Molecular In-Depth on the Epidemiological Expansion of SARS-CoV-2 XBB.1.5
Abstract
1. Introduction
2. Materials and Methods
2.1. Phylodynamics Analyses
2.2. Structural and Molecular Dynamics Analyses
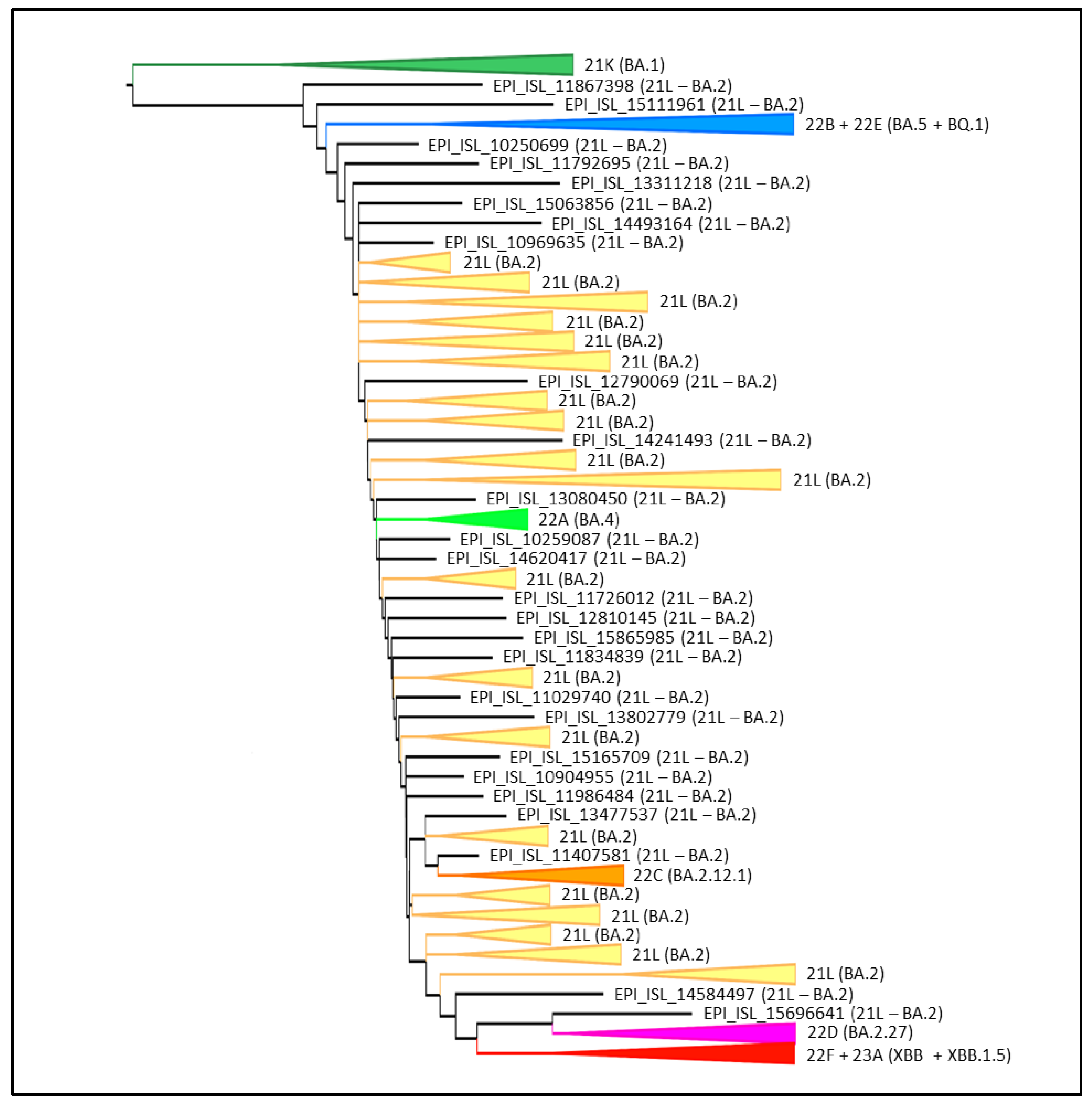
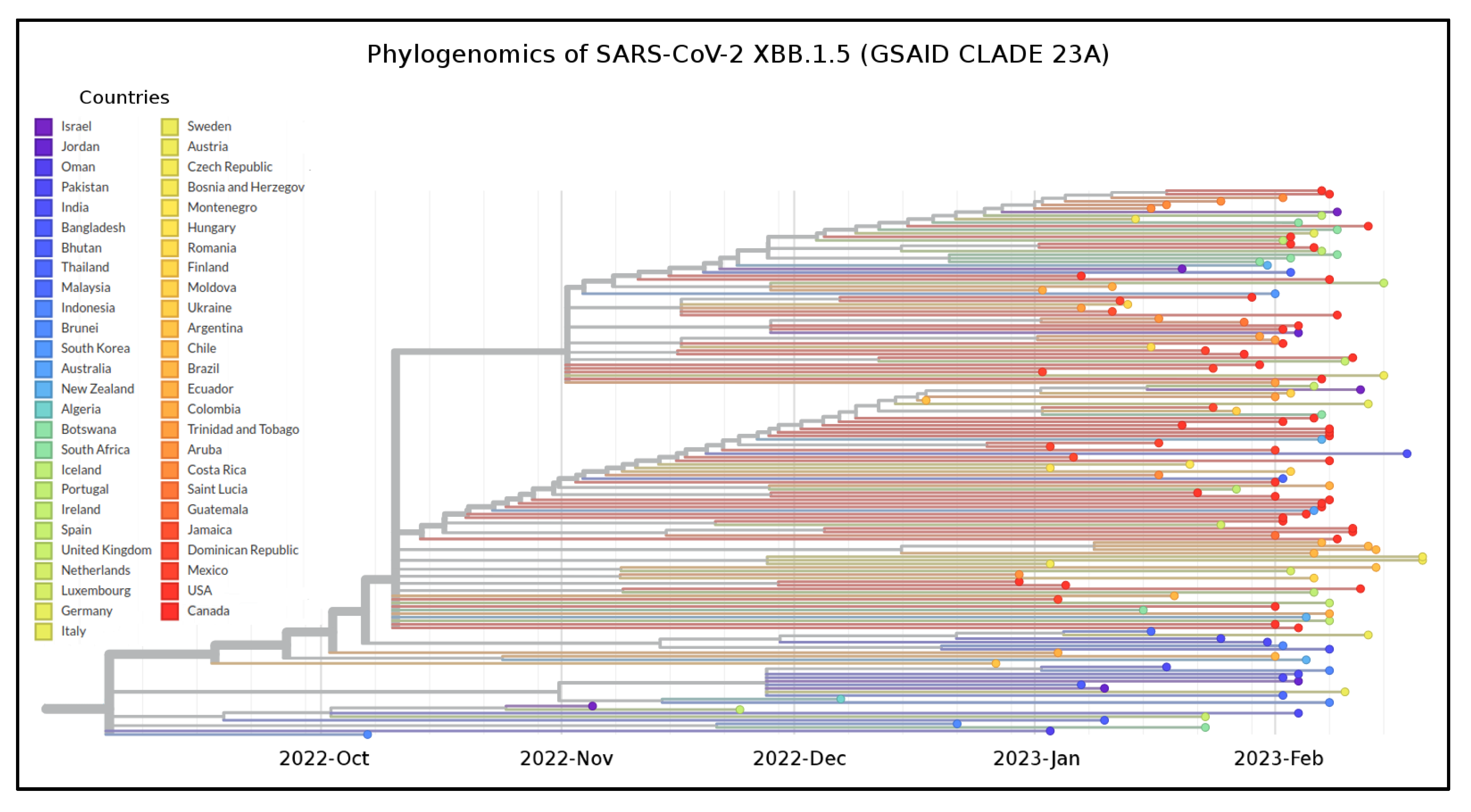
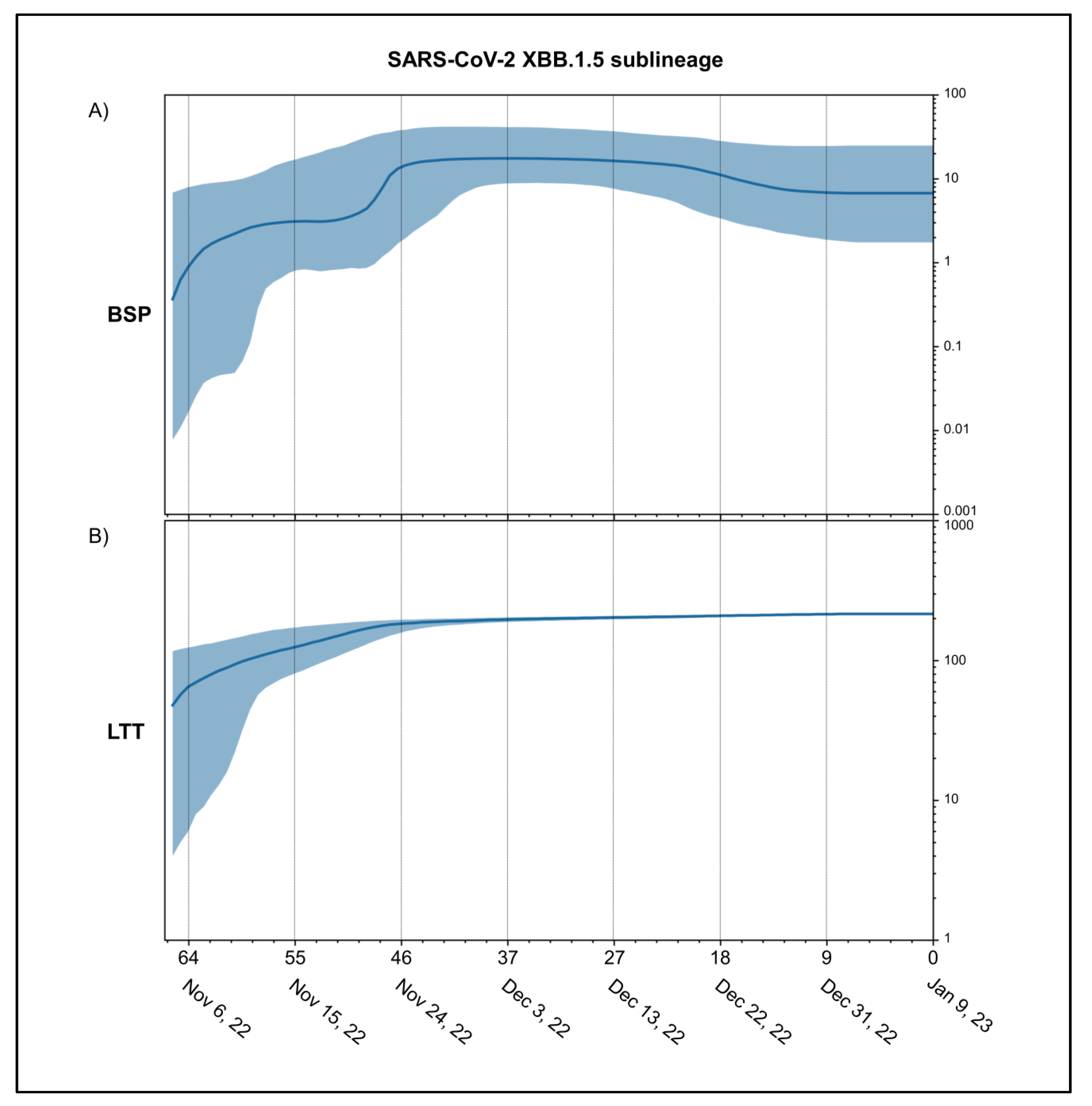
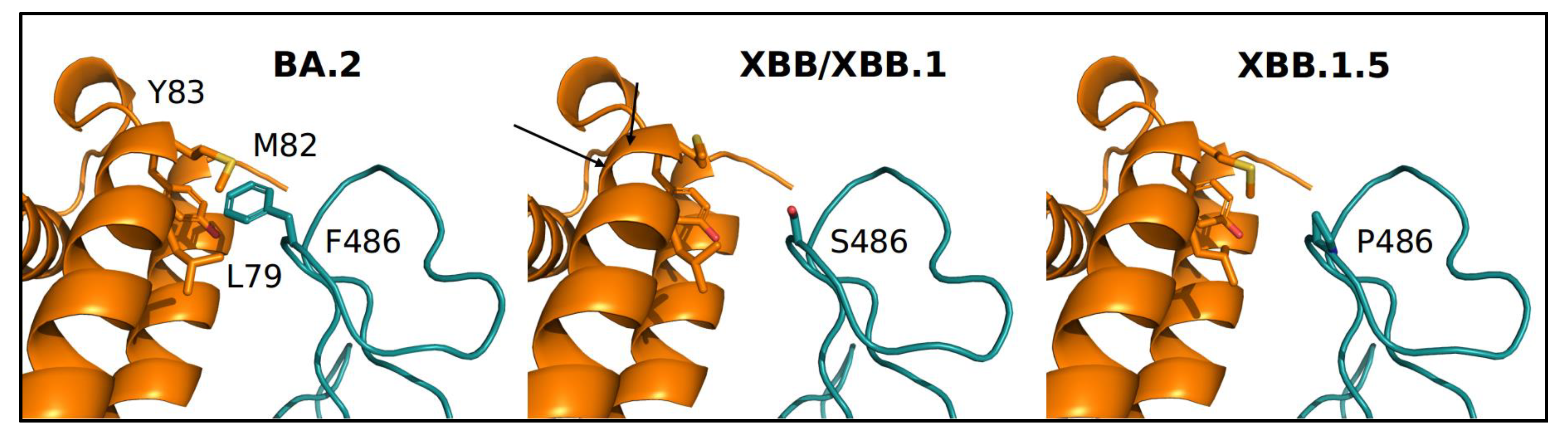
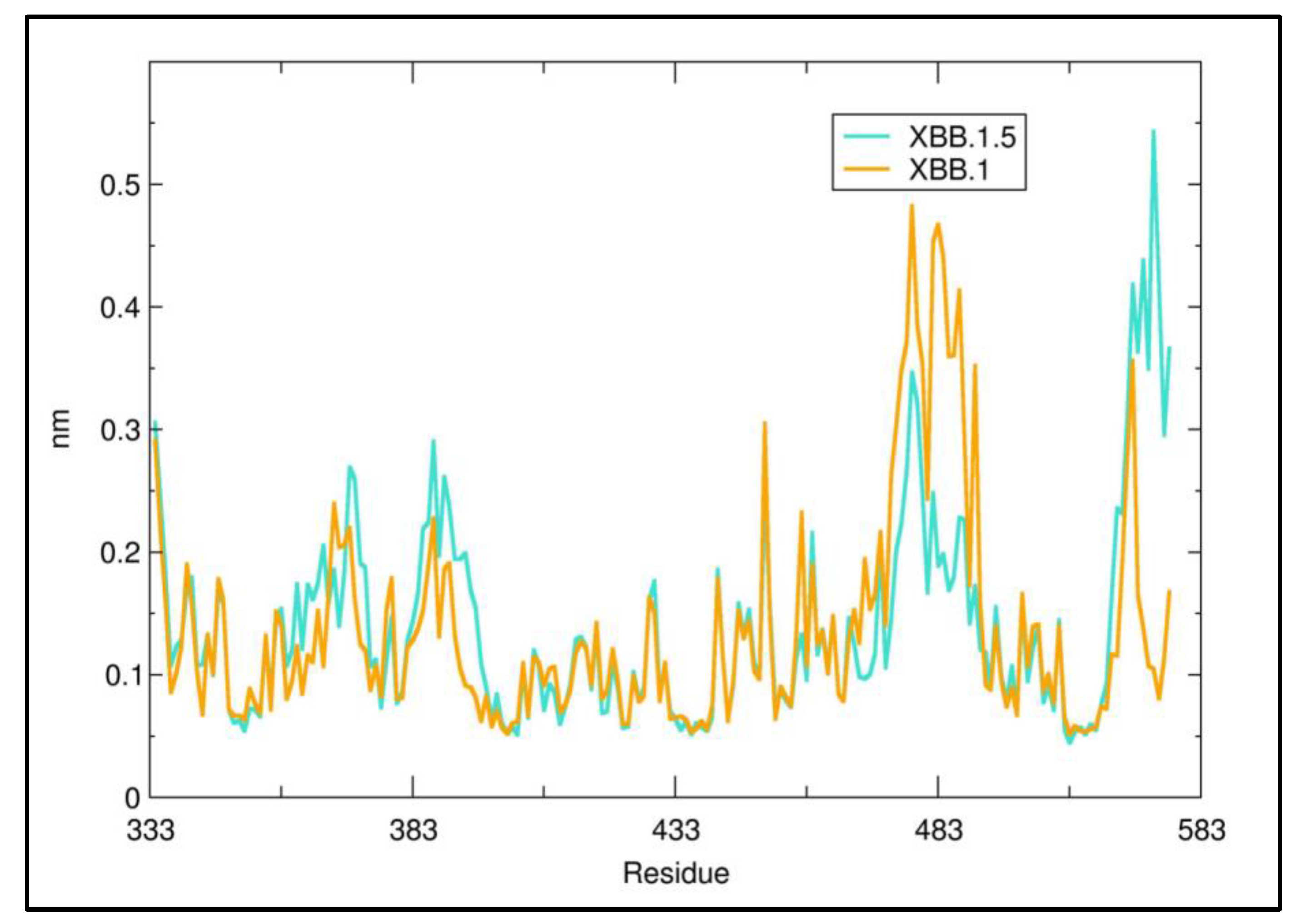
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Weekly Epidemiological Update on COVID-19—8 February 2023. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---1-march-2023 (accessed on 2 March 2023).
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, T.; Liu, Q.; Yang, Z. The SARS-CoV-2 outbreak: What we know. Int. J. Infect. Dis 2020, 94, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Blaas, I.; Bollineni, R.C.; Delic-Sarac, M.; Tran, T.T.; Knetter, C.; Dai, K.Z.; Madssen, T.S.; Vaage, J.T.; Gustavsen, A.; et al. et al. Prevalent and immunodominant CD8 T cell epitopes are conserved in SARS-CoV-2 variants. Cell Rep. 2023, 42, 111995. [Google Scholar] [CrossRef] [PubMed]
- Zella, D.; Giovanetti, M.; Benedetti, F.; Unali, F.; Spoto, S.; Guarino, M.; Angeletti, S.; Ciccozzi, M. . The variants question: What is the problem? J. Med. Vir. 2021, 93, 6479–6485. [Google Scholar] [CrossRef]
- Hillen, H.S.; Kokic, G.; Farnung, L.; Dienemann, C.; Tegunov, D.; Cramer, P. Structure of replicating SARS-CoV-2 polymerase. Nature 2020, 584, 154–156. [Google Scholar] [CrossRef]
- Scarpa, F.; Casu, M.; Sanna, D. Evolutionary and Conservation Genetics. Life 2021, 11, 1160. [Google Scholar] [CrossRef]
- Borsetti, A.; Scarpa, F.; Maruotti, A.; Divino, F.; Ceccarelli, G.; Giovanetti, M.; Ciccozzi, M. The unresolved question on COVID-19 virus origin: The three cards game? J. Med. Vir. 2022, 94, 1257–1260. [Google Scholar] [CrossRef]
- World Health Organization. WHO Announces Simple, Easy-To-Say Labels for SARS-CoV-2 Variants of Interest and Concern. Available online: https://www.who.int/news/item/31-05-2021-who-announces-simple-easy-to-say-labels-for-sars-cov-2-variants-of-interest-and-concern (accessed on 4 January 2023).
- Scarpa, F.; Sanna, D.; Benvenuto, D.; Borsetti, A.; Azzena, I.; Casu, M.; Fiori, P.L.; Giovanetti, M.; Maruotti, A.; Ceccarelli, G.; et al. Genetic and Structural Data on the SARS-CoV-2 Omicron BQ.1 Variant Reveal Its Low Potential for Epidemiological Expansion. Int. J. Mol. Sci. 2022, 23, 15264. [Google Scholar] [CrossRef]
- GISAID. Genomic Epidemiology of SARS-CoV-2 with Subsampling Focused Globally over the Past 6 Months. Available online: https://gisaid.org/phylodynamics/global/nextstrain/ (accessed on 1 February 2023).
- Lai, M.M. Genetic recombination in RNA viruses. Curr. Top. Microbiol. Immunol. 1992, 176, 21–32. [Google Scholar] [CrossRef]
- Scarpa, F.; Sanna, D.; Azzena, I.; Casu, M.; Cossu, P.; Fiori, P.L.; Benvenuto, D.; Imperia, E.; Giovanetti, M.; Ceccarelli, G.; et al. Genome-based comparison between the recombinant SARS-CoV-2 XBB and its parental lineages. J. Med. Vir. 2023, 95, e28625. [Google Scholar] [CrossRef]
- Scarpa, F.; Sanna, D.; Azzena, I.; Giovanetti, M.; Benvenuto, D.; Angeletti, S.; Ceccarelli, G.; Pascarella, S.; Casu, C.; Fiori, P.L.; et al. On the SARS-CoV-2 BA.2.75 variant: A genetic and structural point of view. J. Med. Vir. 2022, 95, e28119. [Google Scholar] [CrossRef]
- Scarpa, F.; Imperia, E.; Azzena, I.; Giovanetti, M.; Benvenuto, D.; Locci, C.; Casu, M.; Fiori, P.L.; Maruotti, A.; Ceccarelli, G.; et al. Genetic and structural genome-based survey reveals the low potential for epidemiological expansion of the SARS-CoV-2 XBB.1.5 sublineage. J. Infect. in press. 2023. [CrossRef] [PubMed]
- World Health Organization. WHO Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 30 January 2023).
- Imai, M.; Ito, M.; Kiso, M.; Yamayoshi, S.; Uraki, R.; Fukushi, S.; Watanabe, S.; Suzuki, T.; Maeda, K.; Sakai-Tagawa, Y.; et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. NEJM 2022, 388, 89–91. [Google Scholar] [CrossRef]
- Parumus, D.V. The XBB. 1.5 (‘Kraken’) Subvariant of Omicron SARS-CoV-2 and its Rapid Global Spread. Med. Sci. Monit. Basic Res. 2023, 29, e939580. [Google Scholar] [CrossRef]
- Popovic, M. XBB.1.5 Kraken Cracked: Gibbs Energies of Binding and Biosynthesis of the XBB.1.5 Variant of SARS-CoV-2. Microbiol. Res. 2023, 270, 127337. [Google Scholar] [CrossRef]
- World Health Organization. WHO XBB.1.5 Updated Rapid Risk Assessment. 24 February 2023. Available online: https://www.who.int/docs/default-source/coronaviruse/22022024xbb.1.5ra.pdf?sfvrsn=7a92619e_3 (accessed on 22 March 2023).
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286. [Google Scholar] [CrossRef]
- Yue, C.; Song, W.; Wang, L.; Jian, F.; Chen, X.; Gao, F.; Shen, Z.; Wang, Y.; Wang, X.; Cao, Y. Enhanced transmissibility of XBB.1.5 is contributed by both strong ACE2 binding and antibody evasion 1.5 is contributed by both strong ACE2 binding and antibody evasion. bioRxiv 2023, preprint. [Google Scholar] [CrossRef]
- GSAID Lineage Comparison. Available online: https://gisaid.org/lineage-comparison/ (accessed on 10 February 2023).
- GitHub Depository. Available online: https://github.com/nextstrain/ncov (accessed on 20 January 2023).
- Katoh, K.; Standley, D.M. MAFFT Multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M. UGENE Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, e214. [Google Scholar] [CrossRef] [PubMed]
- Kass, R.E.; Raftery, A.E. Bayes factors. JASA 1995, 90, 773–795. [Google Scholar] [CrossRef]
- Mugosa, B.; Vujosevic, D.; Ciccozzi, M.; Valli, M.B.; Capobianchi, M.R.; Lo Presti, A.; Cella, E.; Giovanetti, M.; Lai, A.; Angeletti, S.; et al. Genetic diversity of the haemagglutinin (HA) of human influenza A (H1N1) virus in Montenegro: Focus on its origin and evolution. J. Med. Vir. 2016, 88, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- FigTree 1.4.0. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 3 February 2023).
- Scarpa, F.; Sanna, D.; Azzena, I.; Cossu, P.; Giovanetti, M.; Benvenuto, D.; Coradduzza, E.; Alexiev, I.; Casu, M.; Fiori, P.L.; et al. Update on the Phylodynamics of SADS-CoV. Life 2021, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- GSAID Portal. Available online: https://gisaid.org/ (accessed on 13 January 2023).
- Webb, B.; Sali, A. Protein structure modeling with MODELLER. Methods Mol. Biol. 2017, 1654, 39–54. [Google Scholar] [CrossRef]
- Schrodinger, L.L.C. The PyMOL Molecular Graphics System, Version 1.8. 2015. Available online: https://pymol.org/2/ (accessed on 6 February 2023).
- Delgado, J.; Radusky, L.G.; Cianferoni, D.; Serrano, L. FoldX 5.0: Working with RNA, small molecules and a new graphical interface. Bioinformatics 2019, 35, 4168–4169. [Google Scholar] [CrossRef]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent treatment of internal and surface residues in empirical p K a predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef]
- Schweke, H.; Mucchielli, M.H.; Chevrollier, N.; Gosset, S.; Lopes, A. SURFMAP: A Software for Mapping in Two Dimensions Protein Surface Features. J. Chem. Inf. Model. 2022, 62, 1595–1601. [Google Scholar] [CrossRef]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. PRODIGY: A web server for predicting the binding affinity of protein–protein complexes. Bioinformatics 2016, 32, btw514. [Google Scholar] [CrossRef]
- Weng, G.; Wang, E.; Wang, Z.; Liu, H.; Zhu, F.; Li, D.; Hou, T. HawkDock: A web server to predict and analyze the protein–protein complex based on computational docking and MM/GBSA. Nucleic Acids Res. 2019, 47, W322–W330. [Google Scholar] [CrossRef] [PubMed]
- Krüger, D.M.; Gohlke, H. DrugScorePPI webserver: Fast and accurate in silico alanine scanning for scoring protein-protein interactions. Nucleic Acids Res. 2010, 38, W480–W486. [Google Scholar] [CrossRef] [PubMed]
- Clifford, J.N.; Høie, M.H.; Deleuran, S.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-3.0: Improved B-cell epitope prediction using protein language models. Protein Sci. 2022, 31, e4497. [Google Scholar] [CrossRef]
- Jurtz, V.; Paul, S.; Andreatta, M.; Jurtz, V.; Paul, S.; Andreatta, M.; Marcatili, P.; Peters, B.; Nielsen, M. NetMHCpan-4.0: Improved Peptide–MHC Class I Interaction Predictions Integrating Eluted Ligand and Peptide Binding Affinity Data. J. Immunol. 2017, 199, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Páll, S.; Zhmurov, A.; Bauer, P.; Abraham, M.; Lundborg, M.; Gray, A.; Hess, B.; Lindahl, E. Heterogeneous parallelization and acceleration of molecular dynamics simulations in GROMACS. Chem. Phys. 2020, 153, 134110. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Genet. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- XMGRACE Software Package. Available online: https://plasma-gate.weizmann.ac.il/Grace/ (accessed on 1 February 2023).
- U.S. Food & Drug Administration Update [1/26/2023]. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us (accessed on 22 March 2023).
- Focosi, D.; Maggi, F. Recombination in Coronaviruses, with a Focus on SARS-CoV-2. Viruses 2022, 2022 14, 1239. [Google Scholar] [CrossRef]
- World Health Organization. WHO XBB.1.5 Updated Rapid Risk Assessment. 25 January 2023. Available online: https://www.who.int/docs/default-source/coronaviruse/25012023xbb.1.pdf?sfvrsn=c3956081_1 (accessed on 30 January 2023).
- Lai, A.; Bergna, A.; Acciarri, C.; Galli, M.; Zehender, G. Early phylogenetic estimate of the effective reproduction number of SARS-CoV-2. J. Med. Vir. 2020, 92, 675–679. [Google Scholar] [CrossRef]
- Benvenuto, D.; Giovanetti, M.; Salemi, M.; Prosperi, M.; De Flora, C.; Alcantara, L.C.J.; Angeletti, S.; Ciccozzi, M. The global spread of 2019-nCoV: A molecular evolutionary analysis. Pathog. Glob. Health 2020, 114, 64–67. [Google Scholar] [CrossRef]
- Hotez, P. XBB.1.5 emerges in the Americas: What it means to the region. Lancet 2023, 18, 100433. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#variant-proportions (accessed on 6 February 2023).
- Pascarella, S.; Ciccozzi, M.; Benvenuto, D.; Borsetti, A.; Cauda, R.; Cassone, A. Peculiar Variations of the Electrostatic Potential of Spike Protein N-terminal Domain Associated with the Emergence of Successive SARS-CoV-2 Omicron Lineages. J. Infect. 2023, 86, 66–117. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Song, W.; Wang, L.; Jian, F.; Chen, X.; Gao, F.; Shen, Z.; Wang, Y.; Wang, X.; Cao, Y. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect. Dis. 2023, 23, 278–280. [Google Scholar] [CrossRef]
| BA.2 | XBB | XBB.1 | XBB.1.5 | |
|---|---|---|---|---|
| NTD | 0.95 ± 0.04 | 1.14 ± 0.04 | 1.18 ± 0.04 | 1.18 ± 0.04 |
| RBD | 5.19 ± 0.01 | 5.45 ± 0.02 | 5.45 ± 0.02 | 5.42 ± 0.01 |
| BA.2 | XBB | XBB.1 | XBB.1.5 | |
|---|---|---|---|---|
| Foldx 5.0 | −6.19 ± 0.32 | −3.54 ± 0.30 | −3.54 ± 0.30 | −4.57 ± 0.27 |
| PRODIGY | −11.70 ± 0.05 | −11.48 ± 0.05 | −11.48 ± 0.05 | −10.84 ± 0.05 |
| MM/GBSA | −68.43 ± 2.10 | −60.82 ± 0.99 | −60.82 ± 0.99 | −62.44 ± 2.28 |
| BA.2 | XBB | XBB.1 | XBB.1.5 | |
|---|---|---|---|---|
| MM/GBSA | −3.96 ± 0.01 | −0.72 ± 0.20 | −0.47 ± 0.12 | −1.41 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpa, F.; Azzena, I.; Locci, C.; Casu, M.; Fiori, P.L.; Ciccozzi, A.; Angeletti, S.; Imperia, E.; Giovanetti, M.; Maruotti, A.; et al. Molecular In-Depth on the Epidemiological Expansion of SARS-CoV-2 XBB.1.5. Microorganisms 2023, 11, 912. https://doi.org/10.3390/microorganisms11040912
Scarpa F, Azzena I, Locci C, Casu M, Fiori PL, Ciccozzi A, Angeletti S, Imperia E, Giovanetti M, Maruotti A, et al. Molecular In-Depth on the Epidemiological Expansion of SARS-CoV-2 XBB.1.5. Microorganisms. 2023; 11(4):912. https://doi.org/10.3390/microorganisms11040912
Chicago/Turabian StyleScarpa, Fabio, Ilenia Azzena, Chiara Locci, Marco Casu, Pier Luigi Fiori, Alessandra Ciccozzi, Silvia Angeletti, Elena Imperia, Marta Giovanetti, Antonello Maruotti, and et al. 2023. "Molecular In-Depth on the Epidemiological Expansion of SARS-CoV-2 XBB.1.5" Microorganisms 11, no. 4: 912. https://doi.org/10.3390/microorganisms11040912
APA StyleScarpa, F., Azzena, I., Locci, C., Casu, M., Fiori, P. L., Ciccozzi, A., Angeletti, S., Imperia, E., Giovanetti, M., Maruotti, A., Borsetti, A., Cauda, R., Cassone, A., Via, A., Pascarella, S., Sanna, D., & Ciccozzi, M. (2023). Molecular In-Depth on the Epidemiological Expansion of SARS-CoV-2 XBB.1.5. Microorganisms, 11(4), 912. https://doi.org/10.3390/microorganisms11040912














