G.P.; Subrahmanyam, S.N. Phytochemical analysis, in-vitro screening for antimicrobial and anthelmintic activity of combined hydroalcoholic seed extracts of four selected folklore indian medicinal plants. Der. Pharm. Lett. 2013, 5, 168–176. [Google Scholar]
- Hussein, S.N.; Shukur, M.S. In-vitro anthelmentic efficacy of pumpkin seed oil (Cucurbita pepo) on toxocariosis (Toxocara cati). Explor. Anim. Med. Res. 2020, 10, 154–161. [Google Scholar]
- Syed, Q.A.; Akram, M.; Shukat, R. Nutritional and therapeutic importance of the pumpkin seeds. Seed 2019, 21, 15798–15803. [Google Scholar] [CrossRef]
- Adnan, M.; Gul, S.; Batool, S.; Fatima, B.; Rehman, A.; Yaqoob, S.; Aziz, M.A. A review on the ethnobotany, phytochemistry, pharmacology and nutritional composition of Cucurbita pepo L. J. Phytopharm. 2017, 6, 133–139. [Google Scholar] [CrossRef]
- Karanja, J.; Mugendi, B.J.; Khamis, F.; Muchugi, A. Nutritional composition of the pumpkin (Cucurbita spp.) seed cultivated from selected regions in Kenya. J. Hortic. Lett. 2013, 3, 17–22. [Google Scholar]
- Vidhya, C.; Loganathan, M.; Bhuvana, S.; Wadje, P.; Rajamani, M. A study on the evaluation of proximate, fatty acid and amino acid profile of two species of pumpkin using advanced techniques. Uttar Pradesh J. Zool. 2022, 43, 74–83. [Google Scholar]
- Dowidar, M.; Ahmed, A.; Mohamed, H. The Critical Nutraceutical Role of Pumpkin Seeds in Human and Animal Health: An Updated Review. Zagazig Vet. J. 2020, 48, 199–212. [Google Scholar] [CrossRef]
- Kinyua, C.M. Modelling and Application of Response Surface Methodology for Optimization of Weight Gain of Eight Weeks Old Kenbro Served with Pumpkin (Cucurbita Pepo L.) Seeds Extract. Ph.D. Dissertation, Chuka University, Chuka, Kenya, 2019. [Google Scholar]
- Achilonu, M.C.; Nwafor, I.C.; Umesiobi, D.O.; Sedibe, M.M. Biochemical proximates of pumpkin (Cucurbitaeae spp.) and their beneficial effects on the general well-being of poultry species. J. Anim. Physiol. Anim. Nutr. 2018, 102, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Fruhwirth, G.O.; Hermetter, A. Seeds and oil of the Styrian oil pumpkin: Components and biological activities. Eur. J. Lipid Sci. Technol. 2007, 109, 1128–1140. [Google Scholar] [CrossRef]
- Etewa, S.E.; Abaza, S.M. Herbal medicine and parasitic diseases. Parasitol. United J. 2011, 4, 3–14. [Google Scholar]
- Bǎieş, M.H.; Gherman, C.; Boros, Z.; Olah, D.; Vlase, A.M.; Cozma-Petrut, A.; Györke, A.; Miere, D.; Vlase, L.; Crişan, G.; et al. The Effects of Allium sativum L., Artemisia absinthium L., Cucurbita pepo L., Coriandrum sativum L., Satureja hortensis L. and Calendula officinalis L. on the Embryogenesis of Ascaris suum Eggs during an In Vitro Experimental Study. Pathogens 2022, 11, 1065. [Google Scholar] [CrossRef]
- Manser, M.M.; Saez, A.C.; Chiodini, P.L. Faecal Parasitology: Concentration Methodology Needs to be Better Standardised. PLoS Negl. Trop. Dis. 2016, 10, e0004579. [Google Scholar] [CrossRef]
- Mircean, V.; Cozma, V.; Gyorke, A. Diagnostic Coproscopic in Bolile Parazitare la Animale, (Coproparasitological Diagnostic in Parasitic Diseases in Animals); Risoprint: Cluj-Napoca, Romania, 2011; pp. 23–35. [Google Scholar]
- Dobson, R.J.; Hosking, B.C.; Jacobson, C.L.; Cotter, J.L.; Besier, R.B.; Stein, P.A.; Reid, S.A. Preserving new anthelmintics: A simple method for estimating faecal egg count reduction test (FECRT) confidence limits when efficacy and/or nematode aggregation is high. Vet. Parasitol. 2012, 186, 79–92. [Google Scholar] [CrossRef]
- McKenna, P.B. Further comparison of faecal egg count reduction test procedures: Sensitivity and specificity. N. Z. Vet. J. 2006, 54, 365–366. [Google Scholar] [CrossRef]
- Grzybek, M.; Kukula-Koch, W.; Strachecka, A.; Jaworska, A.; Phiri, A.M.; Paleolog, J.; Tomczuk, K. Evaluation of anthelmintic activity and composition of pumpkin (Cucurbita pepo L.) seed extracts—In vitro and in vivo studies. Int. J. Mol. Sci. 2016, 17, 1456. [Google Scholar] [CrossRef] [PubMed]
- Boros, Z.; Baies, M.H.; Gherman, C.; Cozma, V. The effects of Artemisia absinthium (wormwood), Allium sativum (garlic), Cucurbita pepo (pumpkin), and Coriandrum sativum (coriander) on Trichinella spiralis and Trichinella britovi larvae, in vitro study. Sci. Parasitol. 2021, 22, 70–78. [Google Scholar]
- Singletary, K. Coriander: Overview of potential health benefits. Nutr. Today 2016, 51, 151–161. [Google Scholar] [CrossRef]
- Jabeen, Q.; Bashir, S.; Lyoussi, B.; Gilani, A.H. Coriander fruit exhibits gut modulatory, blood pressure lowering and diuretic activities. J. Ethnopharmacol. 2009, 122, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A.; Carabin, I.G. Safety assessment of coriander (Coriandrum sativum L.) essential oil as a food ingredient. Food Chem. Toxicol. 2009, 47, 22–34. [Google Scholar] [CrossRef]
- Al-Mofleh, I.A.; Alhaider, A.A.; Mossa, J.S.; Al-Sohaibani, M.O.; Rafatullah, S.; Qureshi, S. Protection of gastric mucosal damage by Coriandrum sativum L. pretreatment in Wistar albino rats. Environ. Toxicol. Pharmacol. 2006, 22, 64–69. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Anaeigoudari, A.; Hashemzehi, M.; Mohebbati, R. Neuroprotective potency of some spice herbs, a literature review. J. Tradit. Complement. Med. 2019, 9, 98–105. [Google Scholar] [CrossRef]
- World Health Organization. Endocrinotoxicity induced by Coriandrum sativa: A case report. WHO Drug Info. 2002, 16, 15.
- Cândido, F.G.; De Oliveira, F.C.; Lima, M.F.C.; Pinto, C.A.; Da Silva, L.L.; Martino, H.S.; Rita de Cássia, G.A. Addition of pooled pumpkin seed to mixed meals reduced postprandial glycemia: A randomized placebo-controlled clinical trial. Nutr. Res. 2018, 56, 90–97. [Google Scholar] [CrossRef]
- Chen, L.; Long, R.; Huang, G.; Huang, H. Extraction and antioxidant activities in vivo of pumpkin polysaccharide. Ind. Crops Prod. 2020, 146, 112199. [Google Scholar] [CrossRef]
- Iwo, M.I.; Insanu, M.; Dass, C.A.S. Development of immunonutrient from pumpkin (Cucurbita moschata Duchense Ex. Lamk.) seed. Procedia Chem. 2014, 13, 105–111. [Google Scholar] [CrossRef]
- Asgary, S.; Moshtaghian, S.J.; Setorki, M.; Kazemi, S.; Rafieian-Kopaei, M.; Adelnia, A.; Shamsi, F. Hypoglycaemic and hypolipidemic effects of pumpkin (Cucurbita pepo L.) on alloxan-induced diabetic rats. Afr. J. Pharm. Pharmacol. 2011, 5, 2620–2626. [Google Scholar]
- Damiano, R.; Cai, T.; Fornara, P.; Franzese, C.A.; Leonardi, R.; Mirone, V. The role of Cucurbita pepo in the management of patients affected by lower urinary tract symptoms due to benign prostatic hyperplasia: A narrative review. Arch. Ital. Urol. Androl. 2016, 88, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Chari, K.Y.; Polu, P.R.; Shenoy, R.R. An appraisal of pumpkin seed extract in 1, 2-dimethylhydrazine induced colon cancer in wistar rats. J. Toxicol. 2018, 2018, 6086490. [Google Scholar] [CrossRef]
- Magi, E.; Talvik, H.; Jarvis, T. In vivo studies of the effect of medicinal herbs on the pig nodular worm (Oesophagostomum spp.). Helminthologia 2005, 42, 67–69. [Google Scholar]
- De Queiroz-Neto, A.; Mataqueiro, M.I.; Santana, A.E.; Alessi, A.C. Toxicologic evaluation of acute and subacute oral administration of Cucurbita maxima seed extracts to rats and swine. J. Ethnopharmacol. 1994, 43, 45–51. [Google Scholar] [CrossRef]
- Karpagam, T.; Varalakshmi, B.; Bai, J.S.; Gomathi, S. Effect of different doses of Cucurbita pepo linn extract as an anti-inflammatory and analgesic nutraceautical agent on inflamed rats. Int. J. Pharm. Res. Dev. 2011, 3, 184–192. [Google Scholar]
- Krimer-Malešević, V.; Mađarev-Popović, S.; Vaštag, Ž.; Radulović, L.; Peričin, D. Chapter 109. Phenolic acids in pumpkin (Cucurbita pepo L.) seeds. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: Serbia, 2011; pp. 925–932. [Google Scholar]
- Njoku, R.C.C.; Abarikwu, S.O.; Uwakwe, A.A.; Mgbudom-Okah, C.J.; Ezirim, C.Y. Dietary fluted pumpkin seeds induce reversible oligospermia and androgen insufficiency in adult rats. Syst. Biol. Reprod. Med. 2019, 65, 437–450. [Google Scholar] [CrossRef]
- Daugschies, A.; Imarom, S.; Ganter, M.; Bollwahn, W. Prevalence of Eimeria spp. in sows at piglet-producing farms in Germany. J. Vet. Med. 2004, 51, 135–139. [Google Scholar] [CrossRef]
- Pop, L.M.; Varga, E.; Coroian, M.; Nedișan, M.E.; Mircean, V.; Dumitrache, M.O.; Györke, A. Efficacy of a commercial herbal formula in chicken experimental coccidiosis. Parasites Vectors 2019, 12, 343. [Google Scholar] [CrossRef]
- Junkuszew, A.; Milerski, M.; Bojar, W.; Szczepaniak, K.; Le Scouarnec, J.; Tomczuk, K.; Bracik, K. Effect of various antiparasitic treatments on lamb growth and mortality. Small Rumin. Res. 2015, 123, 306–313. [Google Scholar] [CrossRef]
- Bǎieş, M.H.; Györke, A.; Cotuţiu, V.D.; Boros, Z.; Cozma-Petruţ, A.; Filip, L.; Vlase, L.; Vlase, A.-M.; Crişan, G.; Spînu, M.; et al. The In Vitro Anticoccidial Activity of Some Herbal Extracts against Eimeria spp. Oocysts Isolated from Piglets. Pathogens 2023, 12, 258. [Google Scholar] [PubMed]
- Aćimović, M.G.; Kostadinović, L.M.; Puvača, N.M.; Popović, S.J.; Urošević, M.I. Phytochemical constituents of selected plants from Apiaceae family and their biological effects in poultry. Food Feed Res. 2016, 43, 35–41. [Google Scholar] [CrossRef]
- Seidavi, A.; Tavakoli, M.; Slozhenkina, M.; Gorlov, I.; Hashem, N.M.; Asroosh, F.; Swelum, A.A. The use of some plant-derived products as effective alternatives to antibiotic growth promoters in organic poultry production: A review. Environ. Sci. Pollut. Res. 2021, 28, 47856–47868. [Google Scholar] [CrossRef] [PubMed]
- Obiad, H.M.; Al-Alousi, T.I.; Al-Jboori, A.H. An epidemiologic study on Cryptosporidium spp. in Kirkuk city with some trials for in vitro treating the parasite. In Second Scientific Conference; Science College, Tikrit University: Tikrit, Iraq, 2012. [Google Scholar]
- Obiad, H.M.; Al-Alousi, T.I.; Al-Jboori, A.H. The in vivo effect of some medicinal plant extracts on Cryptosporidium parasite. J. Univ. Anbar. Pure Sci. 2012, 6, 3. [Google Scholar]
- Nilles-Bije, M.; Rivera, W.L. Ultrastructural and molecular characterization of Balantidium coli isolated in the Philippines. Parasitol. Res. 2010, 106, 387–394. [Google Scholar] [CrossRef]
- Hindsbo, O.; Nielsen, C.V.; Andreassen, J.; Willingham, A.L.; Bendixen, M.; Nielsen, M.A.; Nielsen, N.O. Age-dependent occurrence of the intestinal ciliate Balantidium coli in pigs at a Danish research farm. Acta Vet. Scand. 2000, 41, 79–83. [Google Scholar] [CrossRef]
- Thamsborg, S.M.; Nejsum, P.; Mejer, H. Chapter 14. Impact of Ascaris suum in livestock. In Ascaris: The Neglected Parasite; Holland, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 363–381. [Google Scholar]
- Ganestya, S.; Djumarga, S.; Utari, C.S. Anthelmintic effects of pumpkin (Cucurbita moschata) seed extract on Ascaris suum in vitro. Asian J. Nat. Prod. Biochem. 2012, 10, 1–6. [Google Scholar] [CrossRef]
- Urban, J.; Kokoska, L.; Langrova, I.; Matejkova, J. In vitro anthelmintic effects of medicinal plants used in Czech Republic. Pharm. Biol. 2008, 46, 808–813. [Google Scholar] [CrossRef]
- Bazh, E.K.; El-Bahy, N.M. In vitro and in vivo screening of anthelmintic activity of ginger and curcumin on Ascaridia galli. Parasitol. Res. 2013, 112, 3679–3686. [Google Scholar] [CrossRef]
- Aziz, A.R.A.; AbouLaila, M.R.; Aziz, M.; Omar, M.A.; Sultan, K. In vitro and in vivo anthelmintic activity of pumpkin seeds and pomegranate peels extracts against Ascaridia galli. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 231–234. [Google Scholar]
- Hellawi, H.; Ibrahim, O.M. Evaluation of anthelmintic activity of N-hexane extract of Cucurbita Maxima and Azadirachta Indica pulp seed against Ascaridia Galli in vitro. Biochem. Cell. Arch. 2021, 21, 211–217. [Google Scholar]
- Summers, R.W.; Elliott, D.E.; Urban, J.F.; Thompson, R.; Weinstock, J. Trichuris suis therapy in Crohn’s disease. Gut 2005, 54, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Jex, A.R.; Nejsum, P.; Schwarz, E.M.; Hu, L.; Young, N.D.; Hall, R.S.; Gasser, R.B. Genome and transcriptome of the porcine whipworm Trichuris suis. Nat. Genet. 2014, 46, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Q.; Ai, L.; Zou, F.C.; Verweij, J.J.; Jiang, Q.; Li, M.W.; Zhu, X.Q. A multiplex PCR tool for the specific identification of Oesophagostomum spp. from pigs. Parasitol. Res. 2008, 103, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Eguale, T.; Tilahun, G.; Debella, A.; Feleke, A.; Makonnen, E. In vitro and in vivo anthelmintic activity of crude extracts of Coriandrum sativum against Haemonchus contortus. J. Ethnopharmacol. 2007, 110, 428–433. [Google Scholar] [CrossRef]
- Macedo, I.T.F.; Oliveira, L.M.B.D.; Camurça-Vasconcelos, A.L.F.; Ribeiro, W.L.C.; Santos, J.M.L.D.; Morais, S.M.D.; Bevilaqua, C.M.L. In vitro effects of Coriandrum sativum, Tagetes minuta, Alpinia zerumbet and Lantana camara essential oils on Haemonchus contortus. Rev. Bras. Parasitol. Vet. 2013, 22, 463–469. [Google Scholar] [CrossRef]
- Helal, M.A.; Abdel-Gawad, A.M.; Kandil, O.M.; Khalifa, M.M.; Cave, G.W.; Morrison, A.A.; Elsheikha, H.M. Nematocidal effects of a coriander essential oil and five pure principles on the infective larvae of major ovine gastrointestinal nematodes in vitro. Pathogens 2020, 9, 740. [Google Scholar] [CrossRef]
- Strickland, V.J.; Krebs, G.L.; Potts, W. Pumpkin kernel and garlic as alternative treatments for the control of Haemonchus contortus in sheep. Anim. Prod. Sci. 2009, 49, 139–144. [Google Scholar] [CrossRef]
- Matthews, K.K.; O’Brien, D.J.; Whitley, N.C.; Burke, J.M.; Miller, J.E.; Barczewski, R.A. Investigation of possible pumpkin seeds and ginger effects on gastrointestinal nematode infection indicators in meat goat kids and lambs. Small Rumin. Res. 2016, 136, 1–6. [Google Scholar] [CrossRef]
- Elhadi, I.M.; Koko, W.S.; Dahab, M.M.; El Imam, Y.M.; El Mageed, M.A.E.A. Antigiardial activity of some Cucurbita species and Lagenaria siceraria. J. For. Prod. Ind. 2013, 2, 43–47. [Google Scholar]
- Barratt, J.; Ellis, J.; Harkness, J.; Marriott, D.; Stark, D. Evaluation of the in vitro antiprotozoal activity of various dry plant extracts against Dientamoeba fragilis. J. Infect. Dis. Ther. 2013, 1, 1000111. [Google Scholar]
- Grabensteiner, E.; Liebhart, D.; Arshad, N.; Hess, M. Antiprotozoal activities determined in vitro and in vivo of certain plant extracts against Histomonas meleagridis, Tetratrichomonas gallinarum and Blastocystis sp. Parasitol. Res. 2008, 103, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Kabbashi, A.S.; Koko, W.S.; Mohammed, S.E.A.; Musa, N.; Osman, E.E.; Dahab, M.M.; Mohammed, A.K. In vitro amoebicidal, antimicrobial and antioxidant activities of the plants Adansonia digitata and Cucurbita maxima. Adv. Med. Plant Res. 2014, 2, 50–57. [Google Scholar]
- Ezeani, C.; Ezenyi, I.; Erhunse, N.; Sahal, D.; Akunne, T.; Okoli, C. Assessment of antimalarial medicinal plants used in Nigerian ethnomedicine reveals antimalarial potential of Cucurbita pepo leaf extract. Heliyon 2022, 8, e09916. [Google Scholar] [CrossRef]
- Salman, S.S.; Ardalan, N.M. Evaluation of Amygdalin (B17) and Cucurbita pepo (Pumpkin seed) activity against Blastocystis from diarrheic patients in Baghdad, Iraq: In Vitro Study. Baghdad Sci. J. 2022, 19, 0016. [Google Scholar] [CrossRef]
- Li, T.; Ito, A.; Chen, X.; Long, C.; Okamoto, M.; Raoul, F.; Craig, P.S. Usefulness of pumpkin seeds combined with areca nut extract in community-based treatment of human taeniasis in northwest Sichuan Province, China. Acta Trop. 2012, 124, 152–157. [Google Scholar] [CrossRef]
- Acorda, J.A.; Mangubat, I.Y.E.C.; Divina, B.P. Evaluation of the in vivo efficacy of pumpkin (Cucurbita pepo) seeds against gastrointestinal helminths of chickens. Turk. J. Vet. Anim. Sci. 2019, 43, 206–211. [Google Scholar] [CrossRef]
- Ayaz, E.; Gökbulut, C.; Coşkun, H.; Uçar Türker, A.; Özsoy, Ş.; Ceylan, K. Evaluation of the anthelmintic activity of pumpkin seeds (Cucurbita maxima) in mice naturally infected with Aspiculuris tetraptera. J. Pharmacogn. Phytother. 2015, 7, 189–193. [Google Scholar]
- Alhawiti, A.O.; Toulah, F.H.; Wakid, M.H. Anthelmintic potential of Cucurbita pepo seeds on Hymenolepis nana. Acta Parasitol. 2019, 64, 276–281. [Google Scholar] [CrossRef]
- Monzote, L.; Herrera, I.; Satyal, P.; Setzer, W.N. In-vitro evaluation of 52 commercially-available essential oils against Leishmania amazonensis. Molecules 2019, 24, 1248. [Google Scholar] [CrossRef] [PubMed]
- Rondon, F.C.M.; Bevilaqua, C.M.L.; Accioly, M.P.; Morais, S.M.D.; Andrade-Júnior, H.F.D.; Carvalho, C.A.D.; Magalhães, H.C.R. In vitro efficacy of Coriandrum sativum, Lippia sidoides and Copaifera reticulata against Leishmania chagasi. Rev. Bras. Parasitol. Vet. 2012, 21, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rondon, F.C.; Bevilaqua, C.M.; Accioly, M.P.; Morais, S.M.; Andrade-Junior, H.F.; Machado, L.K.; Rodrigues, A.C.M. In vitro effect of Aloe vera, Coriandrum sativum and Ricinus communis fractions on Leishmania infantum and on murine monocytic cells. Vet. Parasitol. 2011, 178, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Dawwas, A. Investigation of biochemical effect of phenols extract isolated from Coriandrum sativum seeds against Echinococcus granulosus parasite in vitro. J. Thi-Qar Sci. 2008, 1, 2–9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).