Sarcoidosis, Mycobacterium paratuberculosis and Noncaseating Granulomas: Who Moved My Cheese
Abstract
1. Introduction
2. Mycobacterium avium subsp. paratuberculosis (MAP)
3. MAP Is Difficult to Detect
4. Sarcoid Epidemiology
5. Genetic Risk for Sarcoidosis Overlaps with Risk for Mycobacterial Infection
6. SLC11a1 (NRAMP) and Sarcoidosis
7. HLA Alleles and Sarcoidosis/Tuberculosis Risk
8. CARD15 (NOD2)—Early Onset Sarcoidosis (EOS) and Blau Syndrome
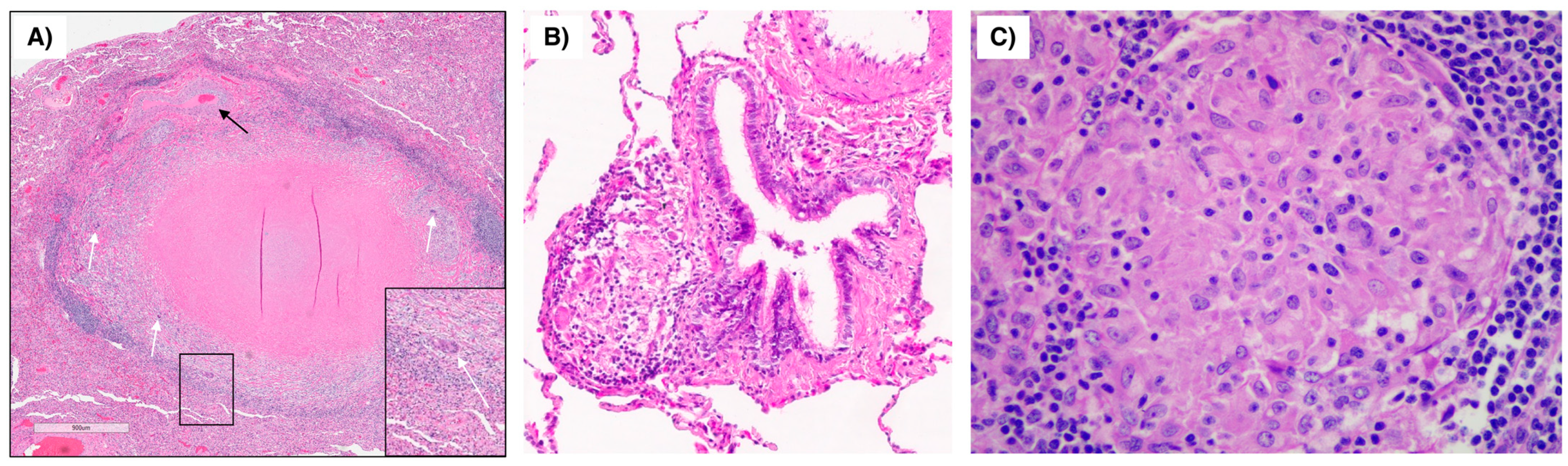
9. An Illustrative Case of Cardiac Sarcoidosis
10. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Spagnolo, P. Sarcoidosis: A Critical Review of History and Milestones. Clin. Rev. Allergy Immunol. 2015, 49, 1–5. [Google Scholar] [CrossRef]
- Gupta, D. Tuberculosis and sarcoidosis: The continuing enigma. Lung India 2009, 26, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.D. Early Clinical Use Of The X-Ray. Trans. Am. Clin. Climatol. Assoc. 2016, 127, 341–349. [Google Scholar] [PubMed]
- Citron, K.M.; Scadding, J.G. Stenosing non-caseating tuberculosis (sarcoidosis) of the bronchi. Thorax 1957, 12, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.; Stanford, J.L. Slow bacterial infections or autoimmunity? Immunol. Today 1992, 13, 160–164. [Google Scholar] [CrossRef]
- Gupta, D.; Agarwal, R.; Aggarwal, A.N.; Jindal, S.K. Molecular evidence for the role of mycobacteria in sarcoidosis: A meta-analysis. Eur. Respir. J. 2007, 30, 508–516. [Google Scholar] [CrossRef]
- Brownell, I.; Ramírez-Valle, F.; Sanchez, M.; Prystowsky, S. Evidence for mycobacteria in sarcoidosis. Am. J. Respir. Cell Mol. Biol. 2011, 45, 899–905. [Google Scholar] [CrossRef]
- Chapman, J.S.; Speight, M. Further studies of mycobacterial antibodies in the sera of sarcoidosis patients. Acta Med. Scand. 1964, 176 (Suppl. 425), 61–67. [Google Scholar] [CrossRef]
- Berger, H.W.; Zaldivar, C.; Chusid, E.L. Anonymous mycobacteria in the etiology of sarcoidosis. Ann. Intern. Med. 1968, 68, 872–874. [Google Scholar] [CrossRef]
- el-Zaatari, F.A.; Naser, S.A.; Markesich, D.C.; Kalter, D.C.; Engstand, L.; Graham, D.Y. Identification of Mycobacterium avium complex in sarcoidosis. J. Clin. Microbiol. 1996, 34, 2240–2245. [Google Scholar] [CrossRef]
- Llanos, O.; Hamzeh, N. Sarcoidosis. Med. Clin. N. Am. 2019, 103, 527–534. [Google Scholar] [CrossRef]
- Mehta, A.C.; Ali, S.R. Mnemonic for the differential diagnosis of non-caseating granulomas. Sarcoidosis Vasc. Diffus. Lung Dis. 2017, 34, 200–207. [Google Scholar] [CrossRef]
- Hunter, R.L.; Olsen, M.R.; Jagannath, C.; Actor, J.K. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann. Clin. Lab. Sci. 2006, 36, 371–386. [Google Scholar] [PubMed]
- Schabbing, R.W.; Garcia, A.; Hunter, R.L. Characterization of the trehalose 6,6’-dimycolate surface monolayer by scanning tunneling microscopy. Infect. Immun. 1994, 62, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L. Tuberculosis as a three-act play: A new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis 2016, 97, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Litinsky, I.; Elkayam, O.; Flusser, G.; Segal, R.; Yaron, M.; Caspi, D. Sarcoidosis: TB or not TB? Ann. Rheum. Dis. 2002, 61, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M.; Alexander, D.C.; Turenne, C.Y.; Girard, C.; Behr, M.A. Evaluation of in situ methods used to detect Mycobacterium avium subsp. paratuberculosis in samples from patients with Crohn’s disease. J. Clin. Microbiol. 2006, 44, 2942–2950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sechi, L.A.; Dow, C.T. Mycobacterium avium ss. paratuberculosis Zoonosis—The Hundred Year War—Beyond Crohn’s Disease. Front. Immunol. 2015, 6, 96. [Google Scholar] [CrossRef]
- Naser, S.A.; Ghobrial, G.; Romero, C.; Valentine, J.F. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet 2004, 364, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Naser, S.A.; Schwartz, D.; Shafran, I. Isolation of Mycobacterium avium subsp paratuberculosis from breast milk of Crohn’s disease patients. Am. J. Gastroenterol. 2000, 95, 1094–1095. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, R.J. Crohn’s disease and the mycobacterioses: A review and comparison of two disease entities. Clin. Microbiol. Rev. 1989, 2, 90–117. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.D.; Chiodini, R.J. Serologic reactivity against Mycobacterium paratuberculosis antigens in patients with sarcoidosis. Sarcoidosis 1993, 10, 32–35. [Google Scholar] [PubMed]
- Schenkel, A.R.; Mitchell, J.D.; Cool, C.D.; Bai, X.; Groshong, S.; Koelsch, T.; Verma, D.; Ordway, D.; Chan, E.D. Characterization of Immune Cells From the Lungs of Patients With Chronic Non-Tuberculous Mycobacteria or Pseudomonas aeruginosa Infection. Immune Network 2022, 22, e27. [Google Scholar]
- Biet, F.; Boschiroli, M.L.; Thorel, M.F.; Guilloteau, L.A. Zoonotic aspects of Mycobacterium bovis and Mycobacterium avium-intracellulare complex (MAC). Vet. Res. 2005, 36, 411–436. [Google Scholar] [CrossRef]
- Whittington, R.J.; Sergeant, E.S. Progress towards understanding the spread, detection and control of Mycobacterium avium subsp paratuberculosis in animal populations. Aust. Vet. J. 2001, 79, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Lombard, J.E.; Gardner, I.A.; Jafarzadeh, S.R.; Fossler, C.P.; Harris, B.; Capsel, R.T.; Wagner, B.A.; Johnson, W.O. Herd-level prevalence of Mycobacterium avium subsp. paratuberculosis infection in United States dairy herds in 2007. Prev. Vet. Med. 2013, 108, 234–238. [Google Scholar] [CrossRef]
- Garvey, M. Mycobacterium avium paratuberculosis: A Disease Burden on the Dairy Industry. Animals 2020, 10, 1773. [Google Scholar] [CrossRef]
- Beumer, A.; King, D.; Donohue, M.; Mistry, J.; Covert, T.; Pfaller, S. Detection of Mycobacterium avium subsp. paratuberculosis in drinking water and biofilms by quantitative PCR. Appl. Environ. Microbiol. 2010, 76, 7367–7370. [Google Scholar] [CrossRef]
- Fridriksdottir, V.; Gunnarsson, E.; Sigurdarson, S.; Gudmundsdottir, K.B. Paratuberculosis in Iceland: Epidemiology and control measures, past and present. Vet. Microbiol. 2000, 77, 263–267. [Google Scholar] [CrossRef]
- Whittington, R.J.; Taragel, C.A.; Ottaway, S.; Marsh, I.; Seaman, J.; Fridriksdottir, V. Molecular epidemiological confirmation and circumstances of occurrence of sheep (S) strains of Mycobacterium avium subsp. paratuberculosis in cases of paratuberculosis in cattle in Australia and sheep and cattle in Iceland. Vet. Microbiol. 2001, 79, 311–322. [Google Scholar] [CrossRef]
- Dow, C.T.; Alvarez, B.L. Mycobacterium paratuberculosis zoonosis is a One Health emergency. Ecohealth 2022, 19, 164–174. [Google Scholar] [CrossRef]
- Chamberlin, W.; Borody, T.; Naser, S. MAP-associated Crohn’s disease MAP, Koch’s postulates, causality and Crohn’s disease. Dig. Liver Dis. 2007, 39, 792–794. [Google Scholar] [CrossRef]
- Hansen, R.; Thomson, J.M.; El-Omar, E.M.; Hold, G.L. The role of infection in the aetiology of inflammatory bowel disease. J. Gastroenterol. 2010, 45, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.B.; Barletta, R.G. Mycobacterium avium subsp. paratuberculosis in Veterinary Medicine. Clin. Microbiol. Rev. 2001, 14, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Hines, M.E., II.; Styer, E.L. Preliminary characterization of chemically generated Mycobacterium avium subsp. paratuberculosis cell wall deficient forms (spheroplasts). Vet. Microbiol. 2003, 95, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Lamont, E.A.; Bannantine, J.P.; Armién, A.; Ariyakumar, D.S.; Sreevatsan, S. Identification and characterization of a spore-like morphotype in chronically starved Mycobacterium avium subsp. paratuberculosis cultures. PLoS ONE 2012, 7, e30648. [Google Scholar] [CrossRef] [PubMed]
- Waddell, L.; Rajić, A.; Stärk, K.; McEwen, S.A. Mycobacterium avium ssp. paratuberculosis detection in animals, food, water and other sources or vehicles of human exposure: A scoping review of the existing evidence. Prev. Vet. Med. 2016, 132, 32–48. [Google Scholar] [CrossRef]
- Mullan, W. Are we closer to understanding why viable cells of Mycobacterium avium subsp. paratuberculosis are still being reported in pasteurised milk? Int. J. Dairy Technol. 2019, 72, 332–344. [Google Scholar] [CrossRef]
- Gill, C.O.; Saucier, L.; Meadus, W.J. Mycobacterium avium subsp. paratuberculosis in dairy products, meat, and drinking water. J. Food Prot. 2011, 74, 480–499. [Google Scholar] [CrossRef]
- Eltholth, M.M.; Marsh, V.R.; Van Winden, S.; Guitian, F.J. Contamination of food products with Mycobacterium avium paratuberculosis: A systematic review. J. Appl. Microbiol. 2009, 107, 1061–1071. [Google Scholar] [CrossRef]
- Van Brandt, L.; Coudijzer, K.; Herman, L.; Michiels, C.; Hendrickx, M.; Vlaemynck, G. Survival of Mycobacterium avium ssp. paratuberculosis in yoghurt and in commercial fermented milk products containing probiotic cultures. J. Appl. Microbiol. 2011, 110, 1252–1261. [Google Scholar] [CrossRef]
- Alonso-Hearn, M.; Molina, E.; Geijo, M.; Vazquez, P.; Sevilla, I.; Garrido, J.M.; Juste, R.A. Isolation of Mycobacterium avium subsp. paratuberculosis from muscle tissue of naturally infected cattle. Foodborne Pathog. Dis. 2009, 6, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Hammer, P.; Walte, H.G.; Matzen, S.; Hensel, J.; Kiesner, C. Inactivation of Mycobacterium avium subsp. paratuberculosis during cooking of hamburger patties. J. Food Prot. 2013, 76, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.; Bartos, M.; Kralik, P.; Pavlik, I. Mycobacterium avium subsp. paratuberculosis in powdered infant milk: Paratuberculosis in cattle—The public health problem to be solved. Vet. Med. 2005, 50, 327–335. [Google Scholar] [CrossRef]
- Hruska, K.; Slana, I.; Kralik, P.; Pavlik, I. Mycobacterium avium subsp. paratuberculosis in powdered infant milk: F57 competitive real time PCR. Vet. Med. 2011, 56, 226–230. [Google Scholar] [CrossRef]
- Botsaris, G.; Swift, B.M.; Slana, I.; Liapi, M.; Christodoulou, M.; Hatzitofi, M.; Christodoulou, V.; Rees, C.E. Detection of viable Mycobacterium avium subspecies paratuberculosis in powdered infant formula by phage-PCR and confirmed by culture. Int. J. Food Microbiol. 2016, 216, 91–94. [Google Scholar] [CrossRef]
- Sung, N.; Collins, M.T. Effect of three factors in cheese production (pH, salt, and heat) on Mycobacterium avium subsp. paratuberculosis viability. Appl. Environ. Microbiol. 2000, 66, 1334–1339. [Google Scholar] [CrossRef]
- Spahr, U.; Schafroth, K. Fate of Mycobacterium avium subsp. paratuberculosis in Swiss hard and semihard cheese manufactured from raw milk. Appl. Environ. Microbiol. 2001, 67, 4199–4205. [Google Scholar] [CrossRef]
- Donaghy, J.A.; Totton, N.L.; Rowe, M.T. Persistence of Mycobacterium paratuberculosis during manufacture and ripening of cheddar cheese. Appl. Environ. Microbiol. 2004, 70, 4899–4905. [Google Scholar] [CrossRef]
- Galiero, A.; Fratini, F.; Mataragka, A.; Turchi, B.; Nuvoloni, R.; Ikonomopoulos, J.; Cerri, D. Detection of Mycobacterium avium subsp. paratuberculosis in cheeses from small ruminants in Tuscany. Int. J. Food Microbiol. 2016, 217, 195–199. [Google Scholar] [CrossRef]
- Barsi, F.; Dalzini, E.; Russo, S.; Cosciani-Cunico, E.; Monastero, P.; Arrigoni, N.; Garbarino, C.A.; Cortimiglia, C.; Losio, M.N.; Ricchi, M. Isothermal inactivation of Mycobacterium avium subsp. paratuberculosis in curd simulating the stretching phase in pasta-filata cheese process. Front. Microbiol. 2022, 13, 1052222. [Google Scholar] [CrossRef]
- Dow, C.T.; Sechi, L.A. Cows Get Crohn’s Disease and They’re Giving Us Diabetes. Microorganisms 2019, 7, 466. [Google Scholar] [CrossRef]
- Ekundayo, T.C.; Okoh, A.I. Systematic Assessment of Mycobacterium avium Subspecies Paratuberculosis Infections from 1911-2019: A Growth Analysis of Association with Human Autoimmune Diseases. Microorganisms 2020, 8, 1212. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.T. M. paratuberculosis Heat Shock Protein 65 and Human Diseases: Bridging Infection and Autoimmunity. Autoimmune Dis. 2012, 2012, 150824. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Park, H.-T.; Park, S.; Lee, J.H.; Kim, D.; Yoo, H.S.; Kim, D. A Machine Learning Approach Reveals a Microbiota Signature for Infection with Mycobacterium avium subsp. paratuberculosis in Cattle. Microbiol. Spectr. 2023, 11, e0313422. [Google Scholar] [CrossRef] [PubMed]
- Elmagzoub, W.A.; Idris, S.M.; Isameldin, M.; Arabi, N.; Abdo, A.; Ibrahim, M.; Khan, M.A.A.; Tanneberger, F.; Bakhiet, S.M.; Okuni, J.B.; et al. Mycobacterium avium subsp. paratuberculosis and microbiome profile of patients in a referral gastrointestinal diseases centre in the Sudan. PLoS ONE 2022, 17, e0266533. [Google Scholar] [CrossRef]
- Xia, X.; Chen, J.; Cheng, Y.; Chen, F.; Lu, H.; Liu, J.; Wang, L.; Pu, F.; Wang, Y.; Liu, H.; et al. Comparative analysis of the lung microbiota in patients with respiratory infections, tuberculosis, and lung cancer: A preliminary study. Front. Cell. Infect. Microbiol. 2022, 12, 1024867. [Google Scholar] [CrossRef]
- Knudsen, K.S.; Lehmann, S.; Nielsen, R.; Tangedal, S.; Paytuvi-Gallart, A.; Sanseverino, W.; Martinsen, E.M.H.; Hiemstra, P.S.; Eagan, T.M. The lower airways microbiota and antimicrobial peptides indicate dysbiosis in sarcoidosis. Microbiome 2022, 10, 175. [Google Scholar] [CrossRef]
- Arkema, E.V.; Cozier, Y.C. Sarcoidosis epidemiology: Recent estimates of incidence, prevalence and risk factors. Curr. Opin. Pulm. Med. 2020, 26, 527–534. [Google Scholar] [CrossRef]
- Hena, K.M. Sarcoidosis Epidemiology: Race Matters. Front. Immunol. 2020, 11, 537382. [Google Scholar] [CrossRef]
- Erdal, B.S.; Clymer, B.D.; Yildiz, V.O.; Julian, M.W.; Crouser, E.D. Unexpectedly high prevalence of sarcoidosis in a representative U.S. Metropolitan population. Respir. Med. 2012, 106, 893–899. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Kim, H.M.; Kim, Y.J.; Song, J.W. Prevalence and incidence of sarcoidosis in Korea: A nationwide population-based study. Respir. Res. 2018, 19, 158. [Google Scholar] [CrossRef] [PubMed]
- Fidler, L.M.; Balter, M.; Fisher, J.H.; To, T.; Stanbrook, M.B.; Gershon, A. Epidemiology and health outcomes of sarcoidosis in a universal healthcare population: A cohort study. Eur. Respir. J. 2019, 54, 1900444. [Google Scholar] [CrossRef] [PubMed]
- Seedahmed, M.I.; Baugh, A.D.; Albirair, M.T.; Luo, Y.; Chen, J.; McCulloch, C.E.; Whooley, M.A.; Koth, L.L.; Arjomandi, M. Epidemiology of Sarcoidosis in U.S. Veterans from 2003 to 2019. Ann. Am. Thorac. Soc. 2023, E-pub. [Google Scholar] [CrossRef]
- Arkema, E.V.; Grunewald, J.; Kullberg, S.; Eklund, A.; Askling, J. Sarcoidosis incidence and prevalence: A nationwide register-based assessment in Sweden. Eur. Respir. J. 2016, 48, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Cozier, Y.C. Assessing the worldwide epidemiology of sarcoidosis: Challenges and future directions. Eur. Respir. J. 2016, 48, 1545–1548. [Google Scholar] [CrossRef]
- Jamilloux, Y.; Maucort-Boulch, D.; Kerever, S.; Gerfaud-Valentin, M.; Broussolle, C.; Eb, M.; Valeyre, D.; Seve, P. Sarcoidosis-related mortality in France: A multiple-cause-of-death analysis. Eur. Respir. J. 2016, 48, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Rossides, M.; Grunewald, J.; Eklund, A.; Kullberg, S.; Di Giuseppe, D.; Askling, J.; Arkema, E.V. Familial aggregation and heritability of sarcoidosis: A Swedish nested case-control study. Eur. Respir. J. 2018, 52, 1800385. [Google Scholar] [CrossRef]
- Swigris, J.J.; Olson, A.L.; Huie, T.J.; Fernandez-Perez, E.R.; Solomon, J.; Sprunger, D.; Brown, K.K. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am. J. Respir. Crit. Care Med. 2011, 183, 1524–1530. [Google Scholar] [CrossRef]
- Dubaniewicz, A.; Jamieson, S.E.; Dubaniewicz-Wybieralska, M.; Fakiola, M.; Miller, E.N.; Blackwell, J.M. Association between SLC11A1 (formerly NRAMP1) and the risk of sarcoidosis in Poland. Eur. J. Hum. Genet. 2005, 13, 829–834. [Google Scholar] [CrossRef]
- Canonne-Hergaux, F.; Gruenheid, S.; Govoni, G.; Gros, P. The Nramp1 protein and its role in resistance to infection and macrophage function. Proc. Assoc. Am. Physicians 1999, 111, 283–289. [Google Scholar] [CrossRef]
- Lapham, A.S.; Phillips, E.S.; Barton, C.H. Transcriptional control of Nramp1: A paradigm for the repressive action of c-Myc. Biochem. Soc. Trans. 2004, 32 Pt 6, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, S.; Seu, P.; Goss, J.A. The natural resistance-associated macrophage protein 1 Slc11a1 (formerly Nramp1) and iron metabolism in macrophages. Microbes Infect. 2002, 4, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Hackam, D.J.; Rotstein, O.D.; Zhang, W.; Gruenheid, S.; Gros, P.; Grinstein, S. Host resistance to intracellular infection: Mutation of natural resistance-associated macrophage protein 1 (Nramp1) impairs phagosomal acidification. J. Exp. Med. 1998, 188, 351–364. [Google Scholar] [CrossRef]
- Stienstra, Y.; van der Werf, T.S.; Oosterom, E.; Nolte, I.M.; van der Graaf, W.T.; Etuaful, S.; Raghunathan, P.L.; Whitney, E.A.; Ampadu, E.O.; Asamoa, K.; et al. Susceptibility to Buruli ulcer is associated with the SLC11A1 (NRAMP1) D543N polymorphism. Genes Immun. 2006, 7, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Brochado, M.J.; Gatti, M.F.; Zago, M.A.; Roselino, A.M. Association of the solute carrier family 11 member 1 gene polymorphisms with susceptibility to leprosy in a Brazilian sample. Mem. Inst. Oswaldo Cruz 2016, 111, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, Y.; Wang, Z.; He, X.; Wang, L.; Yuan, D.; He, Y.; Jin, T.; He, S. Association of SLC11A1 Polymorphisms With Tuberculosis Susceptibility in the Chinese Han Population. Front. Genet. 2022, 13, 899124. [Google Scholar] [CrossRef]
- Shahzad, F.; Bashir, N.; Ali, A.; Nadeem, A.; Ammar, A.; Kashif, M.; Javaid, K.; Jahan, S.; Tahir, R.; Rizwan, M.; et al. SLC11A1 genetic variation and low expression may cause immune response impairment in TB patients. Genes Immun. 2022, 23, 85–92. [Google Scholar] [CrossRef]
- Ruiz-Larrañaga, O.; Garrido, J.M.; Manzano, C.; Iriondo, M.; Molina, E.; Gil, A.; Koets, A.P.; Rutten, V.P.; Juste, R.A.; Estonba, A. Identification of single nucleotide polymorphisms in the bovine solute carrier family 11 member 1 (SLC11A1) gene and their association with infection by Mycobacterium avium subspecies paratuberculosis. J. Dairy Sci. 2010, 93, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Korou, L.M.; Liandris, E.; Gazouli, M.; Ikonomopoulos, J. Investigation of the association of the SLC11A1 gene with resistance/sensitivity of goats (Capra hircus) to paratuberculosis. Vet. Microbiol. 2010, 144, 353–358. [Google Scholar] [CrossRef]
- Purdie, A.C.; Plain, K.M.; Begg, D.J.; de Silva, K.; Whittington, R.J. Candidate gene and genome-wide association studies of Mycobacterium avium subsp. paratuberculosis infection in cattle and sheep: A review. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 197–208. [Google Scholar] [CrossRef]
- Roupie, V.; Rosseels, V.; Piersoel, V.; Zinniel, D.K.; Barletta, R.G.; Huygen, K. Genetic resistance of mice to Mycobacterium paratuberculosis is influenced by Slc11a1 at the early but not at the late stage of infection. Infect. Immun. 2008, 76, 2099–2105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ates, O.; Dalyan, L.; Müsellim, B.; Hatemi, G.; Türker, H.; Ongen, G.; Hamuryudan, V.; Topal-Sarikaya, A. NRAMP1 (SLC11A1) gene polymorphisms that correlate with autoimmune versus infectious disease susceptibility in tuberculosis and rheumatoid arthritis. Int. J. Immunogenet. 2009, 36, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Sechi, L.A.; Gazouli, M.; Sieswerda, L.E.; Molicotti, P.; Ahmed, N.; Ikonomopoulos, J.; Scanu, A.M.; Paccagnini, D.; Zanetti, S. Relationship between Crohn’s disease, infection with Mycobacterium avium subspecies paratuberculosis and SLC11A1 gene polymorphisms in Sardinian patients. World J. Gastroenterol. 2006, 12, 7161–7164. [Google Scholar] [CrossRef] [PubMed]
- Kotlowski, R.; Bernstein, C.N.; Silverberg, M.S.; Krause, D.O. Population-based case-control study of alpha 1-antitrypsin and SLC11A1 in Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1112–1117. [Google Scholar] [CrossRef]
- Kotze, M.J.; de Villiers, J.N.; Rooney, R.N.; Grobbelaar, J.J.; Mansvelt, E.P.; Bouwens, C.S.; Carr, J.; Stander, I.; du Plessis, L. Analysis of the NRAMP1 gene implicated in iron transport: Association with multiple sclerosis and age effects. Blood Cells Mol. Dis. 2001, 27, 44–53. [Google Scholar] [CrossRef]
- Takahashi, K.; Satoh, J.; Kojima, Y.; Negoro, K.; Hirai, M.; Hinokio, Y.; Kinouchi, Y.; Suzuki, S.; Matsuura, N.; Shimosegawa, T.; et al. Promoter polymorphism of SLC11A1 (formerly NRAMP1) confers susceptibility to autoimmune type 1 diabetes mellitus in Japanese. Tissue Antigens 2004, 63, 231–236. [Google Scholar] [CrossRef]
- Malkova, A.; Starshinova, A.; Zinchenko, Y.; Basantsova, N.; Mayevskaya, V.; Yablonskiy, P.; Shoenfeld, Y. The opposite effect of human leukocyte antigen genotypes in sarcoidosis and tuberculosis: A narrative review of the literature. ERJ. Open Res. 2020, 6, 00155–02020. [Google Scholar] [CrossRef]
- Grunewald, J.; Spagnolo, P.; Wahlström, J.; Eklund, A. Immunogenetics of Disease-Causing Inflammation in Sarcoidosis. Clin. Rev. Allergy Immunol. 2015, 49, 19–35. [Google Scholar] [CrossRef]
- Saltini, C.; Pallante, M.; Puxeddu, E.; Contini, S.; Voorter, C.E.; Drent, M.; Amicosante, M.M. avium binding to HLA-DR expressed alleles in silico: A model of phenotypic susceptibility to sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2008, 25, 100–116. [Google Scholar]
- Rossman, M.D.; Thompson, B.; Frederick, M.; Iannuzzi, M.C.; Rybicki, B.A.; Pander, J.P.; Newman, L.S.; Rose, C.; Magira, E.; Monos, D. ACCESS Group. HLA and environmental interactions in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2008, 25, 125–132. [Google Scholar]
- Rossman, M.D.; Thompson, B.; Frederick, M.; Maliarik, M.; Iannuzzi, M.C.; Rybicki, B.A.; Pandey, J.P.; Newman, L.S.; Magira, E.; Beznik-Cizman, B.; et al. ACCESS Group. HLA-DRB1*1101: A significant risk factor for sarcoidosis in blacks and whites. Am. J. Hum. Genet. 2003, 73, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, J.; Eklund, A. Löfgren’s syndrome: Human leukocyte antigen strongly influences the disease course. Am. J. Respir. Crit. Care Med. 2009, 179, 307–312. [Google Scholar] [CrossRef]
- Garman, L.; Pezant, N.; Pastori, A.; Savoy, K.A.; Li, C.; Levin, A.M.; Iannuzzi, M.C.; Rybicki, B.A.; Adrianto, I.; Montgomery, C.G. Genome-Wide Association Study of Ocular Sarcoidosis Confirms HLA Associations and Implicates Barrier Function and Autoimmunity in African Americans. Ocul. Immunol. Inflamm. 2021, 29, 244–249. [Google Scholar] [CrossRef]
- Oswald-Richter, K.; Sato, H.; Hajizadeh, R.; Shepherd, B.E.; Sidney, J.; Sette, A.; Newman, L.S.; Drake, W.P. Mycobacterial ESAT-6 and katG are recognized by sarcoidosis CD4+ T cells when presented by the American sarcoidosis susceptibility allele, DRB1*1101. J. Clin. Immunol. 2010, 30, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, B.A.; Garman, L.; Cejda, N.; Pezant, N.; Rasmussen, A.; Rybicki, B.A.; Levin, A.M.; Benchek, P.; Seshadri, C.; Mayanja-Kizza, H.; et al. Novel HLA associations with outcomes of Mycobacterium tuberculosis exposure and sarcoidosis in individuals of African ancestry using nearest-neighbor feature selection. Genet. Epidemiol. 2022, 46, 463–474. [Google Scholar] [CrossRef]
- Grosser, M.; Luther, T.; Fuessel, M.; Bickhardt, J.; Magdolen, V.; Baretton, G. Clinical course of sarcoidosis in dependence on HLA-DRB1 allele frequencies, inflammatory markers, and the presence of M. tuberculosis DNA fragments. Sarcoidosis Vasc. Diffus. Lung Dis. 2005, 22, 66–74. [Google Scholar]
- Inohara, N.; Ogura, Y.; Fontalba, A.; Gutierrez, O.; Pons, F.; Crespo, J.; Fukase, K.; Inamura, S.; Kusumoto, S.; Hashimoto, M.; et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J. Biol. Chem. 2003, 278, 5509–5512. [Google Scholar] [CrossRef]
- Hugot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Miceli-Richard, C.; Lesage, S.; Rybojad, M.; Prieur, A.M.; Manouvrier-Hanu, S.; Häfner, R.; Chamaillard, M.; Zouali, H.; Thomas, G.; Hugot, J.P. CARD15 mutations in Blau syndrome. Nat. Genet. 2001, 29, 19–20. [Google Scholar] [CrossRef]
- Hampe, J.; Grebe, J.; Nikolaus, S.; Solberg, C.; Croucher, P.J.; Mascheretti, S.; Jahnsen, J.; Moum, B.; Klump, B.; Krawczak, M.; et al. Association of NOD2 (CARD 15) genotype with clinical course of Crohn’s disease: A cohort study. Lancet 2002, 359, 1661–1665. [Google Scholar] [CrossRef]
- Wang, X.; Kuivaniemi, H.; Bonavita, G.; Mutkus, L.; Mau, U.; Blau, E.; Inohara, N.; Nunez, G.; Tromp, G.; Williams, C.J. CARD15 mutations in familial granulomatosis syndromes: A study of the original Blau syndrome kindred and other families with large-vessel arteritis and cranial neuropathy. Arthritis Rheum. 2002, 46, 3041–3045. [Google Scholar] [CrossRef] [PubMed]
- Lesage, S.; Zouali, H.; Cézard, J.P.; Colombel, J.F.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.; Gassull, M.; Binder, V.; et al. EPWG-IBD Group; EPIMAD Group; GETAID Group. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am. J. Hum. Genet. 2002, 70, 845–857. [Google Scholar] [CrossRef]
- Dow, C.T.; Ellingson, J.L. Detection of Mycobacterium avium ss. Paratuberculosis in Blau Syndrome Tissues. Autoimmune Dis. 2010, 2011, 127692. [Google Scholar] [CrossRef]
- Caso, F.; Galozzi, P.; Costa, L.; Sfriso, P.; Cantarini, L.; Punzi, L. Autoinflammatory granulomatous diseases: From Blau syndrome and early-onset sarcoidosis to NOD2-mediated disease and Crohn’s disease. RMD Open 2015, 1, e000097. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Shigemura, T.; Kobayashi, N.; Kaneko, N.; Iwasaki, T.; Minami, K.; Kobayashi, K.; Masumoto, J.; Agematsu, K. Early diagnosis of early-onset sarcoidosis: A case report with functional analysis and review of the literature. Clin. Rheumatol. 2017, 36, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Iwai, K.; Tachibana, T.; Fruie, T.; Shigematsu, N.; Izumi, T.; Homma, A.H.; Mikami, R.; Hongo, O.; Hiraga, Y.; et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann. N. Y. Acad. Sci. 1976, 278, 455–469. [Google Scholar] [CrossRef]
- Roberts, W.C.; McAllister, H.A., Jr.; Ferrans, V.J. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am. J. Med. 1977, 63, 86–108. [Google Scholar] [CrossRef]
- Silverman, K.J.; Hutchins, G.M.; Bulkley, B.H. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978, 58, 1204–1211. [Google Scholar] [CrossRef]
- Dubrey, S.W.; Falk, R.H. Diagnosis and management of cardiac sarcoidosis. Prog. Cardiovasc. Dis. 2010, 52, 336–346. [Google Scholar] [CrossRef]
- Kandolin, R.; Lehtonen, J.; Graner, M.; Schildt, J.; Salmenkivi, K.; Kivistö, S.M.; Kupari, M. Diagnosing isolated cardiac sarcoidosis. J. Intern. Med. 2011, 270, 461–468. [Google Scholar] [CrossRef]
- Dow, C.T.; Collins, M.T. Detection of M. paratuberculosis in Sarcoisosis Cardiac Tissue. In Proceedings of the 3rd International WASOG Conference on Diffuse Lung Diseases, Catania, Italy, 22–25 June 2006. [Google Scholar]
- Celler, B.G. Case Study: Cardiac sarcoidosis resolved with Mycobacterium avium paratuberculosis antibiotics (MAP). Sarcoidosis Vasc. Diffus. Lung Dis. 2018, 35, 171–177. [Google Scholar] [CrossRef]
- Borody, T.J.; Leis, S.; Warren, E.F.; Surace, R. Treatment of severe Crohn’s disease using antimycobacterial triple therapy--approaching a cure? Dig. Liver Dis. 2002, 34, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Borody, T.J.; Bilkey, S.; Wettstein, A.R.; Leis, S.; Pang, G.; Tye, S. Anti-mycobacterial therapy in Crohn’s disease heals mucosa with longitudinal scars. Dig. Liver Dis. 2007, 39, 438–444. [Google Scholar] [CrossRef]
- Alcedo, K.P.; Thanigachalam, S.; Naser, S.A. RHB-104 triple antibiotics combination in culture is bactericidal and should be effective for treatment of Crohn’s disease associated with Mycobacterium paratuberculosis. Gut Pathog. 2016, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Clancy, A.; Huynh, R.; Borody, T. Profound remission in Crohn’s disease requiring no further treatment for 3-23 years: A case series. Gut Pathog. 2020, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Judson, M.A. Corticosteroids in Sarcoidosis. Rheum. Dis. Clin. N. Am. 2016, 42, 119–135. [Google Scholar] [CrossRef]
- Gottlieb, J.E.; Israel, H.L.; Steiner, R.M.; Triolo, J.; Patrick, H. Outcome in sarcoidosis. The relationship of relapse to corticosteroid therapy. Chest 1997, 111, 623–631. [Google Scholar] [CrossRef]
- Petruccioli, E.; Petrone, L.; Chiacchio, T.; Farroni, C.; Cuzzi, G.; Navarra, A.; Vanini, V.; Massafra, U.; Lo Pizzo, M.; Guggino, G.; et al. Mycobacterium tuberculosis Immune Response in Patients With Immune-Mediated Inflammatory Disease. Front. Immunol. 2021, 12, 716857. [Google Scholar] [CrossRef]
- Mishra, H.; Chegou, N.N.; Mkhonza, M.N.; Ndou, S.; Tromp, G.; Baker, B. Comparison of Cytokines Expression from Human Monocyte-Derived Macrophages Infected with Different Species of Mycobacteria. J. Interferon Cytokine Res. 2022, 42, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Qasem, A.; Elkamel, E.; Naser, S.A. Anti-MAP Triple Therapy Supports Immunomodulatory Therapeutic Response in Crohn’s Disease through Downregulation of NF-κB Activation in the Absence of MAP Detection. Biomedicines 2020, 8, 513. [Google Scholar] [CrossRef]
- Drake, W.P.; Culver, D.A.; Baughman, R.P.; Judson, M.A.; Crouser, E.D.; James, W.E.; Ayers, G.D.; Ding, T.; Abel, K.; Green, A.; et al. Phase II Investigation of the Efficacy of Antimycobacterial Therapy in Chronic Pulmonary Sarcoidosis. Chest 2021, 159, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Kuenstner, J.T.; Naser, S.; Chamberlin, W.; Borody, T.; Graham, D.Y.; McNees, A.; Hermon-Taylor, J.; Hermon-Taylor, A.; Dow, C.T.; Thayer, W.; et al. The Consensus from the Mycobacterium avium ssp. paratuberculosis (MAP) Conference 2017. Front. Public Health 2017, 5, 208. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S. Who Moved My Cheese? Putnam Adult: New York, NY, USA, 1998. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dow, C.T.; Lin, N.W.; Chan, E.D. Sarcoidosis, Mycobacterium paratuberculosis and Noncaseating Granulomas: Who Moved My Cheese. Microorganisms 2023, 11, 829. https://doi.org/10.3390/microorganisms11040829
Dow CT, Lin NW, Chan ED. Sarcoidosis, Mycobacterium paratuberculosis and Noncaseating Granulomas: Who Moved My Cheese. Microorganisms. 2023; 11(4):829. https://doi.org/10.3390/microorganisms11040829
Chicago/Turabian StyleDow, Coad Thomas, Nancy W. Lin, and Edward D. Chan. 2023. "Sarcoidosis, Mycobacterium paratuberculosis and Noncaseating Granulomas: Who Moved My Cheese" Microorganisms 11, no. 4: 829. https://doi.org/10.3390/microorganisms11040829
APA StyleDow, C. T., Lin, N. W., & Chan, E. D. (2023). Sarcoidosis, Mycobacterium paratuberculosis and Noncaseating Granulomas: Who Moved My Cheese. Microorganisms, 11(4), 829. https://doi.org/10.3390/microorganisms11040829





