Abstract
Abnormal fat accumulation with gut microbiota dysbiosis results in hepatic inflammation by upregulating the release of lipopolysaccharide (LPS) and inflammatory cytokine. Gochujang, a traditional fermented condiment, has beneficial effects, such as anti-colonic inflammatory effects. However, Gochujang has been controversial because of its high salt content (the Korean Paradox). Thus, the present study aimed to investigate the preventative effects of Gochujang on hepatic inflammation and related gut microbiota through discussing the Korean Paradox. The mice were divided into groups including a normal diet (ND), high-fat diet (HD), HD with salt (SALT), HD with a high percentage of beneficial microbiota Gochujang (HBM), and HD with diverse beneficial microbiota Gochujang (DBM). Gochujang markedly reduced lipid accumulation, hepatic injury, and inflammation response. Furthermore, Gochujang attenuated protein expression involved in the JNK/IκB/NF-κB pathway. Additionally, Gochujang regulated the gut microbiota-derived LPS production and Firmicutes/Bacteroidetes ratio. Gochujang regulated the levels of gut microbiota such as Bacteroides, Muribaculum, Lactobacillus, and Enterorhabdus, which were correlated with hepatic inflammation. Salt did not have foregoing effects, meaning that the salt content in Gochujang did not affect its anti-inflammatory effect. In conclusion, Gochujang showed anti-hepatic inflammation effects via reduced lipid accumulation, hepatic injury, and inflammatory response together with reorganization of gut microbiota dysbiosis regardless of salt content and the difference of micro bacteria composition.
1. Introduction
A high-fat diet and/or high salt diet trigger abnormal fat accumulation and dysfunction of adipose tissue, increasing the production of inflammatory cytokine and chemokine [1,2]. Then, these inflammatory factors activate intracellular signaling pathways and cause chronic hepatic inflammation [3]. Phosphorylation of c-jun N-terminal kinase (JNK), the inhibitor of kappa B (IκB), and nuclear factor kappa B (NF-κB) result in transcription of NF-κB [4,5]. As a result, the expression of pro-inflammatory cytokines, such as cyclooxygenase-2 (COX-2), is upregulated and this causes the chronic inflammatory status of hepatocytes [4,6].
Intriguingly, recent studies demonstrated that HD-induced gut microbiota dysbiosis also stimulates hepatic inflammation [7]. Dysbiosis of gut microbiota increases the production of lipopolysaccharide (LPS), elevating the release of inflammatory cytokines (e.g., tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β) [8]. Therefore, many investigations have been performed to find the nutritional compounds and/or foods that improve diet-induced hepatic inflammation signaling pathways and gut microbiota dysbiosis.
As probiotics, fermented foods have various beneficial effects to health by maintaining gut health and by recovering disease-related gut microbiota dysbiosis [9]. Gochujang, a Korean traditional fermented food (KTFF), contains multiple microbiota, such as Bacillus (B.) amyloliquefaciens, B. licheniformis, and B. subtilis [9]; and it alleviates obesity, oxidative stress, and inflammatory bowel disease (IBD) [10,11,12]. Indeed, Gochujang improves dextran sulfate sodium (DSS)-induced IBD by reducing serum IL-1β and IL-6 levels, suppressing colonic mRNA expression of TNF-α, IL-1β, and IL-6, and reversing imbalance of gut microbiota [12]. However, the ameliorative effects of Gochujang on hepatic inflammation and related gut microbiota dysbiosis have not been studied. Moreover, the effects of Gochujang on diet-induced inflammation are also unexplored.
Many previous studies have reported diverse positive effects on health and the potential beneficial microbiota of various KTFFs, including Gochujang and Ganjang [13]. However, the effects of KTFFs have been inconclusive due to their high salt level (e.g., Gochujang, ~10% and Ganjang, 16~18%), which is strongly associated with diverse metabolic disease [10,14,15]. Paradoxically, it has been reported that Koreans have a low prevalence of hypertension and cardiovascular disease, even though Koreans consume high levels of salt from diverse KTFFs in their diet [16]. Thus, this phenomenon has been defined as the “Korean Paradox” [17]. However, the explanative research regarding the Korean Paradox has still not been adequately performed.
Therefore, this study aimed to (1) investigate the effect of Gochujang on hepatic inflammation and gut microbiota dysbiosis in HD-induced obese mice, (2) explore the relationship between hepatic inflammation and gut microbiota dysbiosis in HD-induced obese mice, and (3) demonstrate that the salt in Gochujang does not have negative effects, unlike table salt.
2. Materials and Methods
2.1. Gochujang Selection
Gochujang microbiota determination was conducted by Next Generation Sequencing (NGS) in the Microbial Institute for Fermentation Industry (Sunchang, Jeollabuk-do, Republic of Korea). The microbiome of 76 Gochujang was analyzed using next generation sequencing (NGS) analysis, and the following two Gochujang were selected. Gochujang with a high percentage of beneficial micro bacteria was named HBM Gochujang, and relatively few but various types of beneficial micro bacteria was named DBM Gochujang.
2.2. Material & Animal Experiment
Gochujang was supplied by the Microbial Institute for Fermentation Industry (Sunchang, Jeollabuk-Do, Republic of Korea). Experimental animals were male C57BL/6 mice, three-weeks-old, and were purchased from DooYeol Biotech (Seoul, Republic of Korea). The mice were housed under a 12 h/12 h light/dark cycle at 23 ± 1 °C and relative humidity at 60 ± 5%. After acclimation for three weeks, animals were randomly divided into five groups (n = 7): normal diet (ND, 10% fat of total kcal), high-fat diet (HD, 60% fat of total kcal), HD with 0.7% salt (SALT), HD with 10% HBM Gochujang (HBM), and HD with 10% DBM Gochujang (DBM). The compositions of the diets were calculated based on the results of the Gochujang compositions conducted by Eurofins Woosol (Yuseong-gu, Daejeon, Republic of Korea) (Tables S1 and S2). The mice had free access to food and water. Food intake was recorded three times a week and body weight (BW) was measured once a week. Energy efficiency (g/kcal) was calculated by body weight gain (g)/energy intake (kcal). After 13 weeks, serum and tissues were collected after anesthesia and stored at −80 °C until use. This study was approved by the Institutional Animal Care and Use Committee of Jeonbuk National University (JBNU 2021-0112).
2.3. Biochemistry Parameters Analysis
The levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) in serum were measured using commercial kits (Asan Pharmaceutical Co., Seoul, Republic of Korea). Very low-density lipoprotein-cholesterol (VLDL) levels were calculated using Friedewald’s formula [18]. The liver tissue in the chloroform- and methanol-mixed solution (2:1 v/v) was homogenized and centrifuged at 8000 rpm, 4 °C, for 15 min. The lower layer was collected and then evaporated at room temperature. The precipitate was dissolved at 5% Triton X-100 (Takara Blo Inc, Kusatsu, Shiga, Japan). TG and TC levels were analyzed by the commercial kit (Asan Pharmaceutical Co., Seoul, Republic of Korea). The inflammatory cytokines, including TNF-α and IL-1β, were measured using a pre-coated ELISA kit (Westlake Village, CA, USA) in accordance with the manufacturer’s instructions.
2.4. Micro-Computed Tomography (CT) and Histological Analysis
To analyze visceral white adipose tissue (VAT) volume, Micro-CT was conducted using a high-resolution in vivo micro-CT system (Skyscan 1076, Konitch, Belgium) at the Center for University-wide Research Facilities (CURF) at Jeonbuk National University. Fat volume was found by CTAn software (Skyscan Co., Konitch, Belgium). Liver tissue was fixed with 10% formalin for 48 h and further processed by the KP&T Company (Cheongju, Chungcheongbuk-do, Republic of Korea). Hematoxylin and eosin (H&E) staining was performed. Stained samples were analyzed by optical microscopy (DM2500, Leica, Germany) in the CURF of Jeonbuk National University. Image-J software (US National Institutes of Health, Bethesda, MD, USA) was used to determine the quantification of the hepatic lipid content.
2.5. Quantitation Real-Time PCR (qRT-PCR)
RNA was extracted from liver tissue using the TRIzol reagent (Takara Korea, Seoul, Republic of Korea). cDNA was synthesized with the PrimeScript RT Master Mix (Takara, Kyoto, Japan). qRT-PCR analyses were performed with SYBR Green PCR Master MIX (Biosystems, Woolston, Warrington, UK) and real-time PCR (7500 Real-Time PCR System, Foster City, CA, USA). Relative quantification of gene expression calculated relative to β-actin was used as a housekeeping gene, and the relative quantification of gene expression was calculated using the 2∆∆Ct method. The primer sequences are listed in Table S3.
2.6. Western Blot
Liver tissue was homogenized in RIPA lysis buffer (Biosesang, Seoul, Republic of Korea) containing a protease inhibitor and phosphatase inhibitor cocktail (EMD Millipore Corp., Burlington, MA, USA), and was centrifuged (4 °C, 12,000× g, 15 min) following the supernatant that was collected. Proteins were electrophoresed on 10–12% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA) followed by blotting with primary antibodies (1:1000); p-IκB, p-p65, COX-2 (Santa Cruz, Dallas, TX, USA), IκB, p65, p-SAPK/JNK, SAPK/JNK, and β-actin (Cell Signaling Technology, Danvers, MA, USA). The membranes were washed and probed with horseradish-peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies (1:2000).
2.7. Gut Microbiota Analysis
At the end of the intervention period, fresh feces were collected and stored at −80 °C and processed further by the Microbial Institute for Fermentation Industry (Sunchang, Jeollabuk-Do, Republic of Korea). Microbiome composition, alpha-diversity, and beta-diversity were analyzed through the EzbioCloud 16S-based Microbiome Taxonomic Profiling software (ChunLab, Inc., Seoul, Republic of Korea). The levels of endotoxins in mouse serum were measured by using a pierce chromogenic endotoxin quantitation kit (Thermo Fisher Scientific Inc., Rockford, IL, USA), according to the manufacturer’s manual.
2.8. Statistical Analysis
All results are expressed as mean ± standard error of means (SEM). Analysis was performed using SPSS version 22.0 (SPSS Institute, Chicago, IL, USA) by one-way ANOVA, followed by Duncan’s Multiple Range Test (a > b), and the criterion for statistical significance was set at p < 0.05. In the case that comparison between the two groups was required, the t-test was used. * p < 0.05, ** p < 0.01, and *** p < 0.001 were considered to be statistically significant.
3. Results
3.1. Microbial Characteristics of HBM and DBM Gochujang
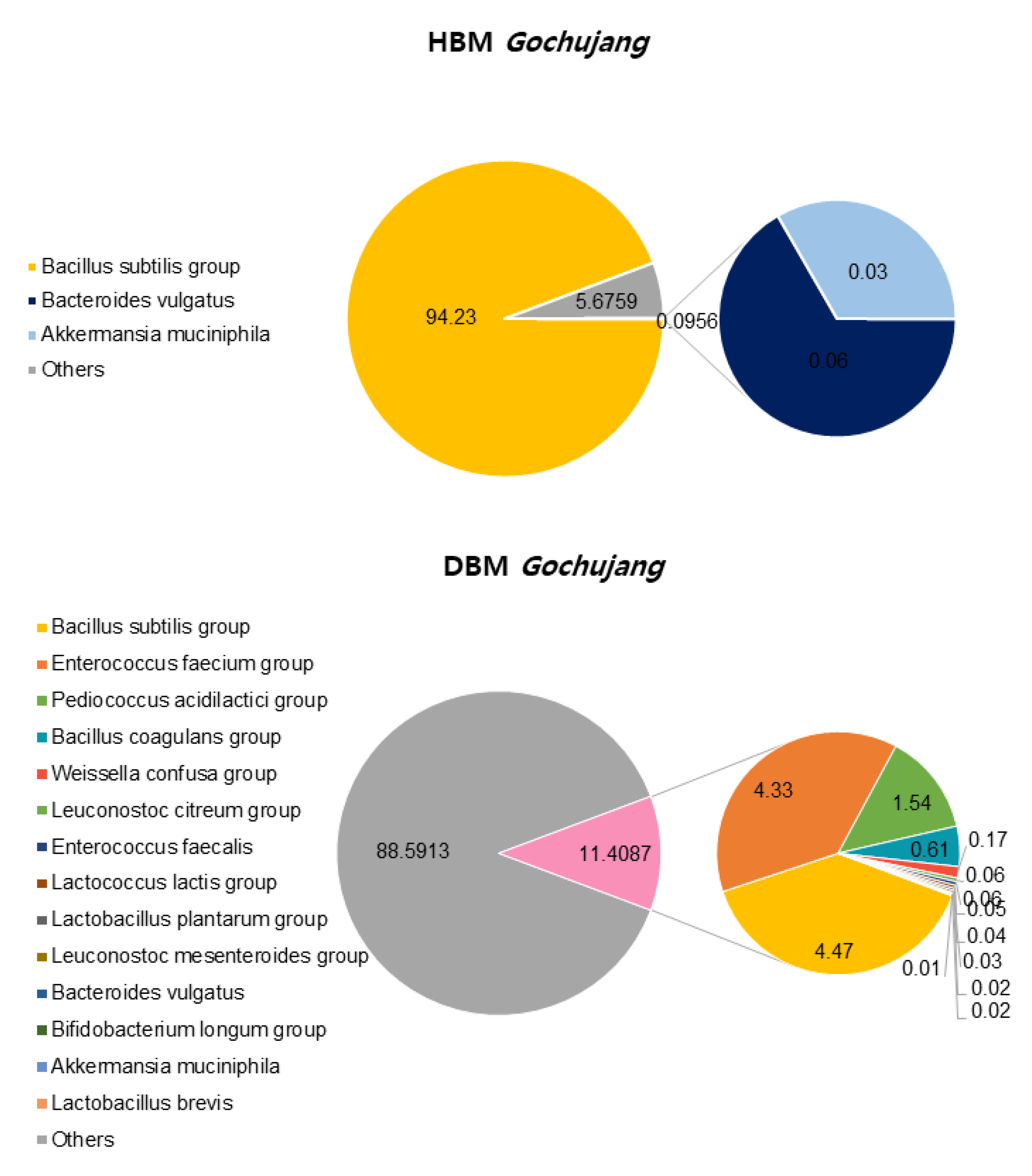
We confirmed the differences between HBM and DBM Gochujang by analyzing beneficial bacteria percentage and composition. OTUs, ACE, CHAO, Simpson, and Phylogenetic diversity in HBM Gochujang were higher than those in DMB Gochujang, but the Shannon index was lower in HBM Gochujang compared with DBM Gochujang (Table 1). The percentage of beneficial micro bacteria in HBM Gochujang (94.32%) was higher than those in DBM Gochujang (11.41%) (Figure 1). However, HBM Gochujang was dominated by Bacillus subtilis group, while DBM Gochujang had various beneficial micro bacteria, such as Bacillus subtilis group, Enterococcus faecium group, and Pediococcus acidilactici group (Figure 1).

Table 1.
α-diversity of Gochujang.
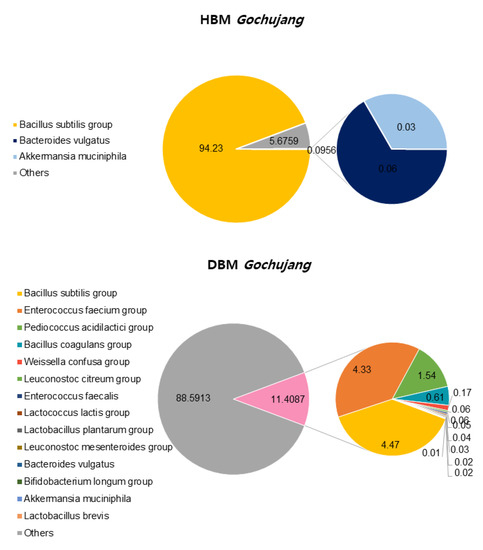
Figure 1.
Beneficial bacteria composition of HBM and DBM Gochujang.
3.2. Gochujang Ameliorates Obesity and Hyperlipidemia in HD-Induced Obese Mice
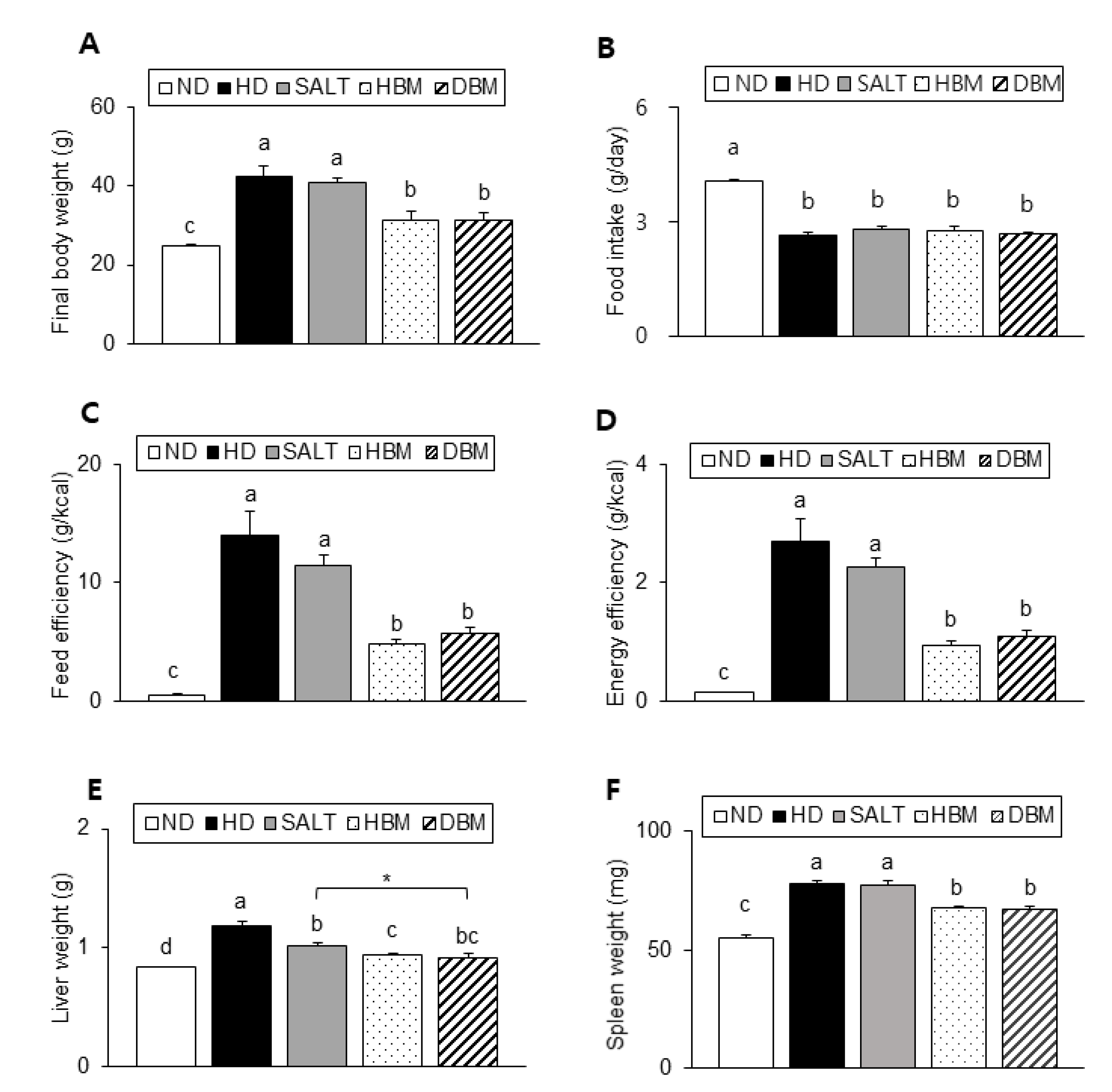
As described in Figure 2A, the HD and SALT groups showed a significant increase in body weight compared with the ND group, whereas both Gochujang groups showed a significantly lower body weight than the HD and SALT groups. There was no significant difference in food intake among the HD groups (Figure 2B). However, both the HD and SALT groups showed a higher feed efficiency and energy efficiency than both Gochujang groups, suggesting that the weight gain of these groups was not coming from food intake (Figure 2C,D). Liver and spleen weights of the HD and SALT groups were significantly higher than the ND group, whereas those of both Gochujang groups were markedly lower compared with the HD and SALT groups (Figure 2E,F).
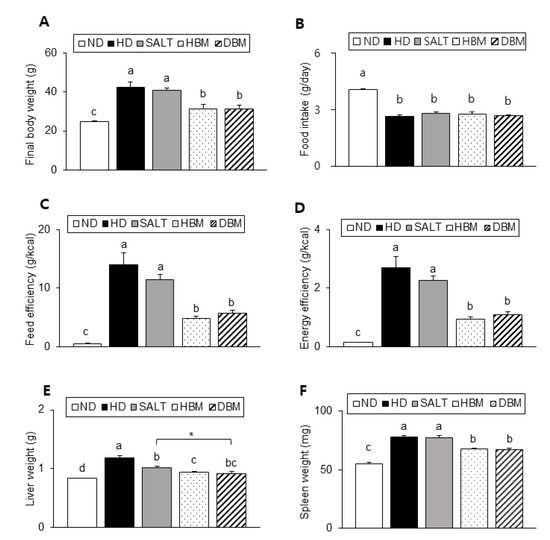
Figure 2.
Effects of Gochujang on body and organs weight in the HD-fed mice. (A) Final body weight of mice; (B) Food intake; (C) Feed efficiency; (D) Energy efficiency; (E) Liver weight; (F) Spleen weight. Values are mean SEM. a–d means with the different letters on the bar (p < 0.05). (a > b > c > d). * p < 0.05. ND, normal diet group; HD, high-fat diet group; SALT, HD with 0.7% salt group; HBM, HD with 10% of HBM Gochujang group; DBM, HD with 10% of DBM Gochujang group.
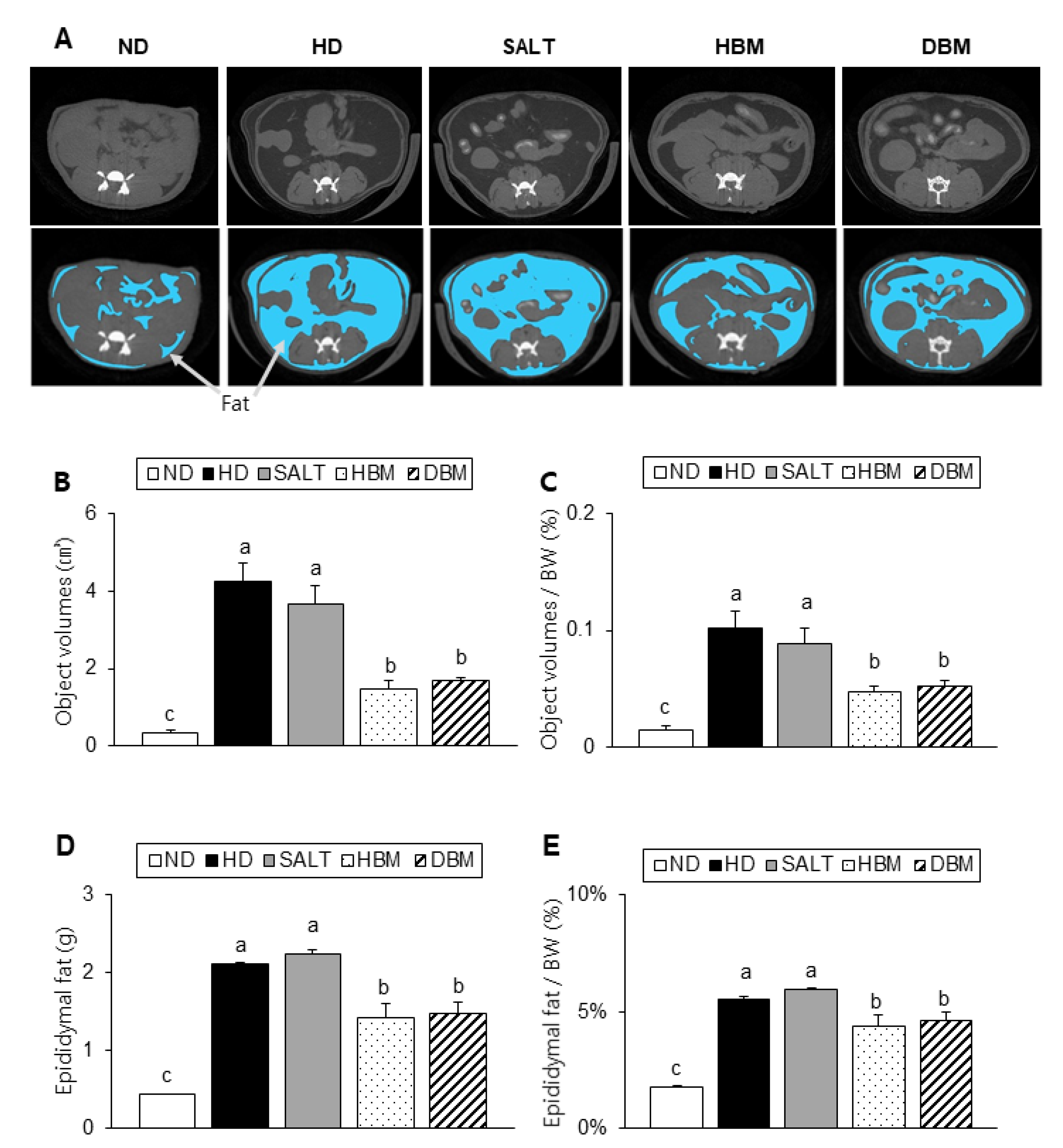
Compared to the ND group, the alternative and relative visceral fat volumes of the HD and SALT groups were notably higher, but both Gochujang groups had a significantly reduced visceral fat volume compared with the HD and SALT groups (Figure 3A–C). Consistently, the alternative and relative epididymal fat weight also significantly lowered in the HBM and DBM groups compared with the HD and SALT groups (Figure 3D,E). Compared to the ND groups, the HD and SALT groups had increased levels of serum TG, TC, and VLDL, while the HBM and DBM groups had a significantly reduced level of those parameters when compared with the HD and SALT groups (Table 2). These observations imply that Gochujang improves HD-induced obesity by improving fat accumulation and serum dyslipidemia.
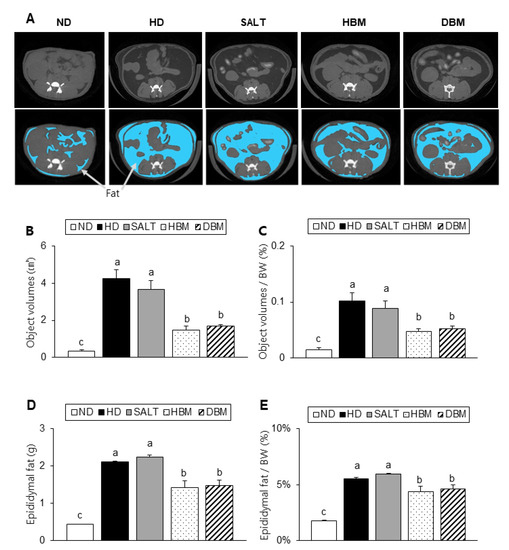
Figure 3.
Effects of Gochujang on dyslipidemia in the HD-fed mice. (A) Micro-CT image of abdominal area (dark gray portions indicate fat tissue, which illustrated under picture with blue portions, white portions indicate bone, and light gray portions indicate organs); (B) Fat tissue volumes at the micro-CT; (C) Fat volume at the micro-CT per body weight (BW); (D) Epididymal fat; (E) Epididymal fat per BW. Values are mean SEM. a–c means with the different letters on the bar (p < 0.05). (a > b > c). ND, normal diet group; HD, high-fat diet group; SALT, HD with 0.7% salt group; HBM, HD with 10% of HBM Gochujang group; DBM, HD with 10% of DBM Gochujang group.

Table 2.
Serum lipid levels.
3.3. Gochujang Alleviates Hepatic Injury, Lipid Accumulation, and Inflammation in HD-Induced Obese Mice
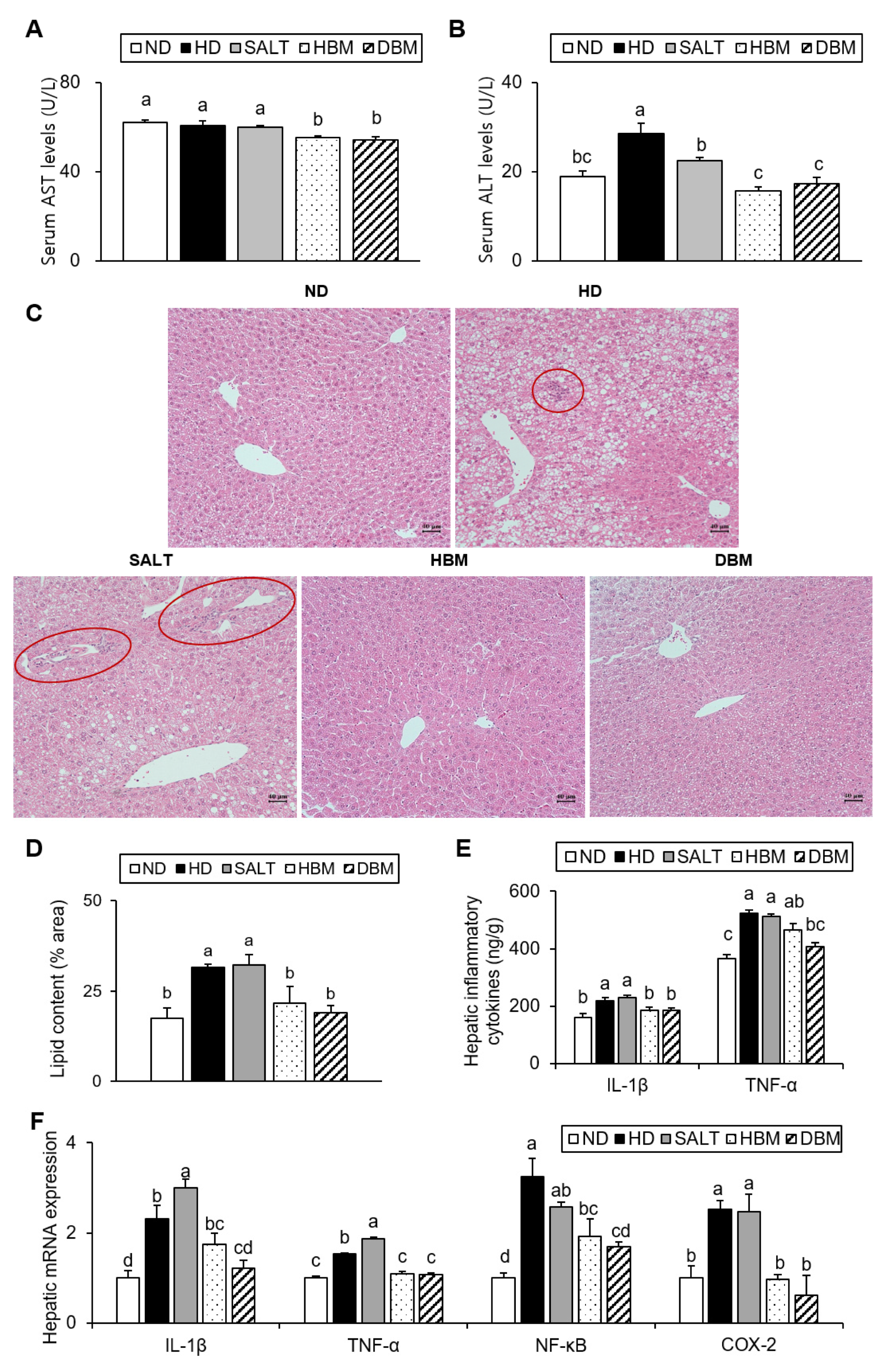
The elevations of the enzymatic activity of serums AST and ALT are associated with hepatic injury [19]. Additionally, increased hepatic lipid accumulation results in hepatic inflammation [20]. Thus, AST and ALT serum levels and hepatic lipid levels were measured. Compared with the ND group, the ALT level of the HD and SALT groups were higher with and/or without statistical significance, while the HBM and DBM groups showed a significant reduction of AST and ALT levels compared with the HD and SALT groups (Figure 4A,B). Hepatic TG and TC levels of the HD and SALT groups were significantly higher than the ND group, but those of both Gochujang groups were notably lower than in the SALT and HD groups (Table 3). Furthermore, hepatic lipid droplets in the HD and SALT groups were higher than in the ND group; however, the HBM and DBM groups were dramatically decreased compared with the HD and SALT groups (Figure 4C,D). Additionally, the HD and SALT groups showed leukocytic infiltration, while other groups did not (Figure 4C).
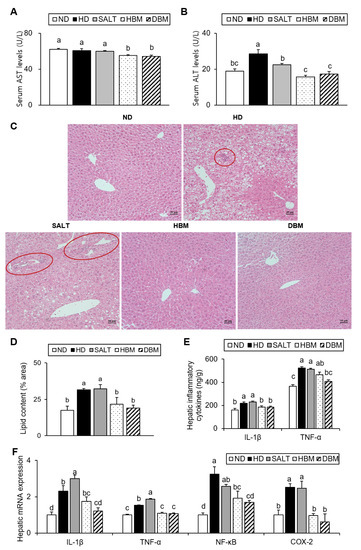
Figure 4.
Effects of Gochujang on hepatic lipid accumulation and inflammation in the HD-fed mice. (A) Serum AST level; (B) Serum ALT level; (C) Histological analysis (200×) of liver tissues by H&E staining (red circle area showed periportal mononuclear leukocytic infiltration); (D) quantitation of liver lipid content shown as percentage of the total histological area; (E) Hepatic levels of IL-1β and TNF-α; (F) Relative mRNA expression of IL-1β, TNF-α, NF-kB, COX-2 in liver tissue. Values are mean SEM. a–d means with the different letters on the bar (p < 0.05). (a > b > c > d). ND, normal diet group; HD, high-fat diet group; SALT, HD with 0.7% salt group; HBM, HD with 10% of HBM Gochujang group; DBM, HD with 10% of DBM Gochujang group; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; IL, interleukin; TNF-α, tumor necrosis factor alpha; NF-κB, nuclear factor kappa B; COX-2, cyclooxygenase-2.

Table 3.
Hepatic lipid levels.
To further investigate the role of Gochujang in hepatic inflammation amelioration, hepatic pro-inflammatory cytokine levels were analyzed. As expected, the serum cytokine levels of IL-1β and TNF-α in the HD and SALT groups were significantly increased compared with the ND group, while the HBM and DBM groups showed significantly lower levels of those of the SALT group (Figure 4E). The hepatic mRNA expression levels of IL-1β, TNF-α, NF-κB, and COX-2 in the HD and SALT groups were notably elevated compared with the ND group, while both Gochujang groups notably decreased when compared with the SALT and HD groups (Figure 4F). These results indicate that Gochujang counteracts HD-induced hepatic injury, steatosis, and inflammatory response.
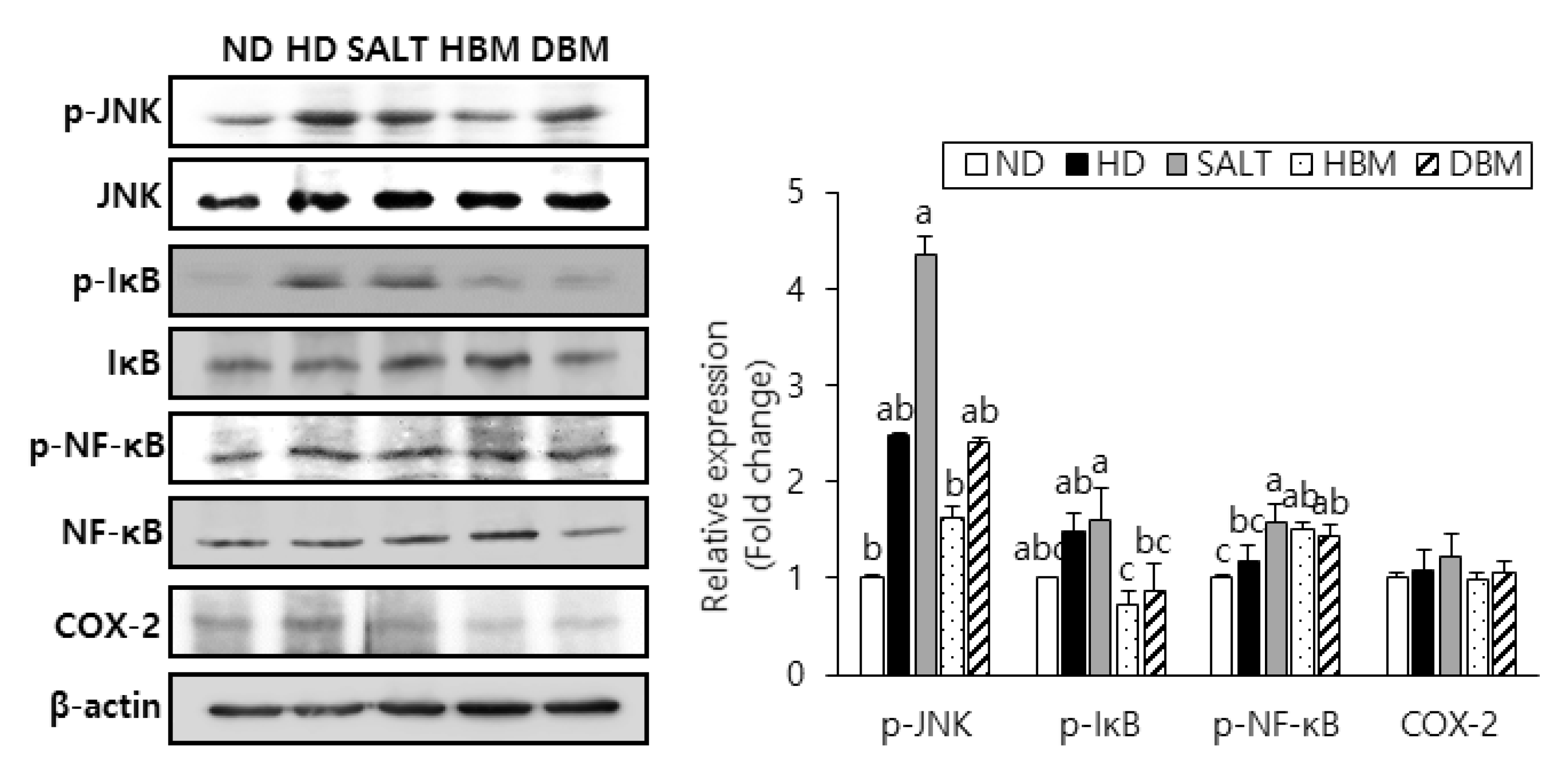
3.4. Gochujang Inhibits Activation of JNK/IκB/NF-κB Pathway in HD-Induced Obese Mice
Since activation of JNK, IκB, and NF-κB results in hepatic inflammation and steatohepatitis [21,22,23], phosphorylation of those proteins was probed to evaluate the inhibitory effects of Gochujang in HD-induced hepatic inflammation. The phosphorylation levels of JNK, IκB, and NF-κB were notably higher in the HD and SALT groups compared with the ND group (Figure 5). Compared with the SALT group, phosphorylation of those proteins in the HBM and DBM groups were decreased with and/or without statistical significance. Expression of COX-2 in the HD and SALT groups had a slight increased tendency compared with the ND group, while both Gochujang groups showed a decreased tendency of COX-2 expression compared with the SALT group. These data suggested that activation of the JNK/IκB/NF-κB pathway is involved in HD-induced inflammatory response; Gochujang suppresses signaling pathway activation to exert its anti-inflammatory effects.
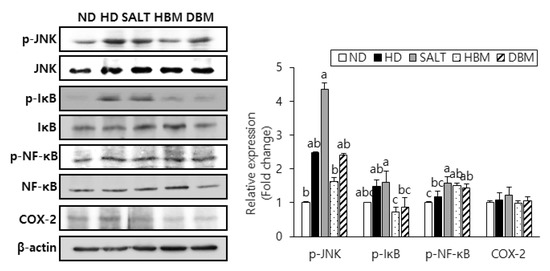
Figure 5.
Effects of Gochujang on protein expression of JNK/NFκB pathway and hepatic inflammation in the HD-fed mice. Hepatic p-JNK, p-IκBα, p-NF-κB (p-p65), and COX-2 in mice. Values are mean SEM. a–c means with the different letters on the bar (p < 0.05). (a > b > c). ND, normal diet group; HD, high-fat diet group; SALT, HD with 0.7% salt group; HBM, HD with 10% of HBM Gochujang group; DBM, HD with 10% of DBM Gochujang group; p-, phospho-; IκB, inhibitor of κB; NF-κB, nuclear factor kappa B; COX-2, cyclooxygenase-2.
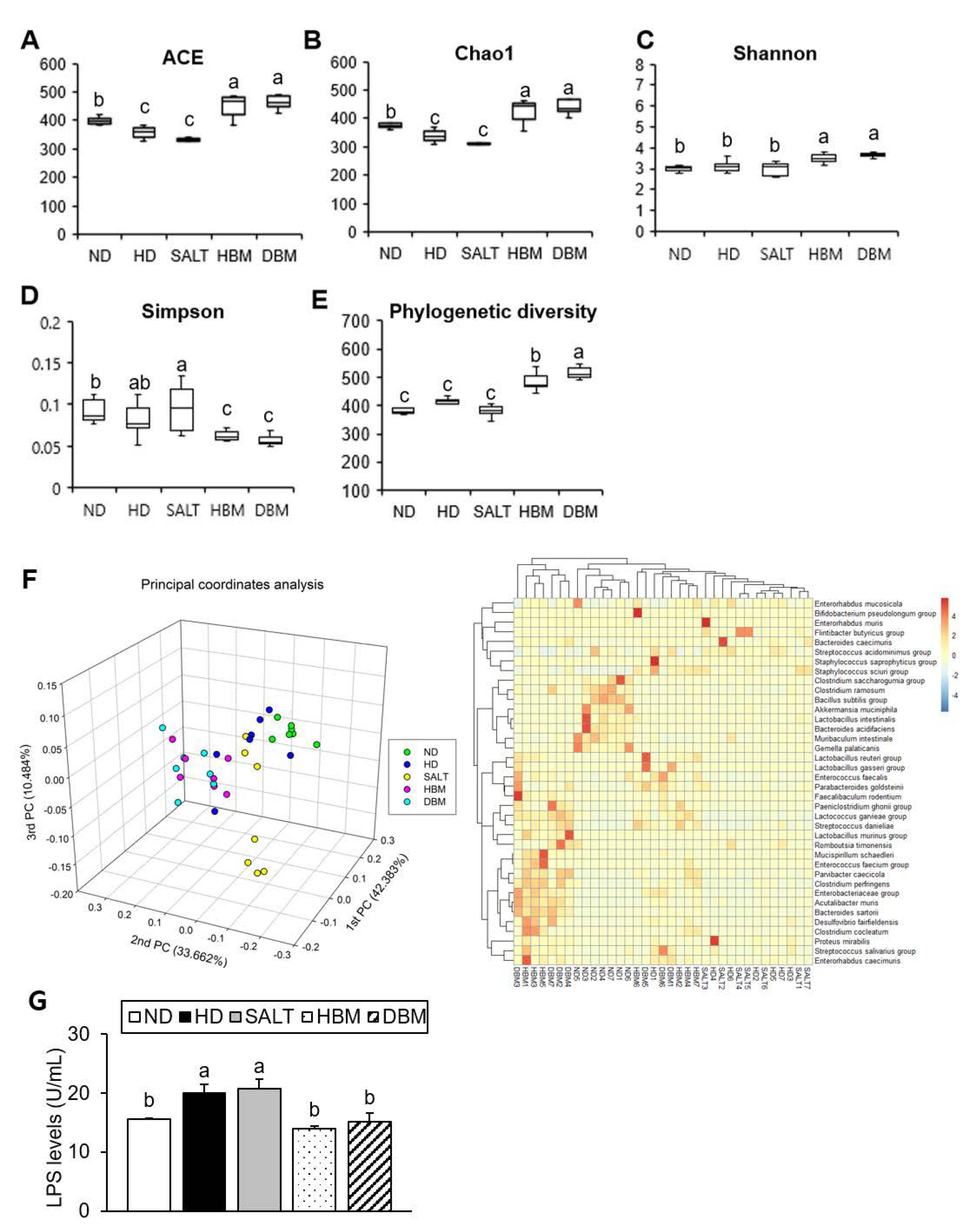
3.5. Gochujang Changes the Gut Microbiota Composition in HD-Induced Obese Mice
Dysbiosis of gut microbiota by HD is strongly related to hepatic inflammation [24]. To investigate gut microbiota dysbiosis in HD-induced obese mice and Gochujang’s effects on it, gut microbiota composition and bacteria-derived LPS levels were analyzed. The results of alpha analyses including ACE, Chao1, Shannon entropy, Simpson index, and Phylogenetic diversity indicated that both Gochujang groups, HBM and DBM, increased the microbial species’ richness compared with the HD and SALT groups, while the HD and SALT groups’ parameters were lowered and/or unchanged compared with the ND group (Figure 6A–E). We confirmed that, through 3D-principal component analysis (PCoA), the samples were separated into ND- and HD-fed mice based on PC1, and the HD and SALT groups were separated from the Gochujang groups based on PC1 (Figure 6F). At species level, the HD and SALT groups clustered together, while the Gochujang groups clustered with the ND group. Additionally, these alterations led to changes in the bacteria-derived LPS levels. Compared with the ND group, HD and SALT groups showed markedly elevated levels of LPS, whereas both Gochujang groups had significantly lowered levels of LPS compared with the HD and SALT groups (Figure 6G).
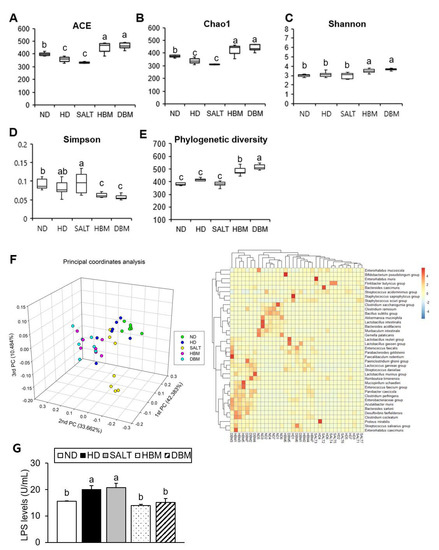
Figure 6.
Effect of Gochujang on the relative abundance of gut microbial community in the HD-fed mice. (A) ACE; (B) Chao1; (C) Shannon entropy; (D) Simpson index; (E) Phylogenetic diversity; (F) 3D-Principal coordinate analysis (PCoA) and Heat map at species level; (G) LPS levels. Values are mean SEM. a–c means with the different letters on the bar (p < 0.05). (a > b > c). ND, normal diet group; HD, high-fat diet group; SALT, HD with 0.7% salt group; HBM, HD with 10% of HBM Gochujang group; DBM, HD with 10% of DBM Gochujang group.
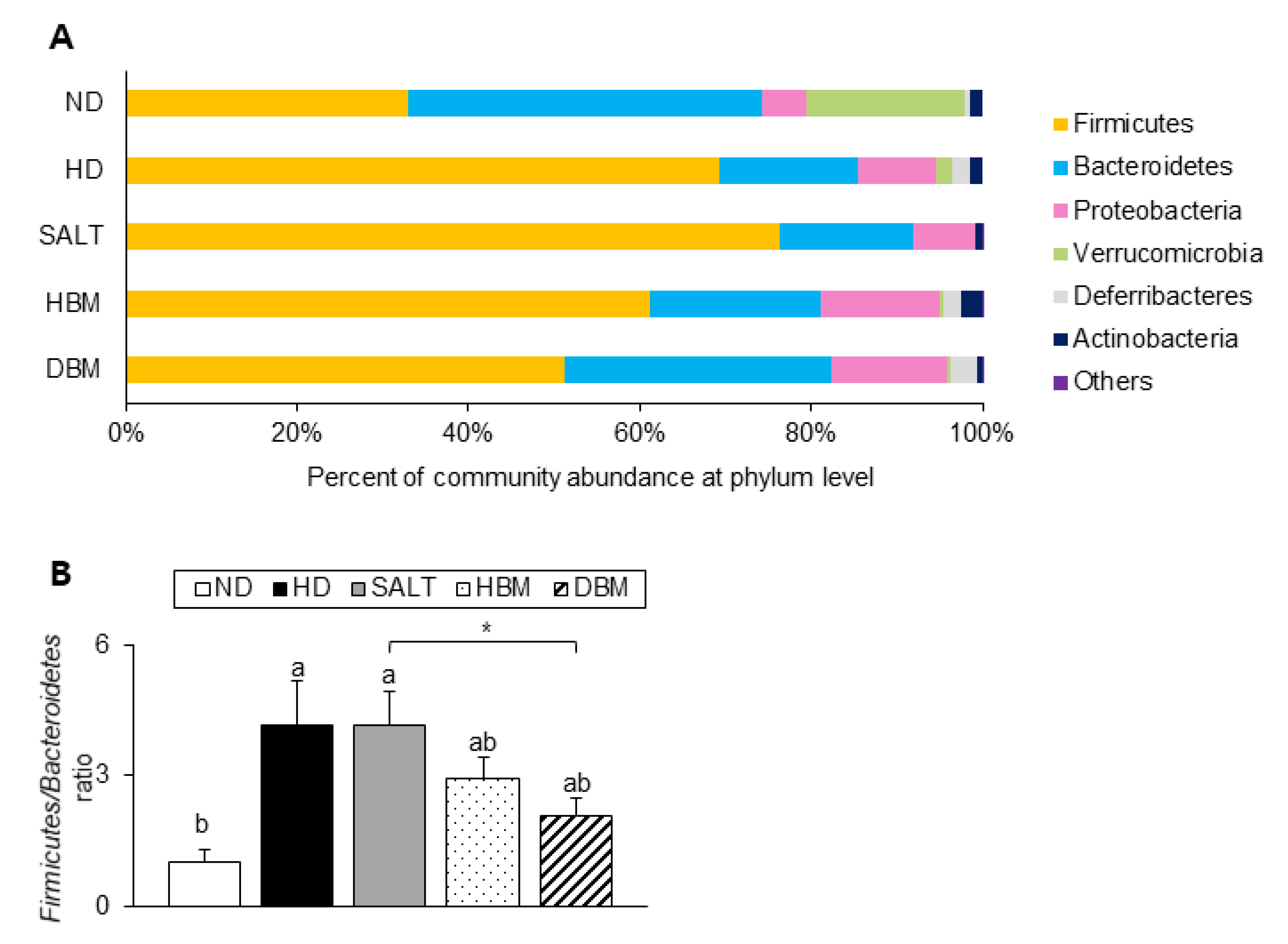
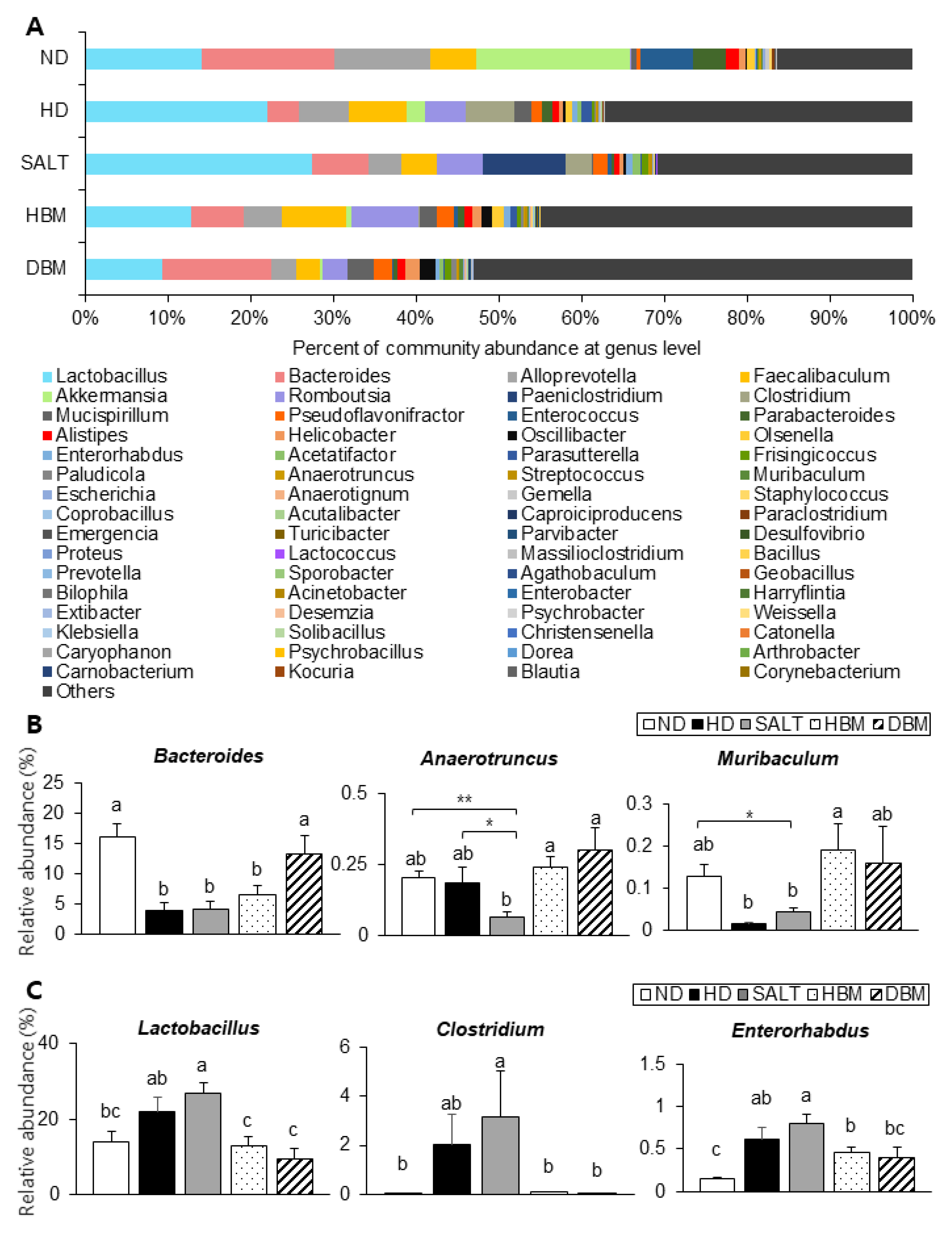
Next, the detailed alterations of gut microbiota community were further analyzed at the phylum and genus levels. At the phylum and genus levels, all groups exhibited different bacterial community structures. At the phylum level, Firmicutes and Bacteroidetes were dominant in all groups (Figure 7A). The Firmicutes-to-Bacteroidetes ratio (F/B ratio) tended to decrease in the HBM and DBM groups compared with the HD and SALT groups with and/or without statistical significance, while the HD and SALT groups showed significantly increased ratios compared with the ND group (Figure 7B). At the genus level, gut microbiota communities were either upregulated or downregulated based on the groups (Figure 8A). The levels of Bacteroides, Anaerotruncus, and Muribaculum were significantly downregulated in the HD and SALT groups compared with the ND group; however, those microbiota were significantly upregulated in the HBM and DBM groups (Figure 8B). On the other hand, the HD and SALT groups had significantly higher levels of Lactobacillus, Clostridium, and Enterorhabdus than the ND group, whereas those microbiota were markedly decreased in both Gochujang groups (Figure 8C). These observations suggest that Gochujang restores HD-induced gut microbiota dysbiosis and restructures gut microbiota communities.
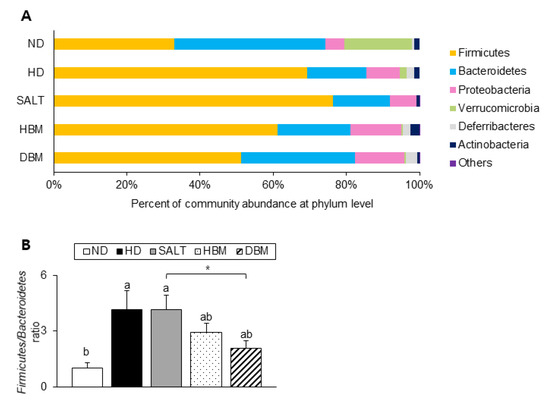
Figure 7.
Effect of Gochujang on the population structure of gut microbiota at phylum level in the HD-fed mice. (A) Microbial community at the phylum level in mice; (B) Firmicutes-to Bacteroidetes ratio in mice. Values are mean SEM. a–b means with the different letters on the bar (p < 0.05). (a > b). * p < 0.05. ND, normal diet group; HD, high-fat diet group; SALT, HD with 0.7% salt group; HBM, HD with 10% of HBM Gochujang group; DBM, HD with 10% of DBM Gochujang group.
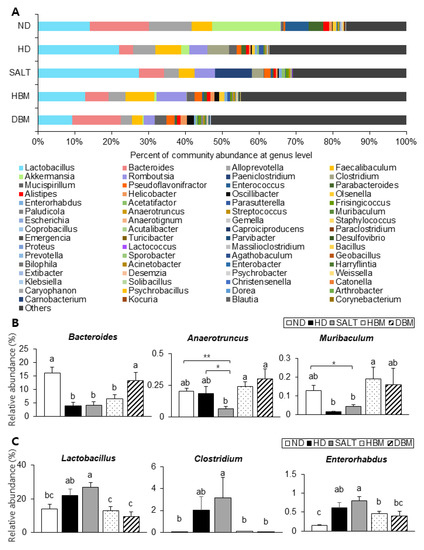
Figure 8.
Effect of Gochujang on the population structure of gut microbiota at genus level in the HD-fed mice. (A) Microbial community at the genus level in mice; The relative abundance of (B) Bacteroides, Muribaculum, Anaerotruncus; (C) Lactobacillus, Enterorhabdus, and Clostridium at genus level. Values are mean SEM. a–c means with the different letters on the bar (p < 0.05). (a > b > c). * p < 0.05 and ** p < 0.01. ND, normal diet group; HD, high-fat diet group; SALT, HD with 0.7% salt group; HBM, HD with 10% of HBM Gochujang group; DBM, HD with 10% of DBM Gochujang group.
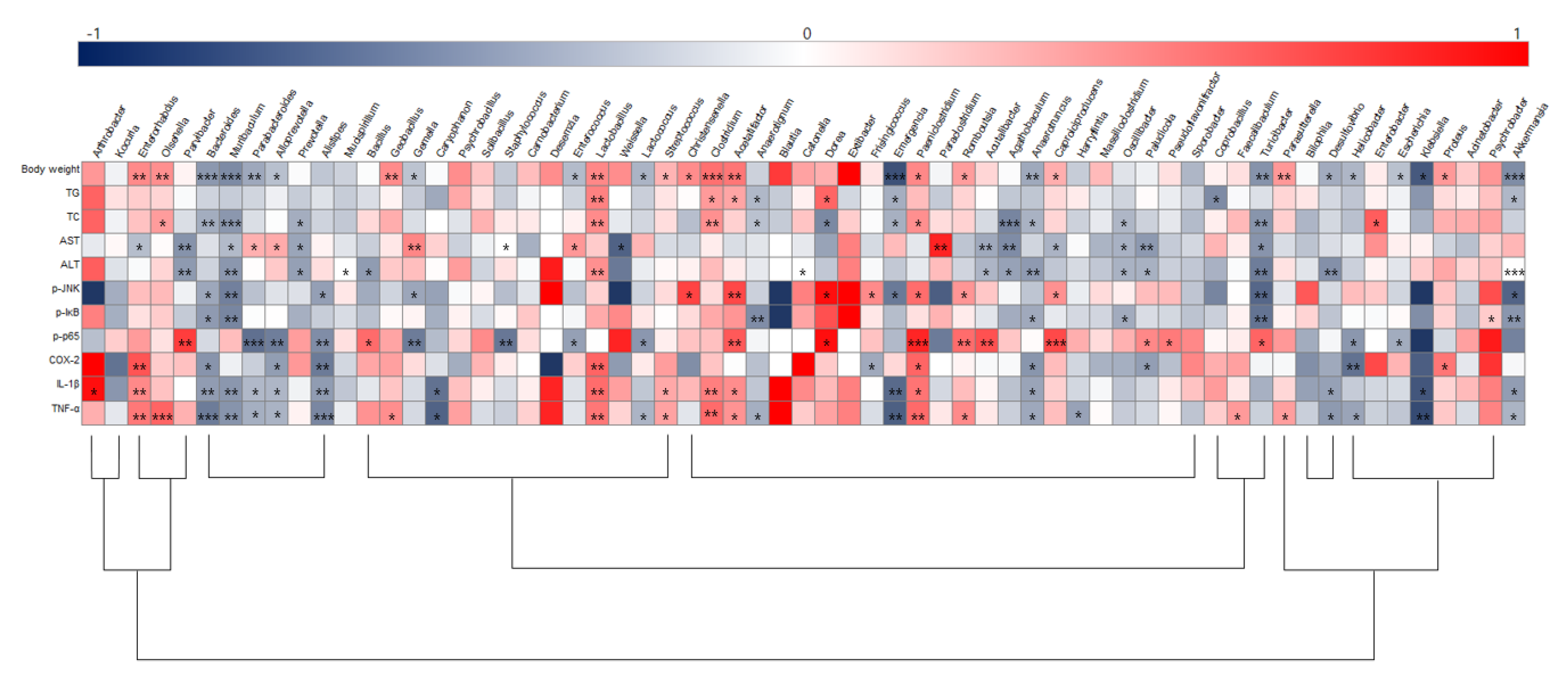
3.6. Correlation between Hepatic Inflammation and Key Gut Microbiota
To illustrate correlations between key physiological markers of hepatic inflammation and gut microbiota alterations at the genus level, Spearman’s correlation coefficient was analyzed (Figure 9). Akkermansia, Bacteroides, Anaerotruncus, Emergencia, and Turicibacter were negatively correlated with body weight and TG and TC levels, while Acetatifactor, Clostridium, Lactobacillus, Olsenella, Paeniclostridium, and Proteus were positively correlated with those parameters. Acutalibacter, Agathobaculum, Muribaculum, Oscillibacter, Paludicola, Parvibacter, Prevotella, and Turicibacter were negatively correlated with ALT and AST serum levels. Furthermore, negative correlations were identified between expression of the JNK/IκB/NF-κB pathway and/or Bacteroides, Muribaculum, and Turicibacter while Acetatifactor was positively correlated with p-JNK and NF-κB. Bacteroidetes, Alloprerovotela, Alistipes, and Anaerotruncus were negatively correlated with COX-2, IL-1β, and TNF-α, however, Enterorhabdus, Lactobacillus, and Clostridium were positively correlated with these parameters. Importantly, Anaerotruncus, Bacteroides, Muribaculum, Lactobacillus, Clostridium, and Enterorhabdus were closely connected with obesity as well as hepatic lipid accumulation and inflammation parameters.
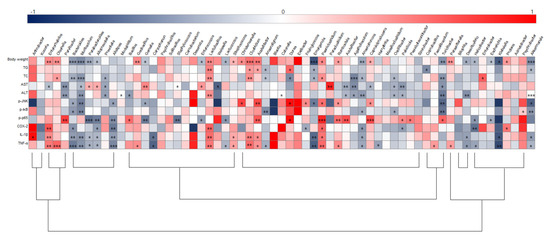
Figure 9.
Heatmap of spearman correlation coefficient between key intestinal microbiota and hepatic inflammation traits. The intensity of the color represents the degree of association (dark blue, negative correlation; red, positive correlation). * p < 0.05, ** p < 0.01, and *** p < 0.001. ND, normal diet group; HD, high-fat diet group; SALT, HD with 0.7% salt group; HBM, HD with 10% of HBM Gochujang group; DBM, HD with 10% of DBM Gochujang group.
4. Discussion
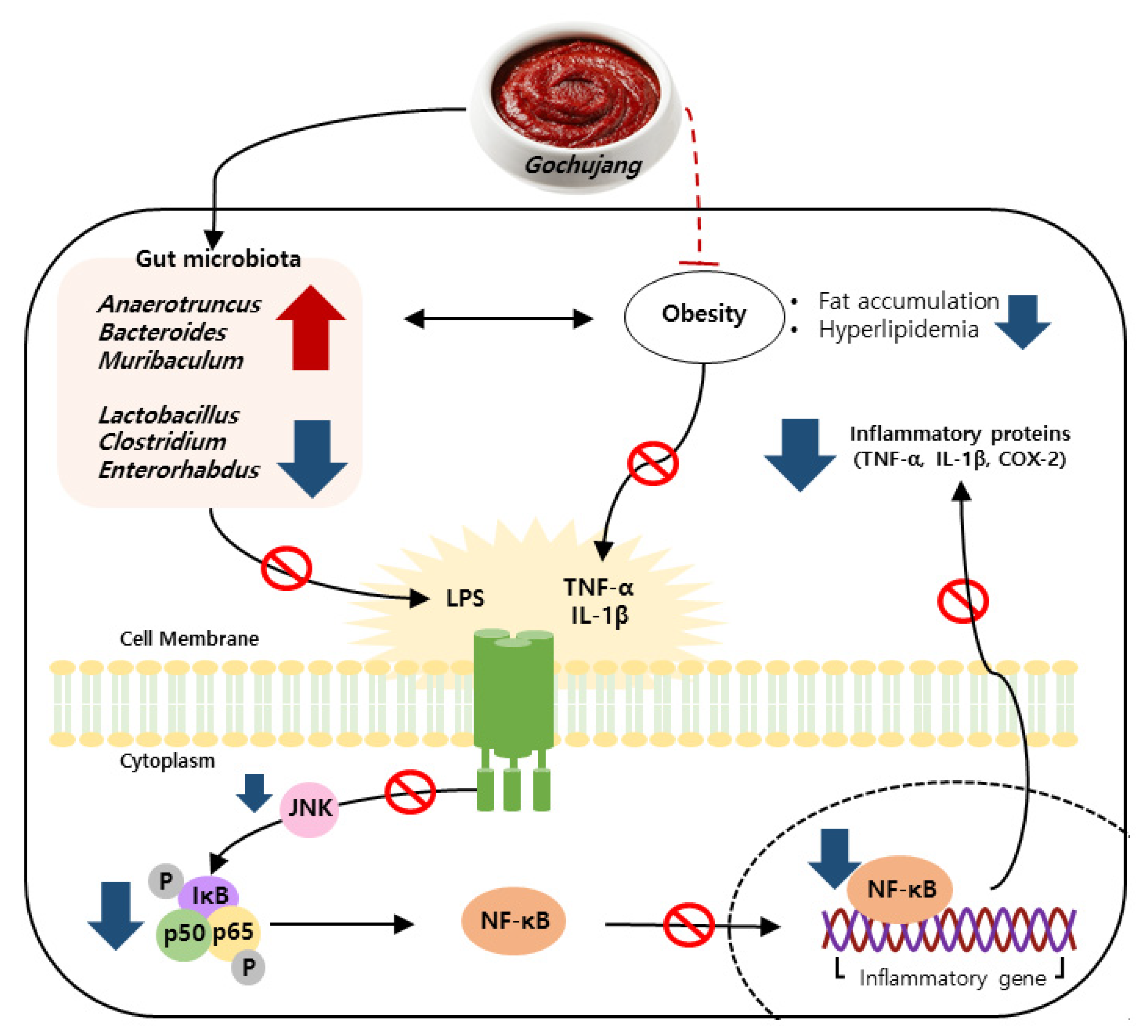
Persistent HD intake results in the activation of a pro-inflammatory response and imbalance of gut microbiota, leading to chronic inflammation [25,26,27]. As one of the commonly consumed KTFFs, Gochujang exerts multiple health benefits, including anti-inflammatory effects [28]. For example, Gochujang ameliorates colonic inflammation by suppressing TNF-α and IL-6 gene expressions and by recovering gut microbiota dysbiosis in DSS-derived IBD [12]. Regardless of many earlier findings, the positive functions of Gochujang have been controversial because of its high salt concentration. The present study found that Gochujang reduced hepatic inflammation by reducing fat accumulation, hyperlipidemia, inflammatory parameters, and JNK/IκB/NF-κB pathway activation (Figure 10). Moreover, HD-induced gut microbiota imbalance was also improved by Gochujang, and these alterations of gut microbiota were correlated with the improvement of hepatic inflammation. Lastly, the high salt level of Gochujang did not have an impact on those advantageous outcomes.
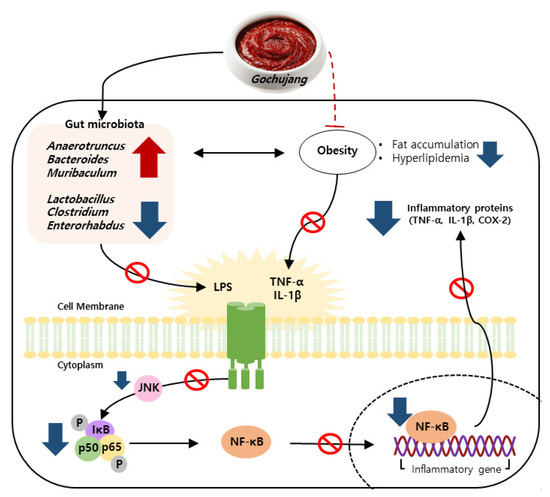
Figure 10.
Schematic depiction of the anti-hepatic inflammatory mechanism of Gochujang in HD induced obese mice.
Increased fat accumulation and lipid indicators upregulate inflammatory response and activate related signaling pathways [1]. Consistent with previous observations [10], the current study found that Gochujang reduced body weight, visceral fat volume, epididymal fat weight, and lipid levels in both the serums and livers of HD-induced obese mice. Furthermore, Gochujang strongly suppressed hepatic inflammatory cytokine levels, mRNA expression of inflammatory markers, and phosphorylation of JNK/IκB/NF-κB pathways. Indeed, Gochujang significantly decreased phosphorylation of JNK and IκB, but phosphorylation of NF-κB and expression of COX-2 protein were slightly reduced in HD-induced obese mice. As a key controller of COX-2 production, NF-κB plays an important role in the occurrences of inflammatory responses by translocating into the nucleus [21,22]. Therefore, future studies are needed to investigate Gochujang’s effect on NF-κB translocation to establish detailed underlying molecular mechanisms of Gochujang’s inhibitory effects on hepatic inflammation.
Recently, the roles of gut microbiota in the subject’s health and disease have been highlighted [29,30]. Importantly, gut microbiota play a role in the development of hepatic inflammation; thus, understanding the gut-liver axis in hepatic inflammation is important to improve the prevalence of inflammation-related liver disease, including steatohepatitis [31]. The present study demonstrated that Gochujang changed the diversity of gut microbiota and its composition; moreover, it decreased the gut microbiota-derived LPS level and the F/B ratio. According to previous studies, the Muribaculum genera is closely related to HD-induced obesity and hepatic steatosis and the Turicibacter genera is associated with liver fibrosis and non-alcohol fatty liver disease (NAFLD) [32,33]. These genera were significantly increased by Gochujang and had negative correlations with hepatic inflammatory and injury parameters, and lipid accumulation and JNK/IκB/NF-κB pathways, respectively. Consistent with previous studies, Lactobacillus levels increased when HD were consumed, but Gochujang showed a decreased Lactobacillus level similar to that of ND-fed mice [34]. This outcome might be because Gochujang alleviated gut dysbiosis due to HD and salt diets. Hence, the understanding of the exact functions of Lactobacillus in the association between gut microbiota and obesity-induced hepatic inflammation are essentially required in future studies.
The Korean Paradox proposes the possibility of the different effects of salt in fermented foods and the additive table salt [28]. Indeed, Gochujang-fed mice experienced improvements in DSS-induced IBD, while additive table salt-fed mice (same amount of salt in Gochujang) did not show any amelioration [12]. Furthermore, Doenjang, another signature KTFF, strongly improves high-salt-diet-induced blood pressure, regardless of its high level of salt [35]. The current study also observed that Gochujang showed a strong anti-inflammatory effect in hepatic inflammation throughout the above underlying mechanisms, while the supplementation of salt with HD accelerates hepatic inflammation and gut microbiota imbalance. Therefore, in addition to earlier findings, the present study also supports the distinct roles between additive salt and salt in KTFFs, allowing for critical future study directions, and including the understanding of different salt metabolisms between the salt in fermented foods and additive salt.
In conclusion, the present study demonstrated that Gochujang ameliorates hepatic inflammation by decreasing fat accumulation, dyslipidemia in serum and liver, production of pro-inflammatory parameters, and phosphorylation of the JNK/IκB/NF-κB pathway, but also by reshaping gut microbial composition in HD-induced obese mice. Moreover, Gochujang exerts those effects regardless of its high salt level. These data provided a scientific basis for understanding the effects of Gochujang on the interactions between gut microbiota and hepatic inflammation, and the Korean Paradox, regardless of its difference of micro bacteria composition.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11040911/s1, Table S1: Gochujang compositions title; Table S2: Diet compositions; Table S3: Primer list for PCR.
Author Contributions
Conceptualization, E.-J.L.; methodology, O.C.E., S.-J.J. and G.H.; validation, A.H. and E.-G.M.; formal analysis, E.-J.L.; investigation, Y.-S.C.; resources, Y.-S.C.; data curation, E.-B.S.; writing—original draft preparation, E.-J.L.; writing—review and editing, A.H; visualization, E.-J.L.; supervision, Y.-S.C.; project administration, A.H.; funding acquisition, S.-J.J. and G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Functional identification of Korean traditional soybean products (safety monitoring) project” under the Ministry of Agriculture, Food, and Rural Affairs, and was partly funded by Korea Agro-Fisheries and Food trade corporation in 2023.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Jeonbuk National University (JBNU 2021-0112).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Wullaert, A.; van Loo, G.; Heyninck, K.; Beyaert, R. Hepatic tumor necrosis factor signaling and nuclear factor-κB: Effects on liver homeostasis and beyond. Endocr. Rev. 2007, 28, 365–386. [Google Scholar] [CrossRef]
- Tarantino, G.; Caputi, A. JNKs, insulin resistance and inflammation: A possible link between NAFLD and coronary artery disease. World J. Gastroenterol. WJG 2011, 17, 3785. [Google Scholar] [CrossRef]
- Chun, J.; Choi, R.J.; Khan, S.; Lee, D.S.; Kim, Y.C.; Nam, Y.J.; Lee, D.U.; Kim, Y.S. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int. Immunopharmacol. 2012, 14, 375–383. [Google Scholar] [CrossRef]
- Kang, G.G.; Trevaskis, N.L.; Murphy, A.J.; Febbraio, M.A. Diet-induced gut dysbiosis and inflammation: Key drivers of obesity driven NASH. iScience 2022, 26, 105905. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Lee, J.-H.; Yun, D.-H.; Lee, Y.-M.; Kim, D.-K. Inhibitory effects of nodakenin on inflammation and cell death in lipopolysaccharide-induced liver injury mice. Phytomedicine 2021, 81, 153411. [Google Scholar] [CrossRef]
- Negrete-Romero, B.; Valencia-Olivares, C.; Baños-Dossetti, G.A.; Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Nutritional Contributions and Health Associations of Traditional Fermented Foods. Fermentation 2021, 7, 289. [Google Scholar] [CrossRef]
- Shin, H.W.; Jang, E.S.; Moon, B.S.; Lee, J.J.; Lee, D.E.; Lee, C.H.; Shin, C.S. Anti-obesity effects of Gochujang products prepared using rice koji and soybean meju in rats. J. Food Sci. Technol. 2016, 53, 1004–1013. [Google Scholar] [CrossRef]
- Yang, H.J.; Lee, Y.S.; Choi, I.S. Comparison of physicochemical properties and antioxidant activities of fermented soybean-based red pepper paste, Gochujang, prepared with five different red pepper (Capsicum annuum L.) varieties. J. Food Sci. Technol. 2018, 55, 792–801. [Google Scholar] [CrossRef]
- Mahoro, P.; Moon, H.-J.; Yang, H.-J.; Kim, K.-A.; Cha, Y.-S. Protective Effect of Gochujang on Inflammation in a DSS-Induced Colitis Rat Model. Foods 2021, 10, 1072. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Paramithiotis, S.; Shin, H.-S. Kimchi and Other Widely Consumed Traditional Fermented Foods of Korea: A Review. Front. Microbiol. 2016, 7, 1493. [Google Scholar] [CrossRef]
- Guidi, L.R.; Gloria, M.B.A. Bioactive amines in soy sauce: Validation of method, occurrence and potential health effects. Food Chem. 2012, 133, 323–328. [Google Scholar] [CrossRef]
- Tan, K.W.; Quaye, S.E.D.; Koo, J.R.; Lim, J.T.; Cook, A.R.; Dickens, B.L. Assessing the Impact of Salt Reduction Initiatives on the Chronic Disease Burden of Singapore. Nutrients 2021, 13, 1171. [Google Scholar] [CrossRef]
- Watanabe-Imai, K.; Harigai, M.; Sada, K.E.; Yamamura, M.; Fujii, T.; Dobashi, H.; Amano, K.; Ito, S.; Homma, S.; Kumagai, S.; et al. Clinical characteristics of and risk factors for serious infection in Japanese patients within six months of remission induction therapy for antineutrophil cytoplasmic antibody-associated vasculitis registered in a nationwide, prospective, inception cohort study. Mod. Rheumatol. 2017, 27, 646–651. [Google Scholar] [CrossRef]
- Park, J.; Kwock, C.K. Sodium intake and prevalence of hypertension, coronary heart disease, and stroke in Korean adults. J. Ethn. Foods 2015, 2, 92–96. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, Inflammation, and Metabolic Disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- dela Peña, A.; Leclercq, I.; Field, J.; George, J.; Jones, B.; Farrell, G. NF-κB Activation, Rather Than TNF, Mediates Hepatic Inflammation in a Murine Dietary Model of Steatohepatitis. Gastroenterology 2005, 129, 1663–1674. [Google Scholar] [CrossRef]
- Kodama, Y.; Brenner, D.A. c-Jun N-terminal kinase signaling in the pathogenesis of nonalcoholic fatty liver disease: Multiple roles in multiple steps. Hepatology 2009, 49, 6–8. [Google Scholar] [CrossRef]
- Caussy, C.; Hsu, C.; Lo, M.T.; Liu, A.; Bettencourt, R.; Ajmera, V.H.; Bassirian, S.; Hooker, J.; Sy, E.; Richards, L.; et al. Link between gut-microbiome derived metabolite and shared gene-effects with hepatic steatosis and fibrosis in NAFLD. Hepatology 2018, 68, 918–932. [Google Scholar] [CrossRef]
- Yu, Q.; Larson, D.F.; Slayback, D.; Lundeen, T.F.; Baxter, J.H.; Watson, R.R. Characterization of high-salt and high-fat diets on cardiac and vascular function in mice. Cardiovasc. Toxicol. 2004, 4, 37–46. [Google Scholar] [CrossRef]
- Tubbs, A.L.; Liu, B.; Rogers, T.D.; Sartor, R.B.; Miao, E.A. Dietary salt exacerbates experimental colitis. J. Immunol. 2017, 199, 1051–1059. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, Y.S.; Heo, N.J.; Lim, J.H.; Yang, S.Y.; Chung, G.E.; Kim, J.S. High Salt Intake Is Associated with Atrophic Gastritis with Intestinal MetaplasiaSalt and Intestinal Metaplasia. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1133–1138. [Google Scholar] [CrossRef]
- Mun, E.-G.; Cha, Y.-S. Korean Traditional Fermented Foods (KTFFs): Antiobesity Effects and Salt Paradox. In Chemistry of Korean Foods and Beverages; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2019; Volume 1303, pp. 121–134. [Google Scholar]
- Wu, G.D.; Bushmanc, F.D.; Lewis, J.D. Diet, the human gut microbiota, and IBD. Anaerobe 2013, 24, 117–120. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Ding, J.H.; Jin, Z.; Yang, X.X.; Lou, J.; Shan, W.X.; Hu, Y.X.; Du, Q.; Liao, Q.S.; Xie, R.; Xu, J.Y. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J. Gastroenterol. 2020, 26, 6141–6162. [Google Scholar] [CrossRef]
- Ye, X.; Huang, D.; Dong, Z.; Wang, X.; Ning, M.; Xia, J.; Shen, S.; Wu, S.; Shi, Y.; Wang, J.; et al. FXR Signaling-Mediated Bile Acid Metabolism Is Critical for Alleviation of Cholesterol Gallstones by Lactobacillus Strains. Microbiol. Spectr. 2022, 10, e00518–e00522. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, H.; Liu, Z.; Sun, X.; Zhang, D.; Wang, S.; Xu, Y.; Zhang, G.; Wang, D. Modulation of Gut Microbiota by Fucoxanthin During Alleviation of Obesity in High-Fat Diet-Fed Mice. J. Agric. Food Chem. 2020, 68, 5118–5128. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Navarro, T.; Salazar, N.; Gutiérrez-Díaz, I.; de Los Reyes-Gavilán, C.G.; Gueimonde, M.; González, S. Different Intestinal Microbial Profile in Over-Weight and Obese Subjects Consuming a Diet with Low Content of Fiber and Antioxidants. Nutrients 2017, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Mun, E.-G.; Park, J.E.; Cha, Y.-S. Effects of Doenjang, a Traditional Korean Soybean Paste, with High-Salt Diet on Blood Pressure in Sprague–Dawley Rats. Nutrients 2019, 11, 2745. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).