Abstract
We have studied the antimycobacterial efficacy of the liposomal preparation of mycobacteriophage D29 on models of tuberculous granuloma in vitro and in the experiment on laboratory mice of the relatively resistant strain C57BL/6, infected with the virulent strain of M. tuberculosis H37Rv. We have shown the preparation of liposomal preparation of the lytic mycobacteriophages and its characteristics. The experiments showed a significant lytic effect of the liposomal form of mycobacteriophage D29 both on the model of tuberculous granuloma formed by human blood mononuclear cells in vitro, which is formed in the presence of Mycobacterium tuberculosis and on the model of tuberculous infection in C57BL/6 mice. Keywords: mycobacteriophage D29, M. tuberculosis, liposomes, tuberculous granuloma in vitro, tuberculosis infection and its treatment.
1. Introduction
Tuberculosis (TB) remains one of the most common and dangerous infectious diseases and is included in the list of socially significant diseases and of diseases that pose a danger to others. Despite the general improvement in the TB epidemic situation in the world, the problem of the spread of drug-resistant forms of the pathogen—Mycobacterium tuberculosis (MTB) is extremely urgent [1]. WHO estimates that only half of treated MDR patients were successfully treated [2]. Multidrug resistance (MDR) is increasing annually by more than 20% [3]. The relative duration of chemotherapy for TB infection and the known relative toxicity of chemotherapy drugs leads to a decrease in patient adherence to treatment, which, in turn, contributes to the spread of drug-resistant MTB strains. The search for alternative ways to treat TB infection, including drug-resistant forms, may become one of the promising areas in the fight against TB.
The use of lytic mycobacteriophages for the treatment of TB infection in conditions of acquiring resistance to TB drugs is of considerable interest as a prospect for developing an alternative method for treating TB infection [4,5,6,7,8,9,10,11,12,13,14], including infection with drug-resistant mycobacteria [8], as well as against the drug-resistant strain of M. abscessus [7]. It should be taken into account that there is only one well-studied strain of lytic mycobacteriophages, namely strain D29. Mycobacteriophage D29 is a specific mycobacterial virus that has unique lytic properties against MTB since its genomic organization does not allow it to transform into a lysogenic or moderate phage form [15].
Mycobacteriophage D29 has also been successfully used for the rapid determination of drug susceptibility or resistance of MTB clinical strains to anti-TB antibiotics [16] When studying the prospects for the use of mycobacteriophages for the treatment of TB, the possibility of their selective transport to granulomatous foci of inflammation is considered and confirmed [7,17]. In this regard, liposomal forms of lytic mycobacteriophage are considered with great interest [18,19,20,21,22]. In our work, we studied the bactericidal effect of the liposomal form of mycobacteriophage D29 with different liposome diameters on the model of TB granuloma in vitro and in vivo on laboratory animals infected with the MTB H37Rv virulent strain.
2. Materials and Methods
2.1. Production of Mycobacteriophage D29
To obtain the required volume of mycobacteriophage, a suspension of M. smegmatis was prepared on a solid nutrient medium (Middlebrook 7H10). This suspension with a minimum density was transferred to a liquid nutrient medium (Middlebrook 7H9) in 0.1 M Tris-HCl pH 7.5 containing 1 mM CaCl2. It was incubated for a day; then 2 mL of mycobacteriophage was added with a concentration of at least 108 plaque-forming units (PFU)/mL and incubated for another day on a shaker (3–5 shakes per minute, 37 °C). The resulting suspension was centrifuged at 3800 rpm for 15 min. The supernatant was sterilized by filtration using 0.22 μm filters (Millex GP 0.22 m Millipore). The titer of mycobacteriophage was determined by tenfold titration and inoculation on a solid nutrient medium 7H10.
2.2. Development and Determination of Biological Activity of Lytic Mycobacteriophage D29
M. smegmatis culture was used to propagate the mycobacteriophage. In total, 100 μL of this culture previously diluted in the liquid nutrient medium Middlebrook 7H9 + 1 mM CaCl2 was applied to Petri dishes with a solid nutrient medium and incubated at 37 °C for 15 min. Then, 900 µL of 0.1 M NaCl Tris-HCl-MgSO4 phage buffer solution pH 7.5 was added to each pre-prepared test tube. Then, 100 µL of the studied lytic mycobacteriophage was added to the first test tube with a phage buffer solution, after which the mycobacteriophage was serially diluted tenfold up to the tenth test tube. The resulting suspension was added from each dilution to Petri dishes with a culture of M. smegmatis. The next day, the calculation of the biological activity of the phage was carried out by multiplying the number of obtained plaques (lysis zones) on plates with a lawn of M. smegmatis by the appropriate dilution.
2.3. Obtaining a Concentrated Highly Purified Preparation of Mycobacteriophage D29
The obtained mycobacteriophage after propagation in the culture of M. smegmatis and determination of its biological activity was purified from the lysate of mycobacteria containing a combination of lipases, proteases, and destroyed DNA molecules using ion-exchange chromatography. We used the column volume of 150 mL with a weakly anionic sorbent Macro-Prep DEAE Media (BioRad) on a BIO-RAD BioLogic LP chromatograph [23]. The phage preparation, on average 50–70 mL, was added to the sorbent column suspended in a buffer solution of 0.1 M NaCl. After obtaining the first peak, the molarity of the solution was increased to 1M NaCl and then the second peak of the phage preparation was collected (Figure 1). The mycobacteriophage preparation was dialyzed against 0.1 M phage buffer solution for 24 h on a magnetic stirrer (110 rpm, 4 °C) and sterilized by filtration through 0.22 µ filters.
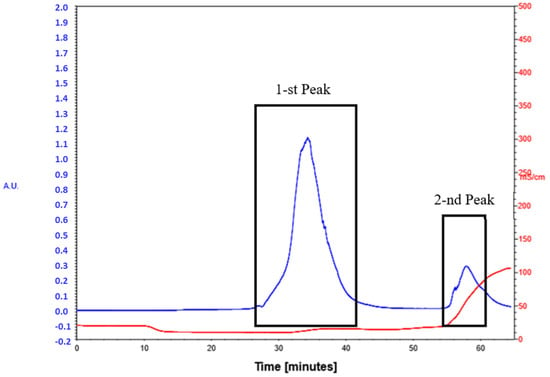
Figure 1.
Chromatography peak image of lytic mycobacteriophage D29.  Optical density [u]
Optical density [u]  Conductivity [mS/cm].
Conductivity [mS/cm].
 Optical density [u]
Optical density [u]  Conductivity [mS/cm].
Conductivity [mS/cm].
2.4. Obtaining a Liposomal Preparation of Mycobacteriophage D29 by Extrusion with a Liposome Diameter of 0.45 and 0.8 Microns
To obtain a phospholipid film, 40 mg of phosphatidylcholine, 98% purity, (Sigma-Aldrich, Taufkirchen, Germany) and 13 mg of cholesterol (Sigma Aldrich, Taufkirchen, Germany) were used. All components were dissolved in 3 mL of 95% ethanol; then dried on a rotary evaporator at t 37 °C until a lipid film was obtained. After complete drying, the phospholipid film was removed by adding 3 mL of a purified mycobacteriophage preparation with a high amount of PFU (at least 108/mL). The flask with the phospholipid film was shaken for 5 min. The obtained multilayer liposomes were subjected to extrusion through filters with a pore size of 5 microns once. This was followed by 19 times extrusion through Millipore filters with a pore size of 0.45 or 0.8 microns, respectively (Millex GP), and an additional single extrusion through a filter with a pore size of 0.45 and 0. 8 microns.
2.5. Obtaining a Liposomal Preparation of Mycobacteriophage Based on Chromatography on a Sephadex G-75 Column
Sephadex G-75 (Pharmacia Fine Chemicals) was weighed in a phage buffer solution. The size of the weighted Sephadex column is 17 × 1.1 cm. In a 100 mL round-bottom flask, 30 mg of 98% pure phosphatidylcholine and 10 mg of cholesterol were dissolved in 3 mL of 96% ethanol solution. The lipid film was prepared using a rotary evaporator, which was removed by shaking using 3 mL of a phage buffer solution to obtain a micellar suspension of multilayer-preparation liposomes. To obtain single-layer liposomes, 300 μL of a 20% solution of sodium deoxycholate in 20% ethanol was added to the resulting suspension. A four-fold volume of the phage preparation compared to the micellar solution was preliminary added to the Sephadex column, and chromatography was stopped. Then, 3 mL of micellar solution with sodium deoxycholate was added, and very slow chromatography was performed at a rate of 0.6 mL/min. Opalescent fractions were collected at the outlet. Then, 8 mL of the most opalescent preparation was obtained, an aliquot of which was precipitated only by centrifugation at 14.5 thousand rpm.
2.6. Obtaining Tuberculous Granuloma In Vitro, Formed by Human Blood Mononuclear Cells
To obtain a granuloma, the venous blood of a healthy adult volunteer with a positive reaction during antigen-specific induction of interferon-gamma in a volume of 20 mL was used. In test tubes with a volume of 15 mL, 3 mL of a solution of the Ficoll-Paque (“GE Healthcare”) preparation was poured, on which 4 mL of diluted 1:1 saline blood was layered. It was then centrifuged at 2000 rpm for 20 min. Then, the cellular interphase formed at the media interface was collected in a 50 mL tube. Washed two times—the first time with physiological saline (30 mL); the second time with RPMI 1640 medium without additives (20 mL). Centrifuged at 3000 g for 15 min, the supernatant was removed. The number of cells was counted using a Scepter cell counter. The resulting suspension of mononuclear cells was adjusted to a concentration of at least 5.0 × 105/mL and poured into a 24-well plate in 500 µL of the suspension. A total of 500 μL of RPMI 1640 medium with 10% FBS was added to each well of the plate. A composition of RPMI 1640 culture medium with 10% FBS: (1) RPMI 1640–500 mL; (2) FBS–50 mL; (3) Pen Strep 100×–5 mL; (4) Alpha-Glutamine–5 mL; (5) MEM Vitamins solution–5 mL; 100×–5 mL; (6) MEM NEAA–5 mL; (7) Sodium Pyruvate 100×–5 mL.
The next day, 50 µL of H37Rv 105/mL was added to each well of the plate. The medium was changed every two days. The cell culture was monitored using an inverted Leica DMIL microscope at a magnification of 1:200 with a Leica DFC 420 digital camera. Granuloma formation occurred on the 13–15th day. (Figure 2).
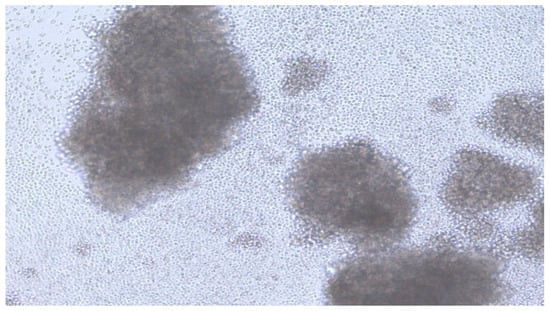
Figure 2.
Image of TB granuloma on the day 14.
3. Results and Discussion
3.1. Manufacturing a Liposomal Preparation of Mycobacteriophage D29
To obtain a higher concentration of mycobacteriophage after chromatography and dialysis, the drug was centrifuged in centrifuge concentrators at 1500 rpm for 15 min. The percentage of mycobacteriophage incorporation into liposomes was evaluated by quantitative determination of mycobacteriophage DNA during real-time PCR (RT-PCR) [16]. The percentage of mycobacteriophage incorporation into liposomes was at least 25% in different experiments (Table 1). When using the extrusion method with filters of 0.8 microns, the level of inclusion of mycobacteriophage was 40%; see Table 1 and electron microscopy (Figure 3).

Table 1.
Efficiency of incorporation of mycobacteriophage D29 into liposomal preparations.
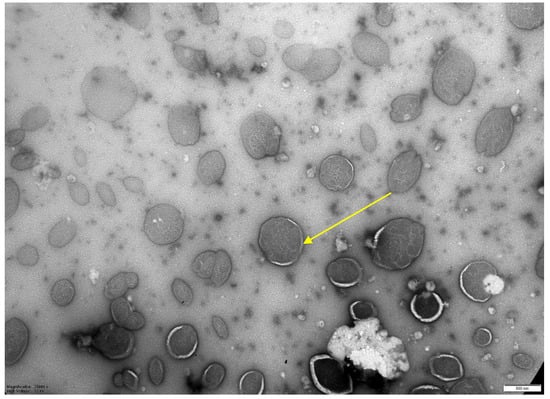
Figure 3.
Electron microscopy of a liposomal preparation of mycobacteriophage D29 obtained by extrusion (liposome diameter 0.8 microns). Magnification 1:25,000. Scale—500 nm.
When conducting electron microscopy, the size of the resulting liposomes obtained by column chromatography was 250 nm (Figure 4). The resulting sterile solution of phage liposomes was stored at 4 °C. Mycobacteriophage not included in liposomes was collected and concentrated for further use. For quantification of incorporation of the mycobacteriophages into liposomes, their samples (in a volume of 200 µL) were centrifuged at 14 thousand rpm (refrigerated centrifuge, Thermo Electron, Waltham, MA, USA), and the sediment volume was measured. The amount of mycobacteriophage DNA in the starting material and in the sediment was determined using real-time PCR (“Amplitub RT, Synthol, Moscow, Russia). In this case, the inclusion percentage was calculated based on the differences between the threshold cycles (the number of threshold cycles is inversely proportional to the amount of DNA in the samples).
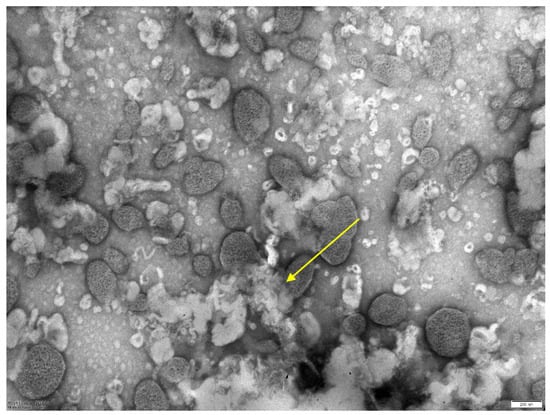
Figure 4.
Electron microscopy of a liposomal preparation of mycobacteriophage D29 obtained by column chromatography. Scale—200 nm. The average size of liposomes is 0.25 microns.
3.2. Formation of a Human Granuloma by Blood Mononuclear Cells In Vitro and Results Analysis Antimycobacterial Efficiency Liposomal
After the formation of a granuloma, mycobacteriophage and liposomal preparations of mycobacteriophage D29, obtained via various methods with the activity determined by real-time PCR, were added to the wells of the plate in a volume of 100 μL. A day later, the procedure was repeated. Two days later, the contents of the wells of the plate were shown on a solid nutrient medium.
In the experiments performed on the in vitro TB granuloma model formed by human blood mononuclear cells, using a liposomal mycobacteriophage obtained by extrusion, a significant antimycobacterial effect was established, obviously exceeding the antimycobacterial effect of mycobacteriophage not included in liposomes (Figure 5). In the case of positive control, more than ten large MTB colonies were detected in the plate. Growth of MTB was not detected for liposomal preparation of mycobacteriophage D29. Non-encapsulated (free) preparation of mycobacteriophage D29 produced more than ten small MTB colonies.
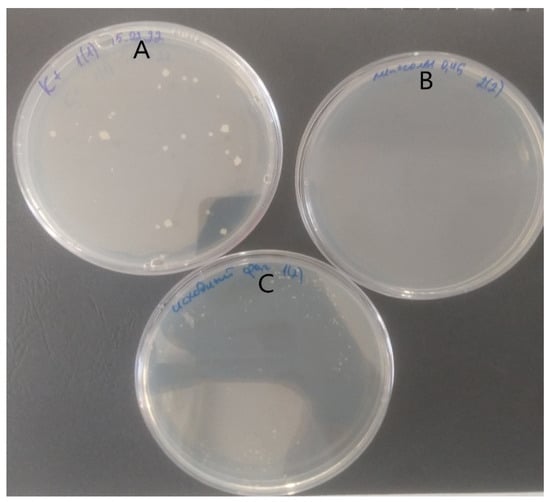
Figure 5.
Comparison of culture results obtained in an in vitro TB granuloma experiment formed by human blood mononuclear cells. (A) positive control, (B) liposomal preparation of mycobacteriophage D29, and (C) non-encapsulated (free) preparation of mycobacteriophage D29.
3.3. Studies of the Antimycobacterial Activity of the Liposomal Preparation of Mycobacteriophage D29 on Laboratory Mice of the C57BL/6 Strain
The study was conducted on the basis of the Scientific Center for Applied Microbiology (Obolensk, Moscow Region) in accordance with the veterinary protocol N 634- n\22, 15 August 2022, of the bioethics commission, “Modeling of tuberculosis infection, which provides for the humane treatment of animals s C57BL/6 strain”.
To determine the in vivo activity of the liposomal form of the lytic mycobacteriophage D29, an experiment was carried out on laboratory mice of the genetically resistant strain C57BL/6. Laboratory mice were infected with an MTB H37Rv virulent strain. Infection of laboratory mice was carried out by intranasal (on inspiration) administration of 50 µL of a suspension of MTB at a concentration of 2.105 bacilli per ml. Infected mice were divided into three groups of six mice with different treatment regimens starting from day 35 after infection. In the first group, lytic mycobacteriophage D29 was administered in liposomal form, obtained by extrusion, in a volume of 50 μL/animal, intranasally (on inspiration), two times a day, three times with an interval of one day. The second group of mice was injected intranasally with a liposomal preparation of lytic mycobacteriophage D29 obtained by the column method in the same volume and mode of administration. In the third group—control, infected mice were not treated. Mice were euthanized 5 days after treatment. The results were evaluated (a) by macroscopic evaluation of cured lung preparations; (b) when inoculating the lungs on plates with Middlebrook 7H10 nutrient medium; (c) according to the results of histological examination of fixed lung preparations.
The results of evaluating the antimycobacterial effect of two liposomal forms of mycobacteriophage D29 on laboratory mice C57BL/6 infected intranasally are presented on Table 2.

Table 2.
Efficacy results of liposomal preparations of lytic mycobacteriophage in macroscopic evaluation and microbiological examination of lung organs of C57BL/6 mice, infected mycobacteria of the virulent strain H37Rv.
Certainty that the liposomal form of lytic mycobacteriophage D29 shows a significant antimycobacterial effect. At the same time, the preparation of liposomal mycobacteriophage obtained by extrusion proved to be more effective than the preparation obtained by the column method. It is possible that this effect is associated with a slightly larger size of liposomes (0.8 microns), which provided a larger amount of lytic mycobacteriophage included. In general, the results of the presented studies showed a positive outlook for the studied liposomal preparations, which allows us to count on their effectiveness after appropriate biosafety studies and in clinical studies, possibly with endobronchial administration in patients with pulmonary TB.
Histological detection among the lungs in the control group of infected animals (3) showed signs of diffuse inflammation (Figure 6A); in Group (2), the lungs of mice that received a liposomal preparation obtained by the column chromatography method detect foci of diffuse inflammation and fibrotic changes in the lung tissue (Figure 6A,B), while in Group (1), the lungs of mice that received the preparation obtained by extrusion with a high level of increase in mycobacteriophage included in liposomes lung tissue remained intact. (Figure 7).
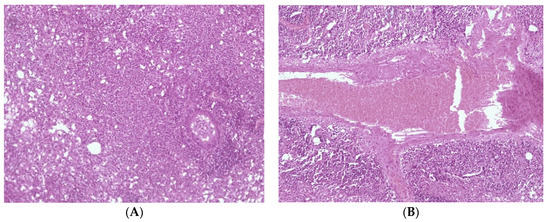
Figure 6.
Histological picture of changes in the lung tissue of the mouse. (A) diffuse inflammation of the lung tissue, (B) perivascular fibrosis, and plethora.
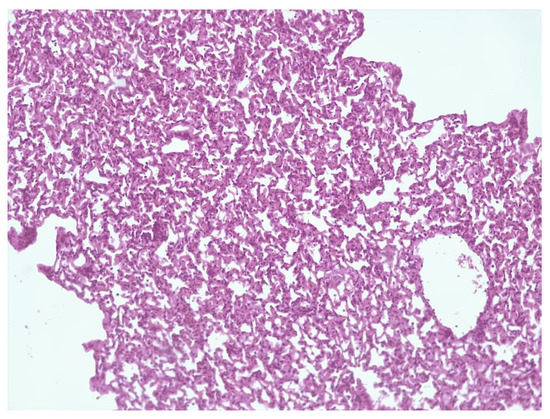
Figure 7.
Intact lung tissue.
4. Conclusions and Short Discussion
Thus, we can state that the use of the liposomal form of lytic mycobacteriophages D29 with a liposome size of 0.8 microns showed the most pronounced significant antimycobacterial effect.
- We believe that in this work, for the first time on an experimental model of laboratory animals with developed tuberculosis infection, the high efficiency of using the liposomal form of lytic mycobacteriophages in short–term (three days) endobronchial (endonasal on inspiration) administration was proved.
- Possible questions about the toxicity of such a drug are planned to be resolved as part of the forthcoming studies on the acute and chronic toxicity of our drug on laboratory animals in the near future. We believe that the success of treatment described in the well-known study using a combination of lytic mycobacteriophages to treat a lung transplant girl infected with drug-resistant M. abscessus infection [7] suggests minimal toxicity of specific lytic mycobacteriophages in their clinical use.
- We believe that a liposomal preparation of lytic mycobacteriophages in the future can be administered to patients with tuberculosis into the bronchial system through a bronchoscope. However, the possibilities of inhalation administration are also discussed [24].
- It also planned to conduct a comparative study of the effectiveness of liposomal preparation of the lytic mycobacteriophages and a combination of the most well-known and effective antibiotics for the treatment of tuberculosis.
Author Contributions
Conceptualization: M.A.V.; methodology: M.A.V., V.V.A., V.V.K. and I.A.V.; investigations: M.A.V., V.V.A., V.V.K. and I.A.V.; writing—original: M.A.V., V.V.A., V.V.K. and I.A.V.; draft preparation: M.A.V. and V.V.A.; writing—review and editing: M.A.V. All authors have read and agreed to the published version of the manuscript.
Funding
The research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2020.
- World Health Organization. Implementing Tuberculosis Diagnostics: A Policy Framework; World Health Organization: Geneva, Switzerland, 2020; WHO/HTM/TB.
- Lange, C.; Chesov, D.; Jon, H.; Leung, C.C.; Udwadia, Z.; Dheda, K. Drug resistant tuberculosis; An update on diseases burden diagnosis and treatment. Respirology 2018, 23, 256–673. [Google Scholar] [CrossRef] [PubMed]
- Hatfull, G.F. Mycobacteriophages: Windows into tuberculosis. PLoS Pathog. 2014, 10, e1003953. [Google Scholar] [CrossRef] [PubMed]
- Hatfull, G.F. Mycobacteriophages. Microbiol. Spectr. 2018, 6, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Hatfull, G.F. Mycobacteriophages: From Petri dish to patient. PLoS Pathog. 2022, 18, e1010602. [Google Scholar] [CrossRef] [PubMed]
- Abrar Senhagi-Kacha, J.E.; Garcia-Quintanilla, M. Considerations for phage therapy against Mycobacterium abscessus. Front. Microbiol. 2021, 11, 609017. [Google Scholar] [CrossRef]
- Allue-Guardia, A.; Saranathan, R.; Chan, J.; Torreles, J. Mycobacteriophages as a potential therapeutic agents drug resistant tuberculosis. Int. J. Mol. Sci. 2021, 22, 735. [Google Scholar] [CrossRef]
- Azimi, T.; Mosadegh, T.; Nasiri, M.; Sabour, S.; Karimaei, S.; Nasser, A. Phage therapy as a renewed therapeutic approach to mycobacterial infections: A comprehensive review. Int. Drug Resist. 2019, 12, 2943–2959. [Google Scholar] [CrossRef]
- Diacon, A.; Guerrero-Bustamante, C.; Rosenkrants, B.; Rubio Pomar, F.J.; Vanker, N.; Hatfull, G.F. Mycobacteriophage to treat tuberculosis. Dream or delusion? Respiration 2022, 101, 1–15. [Google Scholar] [CrossRef]
- Zeynali, K.F.; Susan, K.; Fatemeh, F.; Sarabi, H.S.; Nikkhahi, F. Bacteriophages of Mycobacterium tuberculosis, their diversity, and potential therapeutic uses: A review. BMC Infect. Dis. 2022, 22, 957. [Google Scholar]
- Pires, D.P.; Costa, A.R.; Pinto, G.; Meneses, L.; Azeredo, J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020, 44, 684–700. [Google Scholar] [CrossRef]
- Hosseiniporgham, S.; Leonardo, A.S. A Review on Mycobacteriophages: From Classification to Applications. Pathogens 2012, 50, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Sliwka, P.; Ochocka, M.; Skaradzinska, A. Application of bacteriophages against intracellular bacteria. Crit. Rev. Microbiol. 2022, 48, 222–239. [Google Scholar] [CrossRef]
- Ford, M.E.; Sarkis, G.J.; Belanger, A.E.; Hatfull, G.F.; Hendrix, R.W.; Hatfull, G.F. Genome Structure of Mycobacteriophage D29: Implications for Phage Evolution. J. Mol. Biol. 1998, 279, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Pholwat, S.; Ehdaie, B.; Foongladda, S.; Kelly, K.; Houpt, E. Real-time PCR using mycobacteriophage DNA for rapid phenotypic drug susceptibility results for Mycobacterium tuberculosis. J. Clin. Microbiol. 2012, 50, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Bustamante, C.A.; Dedrick, R.M.; Garlena, R.A.; Russell, D.A.; Hatfull, G.F. Toward a Phage Cocktail for Tuberculosis: Susceptibility and Tuberculocidal Action of Mycobacteriophages against Diverse Mycobacterium tuberculosis Strains. mBio 2021, 12, e00973-21. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Ogren, M.; Dias, J.; Silva, M.; Gil, S.; Tavares, L.; Aires-Da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as antibiotic delivery systems: A promising nanotechnological strategy against antimicrobial resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- Lapenkova, M.B.; Smirnova, N.S.; Rutkevich, P.N.; Vladimirsky, M.A. Study of the activity of lytic mycobacteriophage D29 on the model of a propagated line of macrophages infected with Mycobacterium tuberculosis. Bull. Exp. Biol. Med. 2017, 164, 326–329. [Google Scholar]
- Lapenkova, M.B.; Alyapkina, Y.S.; Vladimirsky, M.A. Bactericidal activity Liposomal form of lytic mycobacteriophage D29 in cell models of tuberculosis infection in vitro. Bull. Exp. Biol. Med. 2020, 169, 361–364. [Google Scholar] [CrossRef]
- Nieth, A.; Verceux; Barnett, S.; Süss, R.; Römer, W. A first step toward liposome-mediated intracellular bacteriophage therapy. Expert Opin. Drug Deliv. 2015, 12, 9. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Vandenheuvel, D.; Rombouts, S.; Adriaenssens, E.M. Purification of bacteriophages using anion-exchange chromatography. In Bacteriophages: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2018; Volume 1681, pp. 56–69. [Google Scholar]
- Carrigy, N.B.; Chang, R.Y.; Leung, S.Y. Antituberculosis bacteriophage D29 delivery with a vibrating mesh nebulizer. Pharm. Res. 2020, 34, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).