Abstract
Ixodes apronophorus is an insufficiently studied nidicolous tick species. For the first time, the prevalence and genetic diversity of Rickettsia spp. in Ixodes apronophorus, Ixodes persulcatus, and Ixodes trianguliceps ticks from their sympatric habitats in Western Siberia were investigated. Rickettsia helvetica was first identified in I. apronophorus with a prevalence exceeding 60%. “Candidatus Rickettsia tarasevichiae” dominated in I. persulcatus, whereas I. trianguliceps were infected with “Candidatus Rickettsia uralica”, R. helvetica, and “Ca. R. tarasevichiae”. For larvae collected from small mammals, a strong association was observed between tick species and rickettsiae species/sequence variants, indicating that co-feeding transmission in studied habitats is absent or its impact is insignificant. Phylogenetic analysis of all available R. helvetica sequences demonstrated the presence of four distinct genetic lineages. Most sequences from I. apronophorus belong to the unique lineage III, and single sequences cluster into the lineage I alongside sequences from European I. ricinus and Siberian I. persulcatus. Rickettsia helvetica sequences from I. trianguliceps, along with sequences from I. persulcatus from northwestern Russia, form lineage II. Other known R. helvetica sequences from I. persulcatus from the Far East group into the lineage IV. The obtained results demonstrated the high genetic variability of R. helvetica.
1. Introduction
Ixodes spp. (Ixodidae, Ixodida, Arachnida) ticks are recognized vectors of different bacterial agents; the most epidemiologically important of them are spirochetes from Borrelia burgdorferi sensu lato species complex and relapsing fever group, as well as intracellular bacteria from the Anaplasmataceae and Rickettsiaceae families [1,2,3,4]. Several Ixodes species are common in Western Siberia of Russia, namely Ixodes persulcatus, Ixodes pavlovskyi, Ixodes trianguliceps, Ixodes apronophorus, Ixodes lividus, and Ixodes crenulatus. Of them, only I. persulcatus and I. pavlovskyi bite humans [5,6].
In many locations, several Ixodes species occur simultaneously. Thus, in the south taiga subzone of Western Siberia, I. persulcatus often occurs in sympatry with I. trianguliceps; moreover, in some locations, three Ixodes species, I. persulcatus, I. trianguliceps, and I. apronophorus, can coexist together [7,8]. All these tick species have three-host developmental cycles. Small mammals are the main hosts for the preimaginal stages of I. persulcatus, whereas I. persulcatus adults feed mainly on middle-size and large mammals [5,9]. All stages of I. trianguliceps and I. apronophorus feed predominantly on small mammals. European water vole (Arvicola amphibious) is considered to be one of the main hosts for I. apronophorus [6,7,9].
Ixodes persulcatus and I. trianguliceps have been tested for the presence of different bacterial agents in a number of studies. Both tick species can be infected with Anaplasma phagocytophilum and B. burgdorferi s.l.; in addition, Ehrlichia muris and Borrelia miyamotoi have been found in I. persulcatus [10,11,12,13]. As for rickettsial agents, I. persulcatus was most frequently infected with “Candidatus Rickettsia tarasevichiae” (up to 90% of infected ticks), and in rare cases, with Rickettsia helvetica, Rickettsia heilongjiangensis, Rickettsia raoultii, and Rickettsia sibirica [11,14,15,16]. Previous studies have demonstrated that R. helvetica, “Ca. R. tarasevichiae”, and “Candidatus Rickettsia uralica” can be detected in I. trianguliceps [14,17].
In contrast to I. persulcatus and I. trianguliceps, I. apronophorus is insufficiently studied. This tick has been recorded in many European countries, Siberia, and Kazakhstan [6,7,9,18,19]. A recent genetic study of I. apronophorus has demonstrated that it belongs to I. ricinus-I. persulcatus species complex within the subgenus Ixodes [8]; I. trianguliceps is a member of the Exopalpiger subgenus [9]. Although I. apronophorus does not bite humans, it may transmit infectious agents to I. persulcatus through infected animals or during co-feeding on the same small mammals and, thus, participate in the same enzootic cycle. It has been recently shown for the first time that I. apronophorus can be infected with Borrelia bavariensis and “Candidatus Borrelia sibirica” [13]. However, the ability of I. apronophorus to be infected with any Anaplasmataceae and Rickettsiaceae agents has not been studied to date.
In this study, we first examined the presence and genetic variability of Rickettsia spp. in I. apronophorus ticks collected in I. persulcatus/I. trianguliceps/I. apronophorus sympatric areas. The species diversity and genetic variability of Rickettsia spp. identified in different Ixodes species from the same sympatric areas were compared.
2. Materials and Methods
2.1. Sampling
Ticks were collected in two sampling sites within the forest zone in Omsk province, Western Siberia, Russia. The first site (Om-Bo) was located at the boundary between aspen-birch forests and southern taiga in the Bolsheukov district (56°46′ N, 72°03′ E), and the second site (site Om-Zn) was situated in the southern taiga subzone in Znamenskiy district (57°23′ N, 73°40′ E) (Figure 1). All animal experiments were approved by the Animal Welfare Act at the Omsk Research Institute of Natural Foci Infections, according to the guidelines for experiments with laboratory animals (Supplement to the Order of the Russian Ministry of Health, no. 755, of 12 August 1977). Animal use and experimental procedures were approved by the Bioethical Committee of the Omsk Research Institute of Natural Foci Infections (Protocol No.1, 20 March 2013; Protocol No. 4, 17 February 2016).
Figure 1.
Sites of specimen collection in Omsk province of Western Siberia. Stars shows the locations of sampling sites.
Wild rodents were captured in the site Om-Bo in June 2016 and in the site Om-Zn from June–September 2014–2015. Voles and mice of the genera Myodes, Microtus and Apodemus were caught using live traps, and European water voles were captured by steel traps. The species of trapped animals were determined based on morphological features. The animals were examined for the presence of attached ticks (larvae, nymphs and adults), which were removed with forceps.
The species and stage of ticks collected from animals were preliminarily determined using a stereo microscope MC-800 (Micros, Hunnenbrunn/Gewerbezone, Austria), according to morphological keys [9,20]. The tick species of all Ixodes spp. ticks were additionally determined using the multiplex PCR assay by ITS2 fragments as described previously [8]. For a subset of specimens, the species identities were confirmed by sequencing of ITS2 and/or mitochondrial cox1 gene fragments as previously described [8].
Some engorged and nearly engorged larvae and nymphs were stored at 10–15 °C for 1–2 weeks and then transported to the laboratory and allowed to molt into nymphs or adults, respectively. Other ticks were placed in sealed plastic tubes, which were then stored in liquid nitrogen until species determination and DNA extraction.
Hereinafter, ticks molted in the laboratory are named “molted ticks”, whereas ticks examined without preliminary molting are named “non-molted ticks”.
2.2. Tick Metamorphosis
For successful molting, partially engorged larvae and nymphs were fed to repletion on laboratory white mice. Each engorged tick was placed individually in a glass tube and incubated in the dark at 100% relative humidity at 24–26 °C until completion of molting. Molted ticks were individually frozen four weeks after molting and stored at −70 °C until DNA extraction.
2.3. DNA Extraction
Frozen ticks were homogenized with the MagNA Lyser Instrument using the MagNa Lyser Green Beads (Roche Diagnostics, Basel, Switzerland). Total DNA was extracted from crushed individual ticks using the Proba NK kit (DNA-Technology, Moscow, Russia) according to the manufacturer’s protocol. To prevent cross-contamination, DNA/RNA extraction, amplification, and PCR product detection were carried out in separate rooms. Aerosol-free pipette tips were used at each stage.
2.4. Detection and Genotyping of Rickettsia spp.
To detect Rickettsia spp. DNA, nested PCR was performed for the gltA gene with primers glt1-glt4. For correct species determination in the case of probably mixed infection, additional nested reactions were performed independently using primers RT1 and RT2, specific to “Ca. R. tarasevichiae”, and primers RH1 and RH3, specific to spotted fever group rickettsiae (SFGR) (Table 1). The amplified gltA gene fragments from all specimens positive for SFGR and some specimens positive for “Ca. R. tarasevichiae” were sequenced. For a number of specimens, fragments of 16S rRNA, ompA, ompB, sca4, and htrA genes, as well as groESL operon and 23S-5S IGS, were additionally amplified using primers specified in Table 1 and sequenced.
2.5. Sequencing and Phylogenetic Analysis
The PCR products were purified using GFX Columns (Amersham Biosciences, Piscataway, NJ, USA). Sanger reactions were performed using the BigDye Terminator V. 3.1 Cycling Sequencing Kit (Applied Biosystems, Foster City, CA, USA) in standard conditions specified in the BigDye Terminator V. 3.1 Cycling Sequencing Kit User Guide. Sanger reaction products were purified using CentriSep spin columns (Princeton Separations, Freehold, NJ, USA) and visualized with a 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Sequence analysis was performed with BlastN (http://www.ncbi.nlm.nih.gov/BLAST, accessed on 3 April 2023) and BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html, accessed on 3 April 2023). Phylogenetic trees were constructed using the Maximum likelihood (ML) method based on the Tamura-Nei model in MEGA 7.0 with 1000 bootstrap replicates [21].

Table 1.
Primers used for detection Rickettsia spp.
Table 1.
Primers used for detection Rickettsia spp.
| Amplified Locus | Organism | Reaction | Primer Name | Primer Sequences 5′-3′ | T# (°C) | References |
|---|---|---|---|---|---|---|
| 16S rRNA | Rickettsia spp. | Primary | 16S1 | gacgggtgagtaacacgtggg | 56 | [22] |
| gene | 16S2 | gtcttttagggatttgctccac | ||||
| Nested | 16S3 | gatggatgagcccgcgtcag | 60 | |||
| 16S4 | gcatctctgcgatccgcgac | |||||
| gltA gene | Rickettsia spp. | Primary | glt1 | gattgctttacttacgaccc | 52 | [22] |
| glt2 | tgcatttctttccattgtgc | |||||
| Nested | glt3 | tatagacggtgataaaggaatc | 53 | |||
| glt4 | cagaactaccgatttctttaagc | |||||
| ompA gene | Rickettsia spp. | Conventional | Rr190.70p | atggcgaatatttctccaaaa | 55 | [23] |
| fragment I | 190-701 | gttccgttaatggcagcatct | ||||
| ompA gene | Rickettsia spp. | Primary | Afnw_1 | ggcacaaatactttaacattacc | 52 | [24] |
| 190-6808 | cacgaactttcacactacc | [23] | ||||
| Nested | Afnw_3 | aagcctactcctaaagagaatg | 53 | [24] | ||
| Afnw_4 | cgacagtctctagtgccg | |||||
| Rickettsia spp. | Primary | A1 | taacattacaagctggaggaagcc | 58 | [22] | |
| A2 | ttcagagcctgaccaccgg | |||||
| Nested | A5 | caagtgctggtgatgttacta | 56 | |||
| A6 | tagttacatttcctgcacctac | |||||
| R. helvetica | Primary | Afn1_helv | gtaatactagcatcaccgaaatcc | 55 | This study | |
| 190-6808 | cacgaactttcacactacc | [23] | ||||
| Nested | 190-5125 | gcggttactttagccaaagg | 54 | |||
| Afnw_4 | cgacagtctctagtgccg | [24] | ||||
| ompA gene | “Ca. R. tarasevichiae” | Conventional | 190-5125 | gcggttactttagccaaagg | 56 | [23] |
| fragment IV | 190-6013m * | gcatcttytgcgttgyattac | ||||
| ompB gene | Rickettsia spp. | Primary | M59 F | ccgcagggttggtaactgc | 55 | [25] |
| 120-1497m * | cctatatcgccggtaattgtagc | |||||
| Nested | BR1 | gttactaatggatttattcaagt | 53 | [22] | ||
| BR2 | gcataaacttgtccagcgat | |||||
| Rickettsia spp. | Primary | B2f_5 | taaacttgctgacggtacag | 56 | [24] | |
| B2f_2 | cgattatgccgttatcgcttccaag | |||||
| Nested | B2f_3 | gtagcctaacaaatgctcaaac | 52 | |||
| 120-2399 | cttgtttgtttaatgttacggt | [25] | ||||
| R. helvetica | Primary | B2f_3 | gtagcctaacaaatgctcaaac | 52 | ||
| B2f_2 | cgattatgccgttatcgcttccaag | |||||
| Nested | B2f_1helv | cagtacaattcgctcacaacac | 55 | This study | ||
| 120-2399 | cttgtttgtttaatgttacggt | |||||
| Rickettsia spp. | Primary | B1 | atatgcaggtatcggtact | 56 | [22] | |
| B2 | ccatataccgtaagctacat | |||||
| Nested | B3 | gcaggtatcggtactataaac | 56 | |||
| B4 | aatttacgaaacgattacttccgg | |||||
| Rickettsia spp. | Primary | 120-3462 | ccacaggaactacaaccatt | 52 | [25] | |
| 120-4879m * | tagaagtttacacggacttttagag | |||||
| Nested | B3f_3f | gctggacctgaagctggagc | 55 | [24] | ||
| 120-4879m * | tagaagtttacacggacttttagag | [25] | ||||
| sca4 gene | Rickettsia spp. | Primary | D1f | atgagtaaagacggtaacct | 52 | [26] |
| D1876rm * | tagtttgttccgccgtaatc | |||||
| Nested | sc1f_3 | gatgtaggtgatgaactctg | 52 | [24] | ||
| D1390r | cttgcttttcagcaatatcac | [26] | ||||
| Primary | sc4-1 | atgtctctgaattaagcaatgc | 52 | [22] | ||
| Rj2837r | cctgatactacccttacatc | [27] | ||||
| Nested | sc4-5 | ccggcacaacaacaattgatg | 50 | [22] | ||
| sc4-6 | cctttaccagctcatctactt | |||||
| Primary | sc4-3 | aattattaggctctgtattaaaga | 52 | [22] | ||
| D3069r | tcagcgttgtggaggggaag | [26] | ||||
| Nested | sc4-5 | ccggcacaacaacaattgatg | 52 | [22] | ||
| sc4-7 | ctctcttttaataggtgttgatt | [24] | ||||
| htrA gene | Rickettsia spp. | Conventional | 17k-5 | gctttacaaaattctaaaaaccatata | 55 | [28] |
| 17k-3 | tgtctatcaattcacaacttgcc | |||||
| 23S-5S IGS | Rickettsia spp. | Conventional | RCK/23-5-F | gataggtcrgrtgtggaagca | 55 | [29] |
| RCK/23-5-R | tcgggaygggatcgtgtgtttc | |||||
| groESL | Rickettsia spp. | Primary | Ric-ESL-F1 | ggtaaatgggcaggyaccgaa | 60 | [30] |
| operon | Ric-ESL-R1 | gaagcaacrgaagcagcatctt | ||||
| Nested | Ric-ESL-F2 | atcgttatgaaagaaagcgayg | 58 | |||
| Ric-ESL-R2 | agwgcagtacgcactactttagc |
m *—modified primers, T#—Annealing temperature.
2.6. Statistical Analysis
Differences in the prevalence of causative agents between tick species were computed with the Pearson χ2 goodness-of-fit test (http://www.socscistatistics.com/tests/chisquare/, accessed on 20 February 2023). p < 0.05 was regarded as statistically significant.
2.7. Nucleotide Sequence Accession Numbers
Nucleotide sequences determined in this study were deposited in the GenBank database under accession numbers: ON863706-ON863712; OQ092468-OQ092488; OQ102487-OQ102493; OQ257005-OQ257011; OQ271213-OQ271221; OQ540726-OQ540738; OQ553798; OQ573689-OQ573698; OQ652110-OQ652116; OQ675828-OQ675832; OQ866612, OQ866613, OQ866615, OQ866617, OQ866619, OQ866621, OQ866623.
3. Results
3.1. Sampling
In this study, ticks were collected in two sites (Om-Bo and Om-Zn) of Omsk province, Western Siberia. The investigation included 145 ticks collected from small mammals in the site Om-Bo and examined without preliminary molting. In addition, 20 ticks from the site Om-Bo and 115 ticks from the site Om-Zn collected from mammals and molted under laboratory conditions were tested (Table 2). As Rickettsia spp. is efficiently transmitted transovarially, the study of non-molted ticks of all stages can be informative.

Table 2.
Detection of Rickettsia spp. in molted and non-molted ticks.
Of 145 non-molted ticks taken from 29 rodents in site Om-Bo, 62 I. apronophorus, 59 I. persulcatus and 24 I. trianguliceps ticks were identified using molecular methods. Among larvae and nymphs collected from mammals in site Om-Bo and molted under laboratory conditions, five I. apronophorus, 14 I. persulcatus and one I. trianguliceps were determined. Among ticks collected in site Om-Zn and molted in the laboratory, there were five I. apronophorus, 87 I. persulcatus and 23 I. trianguliceps ticks.
3.2. Detection of Rickettsia spp. in Non-Molted Ticks
Ixodes spp. larvae, nymphs and adults collected from mammals were examined for the presence of rickettsial agents. Rickettsia spp. DNA was detected in 74.2% (46/62) non-molted I. apronophorus, 83.1% (49/59) I. persulcatus and 66.7% (16/24) I. trianguliceps ticks (Table 2). Among infected I. apronophorus, 44 ticks were infected with R. helvetica, and single ticks carried DNA of “Ca. R. tarasevichiae” or both R. helvetica and “Ca. R. tarasevichiae”. This was the first finding of rickettsiae in I. apronophorus. Forty-eight I. persulcatus carried DNA of “Ca. R. tarasevichiae”, and one tick contained DNA of both “Ca. R. tarasevichiae” and R. raoultii. As for I. trianguliceps ticks, R. helvetica was identified in nine larvae, while “Ca. R. uralica” was determined in all infected nymphs and adults (n = 6) and one larva (Table 2).
3.3. Detection of Rickettsia spp. in Molted Ixodes spp. Ticks
Among molted Ixodes spp., the prevalence of various rickettsiae substantially varied between different tick species (Table 2). Molted I. apronophorus ticks from both sites were infected only with R. helvetica (3/5 ticks in site Om-Bo and 4/5 ticks in site Om-Zn), whereas “Ca. R. uralica” (3/24; 12.5%) and “Ca. R. tarasevichiae” (2/24; 8.3%) were found in molted I. trianguliceps (Table 2). Molted I. persulcatus ticks from both sites were most frequently infected with “Ca. R. tarasevichiae” (57.1% and 85.1% ticks in sites Om-Bo and Om-Zn, respectively). Rickettsia helvetica, “Ca. R. uralica”, and a new Rickettsia genetic variant, Rickettsia sp. Om-113/4_Iper_m, were identified only in single I. persulcatus. In this study, “Ca. R. uralica” was revealed for the first time in a molted I. persulcatus tick (Table 2).
Thus, in molted ticks, “Ca. R. tarasevichiae” was detected significantly more often in I. persulcatus, R. helvetica—in I. apronophorus, and “Ca. R. uralica”—in I. trianguliceps compared to other tick species (p < 0.001 in all cases).
3.4. Genotyping of Rickettsia raoultii and Candidate Species
“Candidatus R. tarasevichiae” was identified using species-specific PCR, whereas other rickettsial species were determined by gltA gene sequencing. All “Ca. R. tarasevichiae” isolates from I. trianguliceps and I. apronophorus and 20 “Ca. R. tarasevichiae” isolates from I. persulcatus were genetically characterized by the gltA gene. In addition, fragments of the 16S rRNA (732 bp), gltA (684 bp), ompA (omp AI, 506 bp and omp AIV, 776 bp), ompB (864 bp), sca4 (1185 bp), htrA (499 bp) genes, groESL operon (1519 bp), and 23S-5S IGS region (334 bp) were sequenced for “Ca. R. tarasevichiae” isolates from non-molted and molted I. persulcatus and molted I. trianguliceps (Table S1). The determined sequences of “Ca. R. tarasevichiae” from different tick species were identical and corresponded to those previously identified in I. persulcatus ticks from the Russian Far East (OP603104, OP612303-OP612310).
“Candidatus R. uralica” isolates were genetically characterized by sequencing of the gltA (790 bp), ompA (384 bp), ompB (1274 bp), and sca4 (786 bp) gene fragments. The obtained sequences showed 100% identity between themselves and the previously determined “Ca. R. uralica” sequences (OM293669, OM293671-OM293673). For more detailed genotyping, the sequences of the 16S rRNA (1067 bp), gltA (1022 bp), ompA (two fragments: 578 bp and 3116 bp), ompB (4871), sca4 (2971 bp), htrA (497 bp) genes, groESL operon (1481 bp) and 23S-5S IGS (354 bp) were determined for four “Ca. R. uralica” samples from I. trianguliceps, including one molted tick (Table S1). The obtained sequences of each genetic locus showed 100% identity.
Rickettsia raoultii and a new rickettsial genetic variant were revealed in single ticks; they were genotyped only by gltA gene. Rickettsia raoultii isolate from an I. persulcatus larva (726 bp) differed by one mismatch from R. raoultii RpA4 genotype (DQ365803). A new genetic variant Rickettsia sp. Om-113/4_Iper_m from a molted I. persulcatus (373 bp) differed by two nucleotide substitutions from R. heilongjiangensis and R. slovaca (CP002912 and U59725, respectively).
3.5. Genotyping of R. helvetica
All R. helvetica isolates were genetically characterized by sequencing gltA fragments with 840 bp length, and five sequence variants were found. For a subset of specimens with various gltA sequences, the ompB (1255 bp), sca4 (783 bp), and 16S rRNA (684 bp) gene fragments were additionally sequenced. A comparison of the obtained sequences showed the presence of six sequence variants, which varied by 2–8 substitutions. All obtained sequences differed from those of prototype R. helvetica strain C9P9 (AICO01000001) and isolates from the Russian Far East (OQ209952, OQ209953, OQ257004) (Figure 2A).
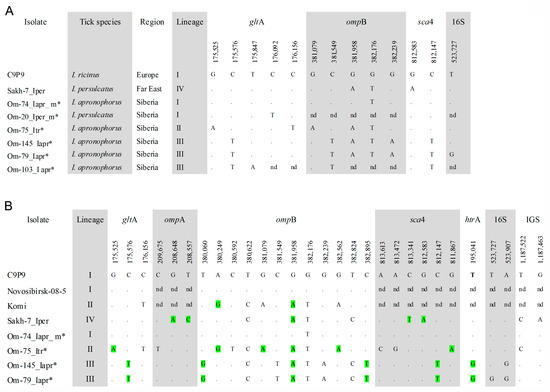
Figure 2.
(A) Condensed alignment of gltA (840 bp), ompB (1255 bp), sca4 (783 bp), and 16S rRNA (684 bp) gene fragments of R. helvetica sequence variants from this study; (B) Condensed alignment of gltA (1037 bp), ompA (1417 bp), ompB (3100 bp), sca4 (2398 bp), htrA (499 bp), 16S rRNA (1070 bp) genes and 23S-5S IGS region (489 bp) of R. helvetica genetic lineages. Variable nucleotide positions are given according to sequence R. helvetica strain C9P9 (AICO01000000). * the specimens from this study. Non-synonymous polymorphic sites are highlighted in green.
Phylogenetic analysis based on gltA-ompB-sca4 concatenated sequences demonstrated that the obtained sequences belong to three genetic lineages I–III (Figure 3). Specimens from lineage I (European lineage) clustered together with R. helvetica str. C9P9, a prototype R. helvetica strain isolated from I. ricinus from Switzerland. Studied specimens differed from the C9P9 strain by single substitutions in the ompB or gltA genes (Figure 2A). Sequences from this lineage were identified in site Om-Zn in three molted I. apronophorus and one molted I. persulcatus (Table 3). Sequences from lineage II (I. trianguliceps lineage) were identical to those previously found in two feeding I. trianguliceps nymphs from Omsk Province (GenBank KR150775, KR150777, KR150781, KR150786) [14]. In this study, nine I. trianguliceps larvae and one molted I. apronophorus from site Om-Bo contained DNA of R. helvetica from lineage II (Table 3 and Table S2). Lineage III (I. apronophorus lineage) was the most abundant and contained only novel sequences that were determined in 48 I. apronophorus, mainly from the site Om-Bo (Table 3 and Table S1). This lineage was genetically diverse; the sequence of a specimen Om-103_Iapr differed from others by one substitution in the gltA gene, whereas sequences of five specimens differed by one substitution in the highly conserved 16S rRNA gene (Figure 2A). Notably, all sequences with a unique substitution in the 16S rRNA gene were found in larvae collected from vole 79. In addition, some other sequences previously detected in I. persulcatus from the Far East formed lineage IV (the Far Eastern lineage) on the constructed phylogenetic tree (Figure 3).

Figure 3.
The phylogenetic tree was constructed using the ML method. Three gene fragments were concatenated (gltA-ompB-sca4), and a total of 2259 positions were analyzed. The scale bar indicates an evolutionary distance of 0.001 nucleotide per position in the sequence. Significant bootstrapping values (>70%) are shown on the nodes. The sequences of R. helvetica determined in this study are in boldface.

Table 3.
Prevalence of different sequence variants of R. helvetica in Ixodes spp.
Since many R. helvetica isolates from the GenBank database were characterized by ompB gene, we used this genetic locus to analyze available R. helvetica sequences from other regions. The phylogenetic tree, which was reconstructed using the ompB gene fragment with a length of 2684 bp, demonstrated the presence of the same four well-supported genetic lineages I–IV (Figure 4). As a result of phylogenetic analysis, genetic lineage I was supplemented with R. helvetica specimens from I. ricinus from Germany (MF163037, HQ232244-HQ232251) and I. persulcatus from Western Siberia (Novosibirsk province) (KU310591) (Figure 4) Lineage II additionally included 32 R. helvetica specimens identified in I. persulcatus from Komi Republic [31]. Sequences from the Komi Republic differed from the studied R. helvetica sequences from I. trianguliceps by one substitution in each of the ompB and gltA genes (Figure 2B and Figure 4). As for genetic lineage IV, sequences from I. persulcatus from the continental part of the Far East (KT825966, KT825970) also corresponded to this lineage in addition to those from Sakhalin and Putyatin islands [15].

Figure 4.
The phylogenetic tree constructed by the ML method based on nucleotide sequences of the 2684 bp fragment of the ompB gene of R. helvetica. The sequence of R. asiatica was used as outgroup. The scale bar indicates an evolutionary distance of 0.001 nucleotide per position in the sequence. Significant bootstrapping values (>70%) are shown on the nodes. The sequences of R. helvetica determined in this study are in boldface.
For more detailed genotyping, sequences of the 16S rRNA (1070 bp), gltA (1037 bp), ompA (1417 bp), ompB (3100 bp), sca4 (2398 bp) and htrA (499 bp) genes, as well as 23S-5S IGS region (489 bp) and groESL operon (1528 bp), were determined for five R. helvetica isolates, belonging to different lineages. Notably, sequences of the groESL operon were identical to all known R. helvetica samples and these sequences were not used for phylogenetic analysis. All other examined genetic loci have polymorphic sites, with the ompB gene being the most variable. Among coding sequences, nucleotide substitutions in 15/25 polymorphic sites were non-synonymous (Figure 2B). The obtained concatenated sequence of isolate Om-74_Iapr_m from lineage I differed from the sequence of R. helvetica str. C9P9 by one substitution in the ompB gene (Figure 2B). Sequences of the specimens from lineages II and III varied between themselves by 20 substitutions and differed from the sequences of R. helvetica str. C9P9 and Far Eastern isolate Skh-7_Iper (OQ209950-OQ209956, OQ257004) by 12–16 substitutions (Figure 2B). The comparison of polymorphic sites from different genetic loci showed that ompB and sca4 gene fragments could be used to reliably differentiate specimens from various genetic lineages (Figure 2B). Notably, phylogenetic analysis based on the sca4 gene demonstrated that R. helvetica isolate from I. persulcatus from Japan (FJ358501) [27] can also be referred to as the Far Eastern lineage.
Phylogenetic tree based on 16S-gltA-ompA-ompB-sca4-htrA-IGS concatenated sequences (9779 bp) (Figure 5) showed the presence of four well-supported genetic lineages, which correspond to those that were identified based on analysis of shorter gltA-ompB-sca4 concatenated sequences and the ompB gene fragment with a length of 2684 bp (Figure 3 and Figure 4).

Figure 5.
The phylogenetic tree was constructed using the ML method. Seven loci were concatenated (gltA-ompA-ompB-sca4-htrA-16SrRNA-IGS), and a total of 9779 positions were analyzed. The sequence of R. asiatica was used as outgroup. The scale bar indicates an evolutionary distance of 0.001 nucleotide per position in the sequence. Significant bootstrapping values (>70%) are shown on the nodes. The sequences of R. helvetica determined in this study are in boldface.
4. Discussion
In Russian Siberia, several Rickettsia species are abundant. Different Rickettsia species are usually associated with certain tick species. Thus, “Ca. R. tarasevichiae”, “Ca. R. uralica” and R. raoultii are closely associated with I. persulcatus, I. trianguliceps and Dermacentor spp., respectively [11,14,16,32,33,34]. As for R. helvetica, this agent is associated with Ixodes spp. and is prevalent in I. ricinus in Europe and I. persulcatus from Sakhalin Island (the Far East) and Komi Republic (European part of Russia) [15,19,31,35].
The study of rickettsiae agents in ticks from sympatric areas is of particular interest because it makes it possible to compare pathogen-tick association for different tick species from the same location. This study includes I. apronophorus, I. persulcatus and I. trianguliceps ticks collected in two sites in the Omsk Province. In site Om-Bo, the abundance of all these tick species was high, whereas in site Om-Zn, I. persulcatus dominated and the prevalence of I. apronophorus was low [8].
In this study, Rickettsia spp. were first found in I. apronophorus. Rickettsia helvetica was found in 70–80% of molted and non-molted I. apronophorus from both locations, indicating a close association of R. helvetica with I. apronophorus. In addition to R. helvetica, “Ca. R. tarasevichiae” was identified in 3% of non-molted I. apronophorus (Table 2).
Expectedly, “Ca. R. tarasevichiae” prevailed in I. persulcatus, occurring in more than 80% of molted and non-molted ticks. Other Rickettsia spp. were found in I. persulcatus only in single cases. Previously, a similarly high prevalence of “Ca. R. tarasevichiae” in questing adult I. persulcatus has been observed in various regions of the Asian part of Russia in Omsk Province, but not in I. persulcatus from Sakhalin Island, Komi Republic, and Estonia [11,14,15,16,31,32]. Surprisingly, one molted I. persulcatus was infected with “Ca. R. uralica”, despite “Ca. R. uralica” was not found in any of the over 500 previously analyzed questing I. persulcatus from Omsk province [14].
As for I. trianguliceps, three Rickettsia species, “Ca. R. uralica”, “Ca. R. tarasevichiae” and R. helvetica, were found in this tick species. Notably, the prevalence of Rickettsia spp. substantially varied depending on the sampling site and developmental stage of I. trianguliceps (Table 2). Association of “Ca. R. uralica” with I. trianguliceps has been previously recorded in other locations of Omsk province and Estonia [14,17], whereas “Candidatus R. thierseensis” (a genetic variant of “Ca. R. uralica”) was found in one I. ricinus in Austria [24,36]. The findings of “Ca. R. uralica” in human-biting I. persulcatus and I. ricinus may indicate the potential threat of this rickettsial species to humans.
“Candidatus R. tarasevichiae”, recently recognized as a pathogenic species [37,38,39,40], is reliably associated with I. persulcatus; however, in rare cases, it has been found in other tick species, namely I. pavlovskyi, Dermacentor spp., and Haemaphysalis spp. [11,16,22,41,42]. In this study, “Ca. R. tarasevichiae” was found in two molted I. trianguliceps in the Om-Zn site (Table 2), which is consistent with the previous detection of “Ca. R. tarasevichiae” in feeding I. trianguliceps nymphs and adults from Omsk province [14]. It is noteworthy that Rickettsia spp. were first identified in molted I. trianguliceps ticks, which demonstrates their ability to transmit transstadially both “Ca. R. uralica” and “Ca. R. tarasevichiae” (Table 2).
Surprisingly, R. helvetica was found only in I. trianguliceps larvae but not in nymphs and adults (Table 2 and Table S2). As all R. helvetica-infected larvae were collected only from two voles, the observed discrepancy may be explained by the insufficient number of I. trianguliceps studied and the uneven distribution of infected and uninfected larval offspring from different females. This uneven distribution of larvae also explains the fact that all R. helvetica specimens with a unique substitution in the 16S rRNA gene were identified in I. apronophorus larvae (but not adults) collected from the same vole.
A number of “Ca. R. uralica”, “Ca. R. tarasevichiae”, and R. helvetica isolates were genetically characterized by sequencing nine genetic loci. “Candidatus R. uralica” and “Ca. R. tarasevichiae” isolates were shown to be highly conserved; 100% identity was shown for the obtained sequences of “Ca. R. uralica” specimens from I. persulcatus and I. trianguliceps and for “Ca. R. tarasevichiae” isolates from Omsk province (this study) and the Russian Far East [43].
On the contrary, analyzed R. helvetica isolates were diverse. Six sequence variants of R. helvetica belonging to three genetic lineages were identified. Only R. helvetica specimens from lineage II were found in infected I. trianguliceps, whereas R. helvetica from lineages I–III were identified in I. apronophorus (Table 3). The observed high divergence may be related to the wide range of R. helvetica carriers identified in this and previous studies: I. ricinus, I. pavlovskyi, I. persulcatus, I. apronophorus, I. trianguliceps, I. hexagonus, I. arboricola, I. ovatus and I. monospinosus [3,11,15,31,35,44].
To date, there is no reliable data confirming Rickettsia spp. co-feeding transmission [45]. Although this transmission may occur in artificial conditions (in the case of R. rickettsia), its impact on pathogen transmission in nature seems insignificant [46]. Our study of non-molted larvae indicated that at least R. helvetica and “Ca. R. tarasevichiae” cannot be effectively transmitted between different Ixodes species as a result of simultaneous feeding on small mammals. Indeed, with single exceptions, I. persulcatus larvae were infected with “Ca. R. tarasevichiae”, I. apronophorus—with R. helvetica from lineage III, and I. trianguliceps—with R. helvetica from lineage II (Table 2, Table 3 and Table S2). Notably, the association between tick species and rickettsial species/sequence variant was retained when larvae of different species were fed on the same animal (Table S2: rodents BU75, 79, 158). However, it cannot be ruled out that single findings of “Ca. R. tarasevichiae” and “Ca. R. uralica” in atypical tick carriers may be due to rare cases of co-feeding transmission.
The long-length sequences of R. helvetica (above 11,500 bp) were determined for specimens belonging to various phylogenetic groups. Analysis of R. helvetica sequences from this study and available sequences from Europe (mainly from Germany) [47], North Western Russia [31], Western Siberia, and the Far East [15,27] demonstrated a high genetic variability of R. helvetica. The analyzed sequences can be reliably assigned to four genetic lineages. However, the association of different lineages with specific tick species and territories was not observed in all cases. Thus, although the European genetic lineage (lineage I) dominated I. ricinus in Europe, it was also found in I. persulcatus and I. apronophorus from Western Siberia. Similarly, I. trianguliceps genetic lineage (lineage II) was found in both I. persulcatus and I. trianguliceps ticks from two remote regions of Russia. On the contrary, I. apronophorus genetic lineage (lineage III) was identified only in I. apronophorus from Western Siberia; the Far Eastern genetic lineage (lineage IV) was identified only in I. persulcatus from the Far East.
It has been shown that R. helvetica can cause rickettsiosis in humans, mainly associated with fever, headache, and myalgias [3,44,48]. However, data on the genetic variability of R. helvetica is limited by a small number of studied geographic regions and has been somewhat extended as a result of this study. Further genetic characterization of R. helvetica isolates from other regions and/or other tick species is required to assess the prevalence and distribution of different genetic lineages of R. helvetica and to fill a gap in our knowledge of R. helvetica biodiversity. It cannot be ruled out that different genetic lineages of R. helvetica may differ in their pathogenic properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11051215/s1, Table S1: Accession numbers of Rickettsia spp. sequences obtained in this study; Table S2: Detection of Rickettsia spp. in ticks collected from individual rodents (without molting).
Author Contributions
Conceptualization, V.R. and N.T.; methodology, Y.I.; validation, T.E. and V.R.; formal analysis, Y.I. and A.T. (Artem Tikunov); investigation, Y.I., V.Y., A.T. (Artem Tikunov), T.E., A.T. (Aleksey Tancev) and V.R.; resources, V.Y. and A.T. (Aleksey Tancev); writing—original draft preparation, Y.I. and V.R.; writing—review and editing, N.T.; supervision, V.R.; project administration, N.T.; funding acquisition, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Russian State-funded budget project of ICBFM SB RAS # No. 121031300043-8.
Institutional Review Board Statement
The animal study protocol was approved by the Bioethical Committee of the Omsk Research Institute of Natural Foci Infections (Protocol No.1, 20 March 2013; Protocol No. 4, 17 February 2016).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Eisen, L. Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: A review. Ticks Tick Borne Dis. 2020, 11, 101359. [Google Scholar] [CrossRef] [PubMed]
- Hoornstra, D.; Azagi, T.; van Eck, J.A.; Wagemakers, A.; Koetsveld, J.; Spijker, R.; Platonov, A.E.; Sprong, H.; Hovius, J.W. Prevalence and clinical manifestation of Borrelia miyamotoi in Ixodes ticks and humans in the northern hemisphere: A systematic review and meta-analysis. Lancet. Microbe 2022, 3, e772–e786. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Rar, V.; Golovljova, I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 2011, 11, 1842–1861. [Google Scholar] [CrossRef] [PubMed]
- Balashov, Y.S. Ixodid Ticks—Parasites and Vectors of Diseases; Nauka: Sankt-Peterburg, Russia, 1998. (In Russian) [Google Scholar]
- Yakimenko, V.V.; Malkova, M.G.; Shpynov, S.N. Ixodid Ticks of the Western Siberia; Omskiy Nauchnyi Vestnik: Omsk, Russia, 2013; 240p. (In Russian) [Google Scholar]
- Karimov, A.V.; KoralloVinarskaya, N.P.; Kuzmenko, Y.F.; Vinarski, M.V. Ixodes apronophorus Schulze (Acari: Ixodida: Ixodidae): Distribution, abundance, and diversity of its mammal hosts in West Siberia (Results of a 54-year long surveillance). Diversity 2022, 14, 702. [Google Scholar] [CrossRef]
- Rar, V.; Yakimenko, V.; Tikunov, A.; Vinarskaya, N.; Tancev, A.; Babkin, I.; Epikhina, T.; Tikunova, N. Genetic and morphological characterization of Ixodes apronophorus from Western Siberia, Russia. Ticks Tick Borne Dis. 2020, 11, 101284. [Google Scholar] [CrossRef]
- Filippova, N.A. Ixodid ticks of the subfamily Ixodinae. In The Fauna of the USSR. Arachnida; Nauka Publishing House: Leningrad, Russia, 1977; New Ser 4. (In Russian) [Google Scholar]
- Korenberg, E.I.; Kovalevskii, Y.V.; Gorelova, N.B.; Nefedova, V.V. Comparative analysis of the roles of Ixodes persulcatus and I. trianguliceps ticks in natural foci of ixodid tick-borne borrelioses in the Middle Urals, Russia. Ticks Tick Borne Dis. 2015, 6, 316–321. [Google Scholar] [CrossRef]
- Rar, V.; Livanova, N.; Tkachev, S.; Kaverina, G.; Tikunov, A.; Sabitova, Y.; Igolkina, Y.; Panov, V.; Livanov, S.; Fomenko, N.; et al. Detection and genetic characterization of a wide range of infectious agents in Ixodes pavlovskyi ticks in Western Siberia, Russia. Parasit. Vectors 2017, 10, 258. [Google Scholar] [CrossRef]
- Rar, V.; Yakimenko, V.; Tikunov, A.; Makenov, M.; Epikhina, T.; Tancev, A.; Tikunova, N. Genetic variability of Anaplasmataceae circulating in small mammals and ticks in an Ixodes persulcatus/Ixodes trianguliceps sympatric area in Russian Siberia. Ticks Tick Borne Dis. 2020, 11, 101499. [Google Scholar] [CrossRef]
- Sabitova, Y.; Rar, V.; Tikunov, A.; Yakimenko, V.; Korallo-Vinarskaya, N.; Livanova, N.; Tikunova, N. Detection and genetic characterization of a putative novel Borrelia genospecies in Ixodes apronophorus/Ixodes persulcatus/Ixodes trianguliceps sympatric areas in Western Siberia. Ticks Tick Borne Dis. 2023, 14, 102075. [Google Scholar] [CrossRef]
- Igolkina, Y.P.; Rar, V.A.; Yakimenko, V.V.; Malkova, M.G.; Tancev, A.K.; Tikunov, A.Y.; Epikhina, T.I.; Tikunova, N.V. Genetic variability of Rickettsia spp. in Ixodes persulcatus/Ixodes trianguliceps sympatric areas from Western Siberia, Russia: Identification of a new Candidatus Rickettsia species. Infect. Genet. Evol. 2015, 34, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Igolkina, Y.; Bondarenko, E.; Rar, V.; Epikhina, T.; Vysochina, N.; Pukhovskaya, N.; Tikunov, A.; Ivanov, L.; Golovljova, I.; Ivanov, M.; et al. Genetic variability of Rickettsia spp. in Ixodes persulcatus ticks from continental and island areas of the Russian Far East. Ticks Tick Borne Dis. 2016, 7, 1284–1289. [Google Scholar] [CrossRef]
- Shpynov, S.; Fournier, P.E.; Rudakov, N.; Tarasevich, I.; Raoult, D. Detection of members of the genera Rickettsia, Anaplasma, and Ehrlichia in ticks collected in the Asiatic part of Russia. Ann. N. Y. Acad. Sci. 2006, 1078, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Vikentjeva, M.; Geller, J.; Remm, J.; Golovljova, I. Rickettsia spp. in rodent-attached ticks in Estonia and first evidence of spotted fever group Rickettsia species Candidatus Rickettsia uralica in Europe. Parasit. Vectors 2021, 14, 65. [Google Scholar] [CrossRef]
- Nowak-Chmura, M.; Siuda, K. Ticks of Poland. Review of contemporary issues and latest research. Ann. Parasitol. 2012, 58, 125–155. [Google Scholar] [PubMed]
- Stanko, M.; Derdáková, M.; Špitalská, E.; Kazimírová, M. Ticks and their epidemiological role in Slovakia: From the past till present. Biologia 2022, 77, 1575–1610. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Pfäffle, M.P.; Petney, T.N. Genus Ixodes Latreille, 1795. In Ticks of Europe and North Africa. A Guide to Species Identification; Estrada-Pena, E., Mihalca, A.D., Petney, T.N., Eds.; Springer: Cham, Switzerland, 2017; pp. 79–91. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Igolkina, Y.; Rar, V.; Vysochina, N.; Ivanov, L.; Tikunov, A.; Pukhovskaya, N.; Epikhina, T.; Golovljova, I.; Tikunova, N. Genetic variability of Rickettsia spp. in Dermacentor and Haemaphysalis ticks from the Russian Far East. Ticks Tick Borne Dis. 2018, 9, 1594–1603. [Google Scholar] [CrossRef]
- Fournier, P.E.; Roux, V.; Raoult, D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Bacteriol. 1998, 48, 839–849. [Google Scholar] [CrossRef]
- Igolkina, Y.; Rar, V.; Yakimenko, V.; Tikunov, A.; Tikunova, N. “Candidatus Rickettsia uralica” and “Candidatus Rickettsia thierseensis” are genetic variants of one species. Ticks Tick Borne Dis. 2022, 13, 101933. [Google Scholar] [CrossRef]
- Roux, V.; Raoult, D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. Evol. Microbiol. 2000, 50, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Sekeyova, Z.; Roux, V.; Raoult, D. Phylogeny of Rickettsia spp. inferred by comparing sequences of “gene D”, which encodes an intracytoplasmic protein. Int. J. Syst. Evol. Microbiol. 2001, 51, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Inokuma, H. Identification of spotted fever group Rickettsia species by polymerase chain reaction-restriction fragment length polymorphism analysis of the sca4 gene. Vector Borne Zoonotic Dis. 2009, 9, 747–749. [Google Scholar] [CrossRef]
- Labruna, M.B.; McBride, J.W.; Bouyer, D.H.; Camargo, L.M.A.; Camargo, E.P.; Walker, D.H. Molecular evidence for a spotted fever group Rickettsia species in the tick Amblyomma longirostre in Brazil. J. Med. Entomol. 2004, 41, 533–537. [Google Scholar] [CrossRef]
- Jado, I.; Escudero, R.; Gil, H.; Jimenez-Alonso, M.I.; Sousa, R.; Garcia-Perez, A.L.; Rodriguez-Vargas, M.; Lobo, B.; Anda, P. Molecular method for identification of Rickettsia species in clinical and environmental samples. J. Clin. Microbiol. 2006, 44, 4572–4576. [Google Scholar] [CrossRef]
- Shao, J.W.; Zhang, X.L.; Li, W.J.; Huang, H.L.; Yan, J. Distribution and molecular characterization of rickettsiae in ticks in Harbin area of Northeastern China. PLoS Negl. Trop. Dis. 2020, 14, e0008342. [Google Scholar] [CrossRef] [PubMed]
- Kartashov, M.Y.; Glushkova, L.I.; Mikryukova, T.P.; Korabelnikov, I.V.; Egorova, Y.I.; Tupota, N.L.; Protopopova, E.V.; Konovalova, S.N.; Ternovoi, V.A.; Loktev, V.B. Detection of Rickettsia helvetica and Candidatus R. tarasevichiae DNA in Ixodes persulcatus ticks collected in Northeastern European Russia (Komi Republic). Ticks Tick Borne Dis. 2017, 8, 588–592. [Google Scholar] [CrossRef]
- Katargina, O.; Geller, J.; Ivanova, A.; Värv, K.; Tefanova, V.; Vene, S.; Lundkvist, Å.; Golovljova, I. Detection and identification of Rickettsia species in Ixodes tick populations from Estonia. Ticks Tick Borne Dis. 2015, 6, 689–694. [Google Scholar] [CrossRef]
- Balážová, A.; Földvári, G.; Bilbija, B.; Nosková, E.; Široký, P. High prevalence and low diversity of Rickettsia in Dermacentor reticulatus ticks, Central Europe. Emerg. Infect. Dis. 2022, 28, 893–895. [Google Scholar] [CrossRef]
- Cheng, C.; Fu, W.; Ju, W.; Yang, L.; Xu, N.; Wang, Y.M.; Li, H.; Wang, Y.L.; Hu, M.X.; Wen, J.; et al. Diversity of spotted fever group Rickettsia infection in hard ticks from Suifenhe, Chinese-Russian border. Ticks Tick Borne Dis. 2016, 7, 715–719. [Google Scholar] [CrossRef]
- Silaghi, C.; Gilles, J.; Höhle, M.; Pradel, I.; Just, F.T.; Fingerle, V.; Küchenhoff, H.; Pfister, K. Prevalence of spotted fever group rickettsiae in Ixodes ricinus (Acari: Ixodidae) in southern Germany. J. Med. Entomol. 2008, 45, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Schötta, A.M.; Wijnveld, M.; Höss, D.; Stanek, G.; Stockinger, H.; Markowicz, M. Identification and characterization of “Candidatus Rickettsia thierseensis”, a novel spotted fever group Rickettsia species detected in Austria. Microorganisms 2020, 28, 1670. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Zheng, Y.C.; Jiang, J.F.; Ma, L.; Cao, W.C. Human infection with Candidatus Rickettsia tarasevichiae. N. Engl. J. Med. 2013, 369, 1178–1180. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Lu, Q.B.; Cui, N.; Yang, Z.D.; Hu, J.G.; Fan, Y.D.; Guo, C.T.; Li, X.K.; Wang, Y.W.; et al. Candidatus Rickettsia tarasevichiae infection in Eastern Central China: A case series. Ann. Intern. Med. 2016, 164, 641–648. [Google Scholar] [CrossRef]
- Rudakov, N.; Samoylenko, I.; Shtrek, S.; Igolkina, Y.; Rar, V.; Zhirakovskaia, E.; Tkachev, S.; Kostrykina, T.; Blokhina, I.; Lentz, P.; et al. A fatal case of tick-borne rickettsiosis caused by mixed Rickettsia sibirica subsp. sibirica and “Candidatus Rickettsia tarasevichiae” infection in Russia. Ticks Tick Borne Dis. 2019, 10, 101278. [Google Scholar] [CrossRef] [PubMed]
- Igolkina, Y.; Rar, V.; Krasnova, E.; Filimonova, E.; Tikunov, A.; Epikhina, T.; Tikunova, N. Occurrence and clinical manifestations of tick-borne rickettsioses in Western Siberia: First Russian cases of Rickettsia aeschlimannii and Rickettsia slovaca infections. Ticks Tick Borne Dis. 2022, 13, 101927. [Google Scholar] [CrossRef]
- Shpynov, S.; Fournier, P.E.; Rudakov, N.; Raoult, D. “Candidatus Rickettsia tarasevichiae” in Ixodes persulcatus ticks collected in Russia. Ann. N. Y. Acad Sci. 2003, 990, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Q.; Zhang, X.; Li, Z.; Wang, Z.; Song, M.; Wei, F.; Wang, S.; Liu, Q. Characterization of rickettsiae in ticks in northeastern China. Parasit. Vectors 2016, 13, 498. [Google Scholar] [CrossRef]
- Igolkina, Y.; Nikitin, A.; Verzhutskaya, Y.; Gordeyko, N.; Tikunov, A.; Epikhina, T.; Tikunova, N.; Rar, V. Multilocus genetic analysis indicates taxonomic status of “Candidatus Rickettsia mendelii” as a separate basal group. Ticks Tick Borne Dis. 2023, 14, 102104. [Google Scholar] [CrossRef]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Spitalská, E.; et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: New hazards and relevance for public health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef]
- Hensley, J.R.; Zambrano, M.L.; Williams-Newkirk, A.J.; Dasch, G.A. Detection of Rickettsia species, and Coxiella-like and Francisella-like endosymbionts in Amblyomma americanum and Amblyomma maculatum from a shared field site in Georgia, United States of America. Vector Borne Zoonotic Dis. 2021, 21, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Filho, J.; Costa, F.B.; Gerardi, M.; Soares, H.S.; Labruna, M.B. Rickettsia rickettsii co-feeding transmission among Amblyomma aureolatum ticks. Emerg. Infect. Dis. 2018, 24, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.; Hamel, D.; Thiel, C.; Pfister, K.; Pfeffer, M. Spotted fever group rickettsiae in ticks, Germany. Emerg. Infect. Dis. 2011, 17, 890–892. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K. Septicaemia with Rickettsia helvetica in a patient with acute febrile illness, rash and myasthenia. J. Infect. 2009, 58, 79–82. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).