Effect of Selected Factors Influencing Biogenic Amines Degradation by Bacillus subtilis Isolated from Food
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Cultivation Conditions
2.2. Sample Preparation for the Analysis of BA Degradation Depending on Cultivation Conditions
2.3. HPLC Analysis of Biogenic Amines
2.4. Statistical Analysis
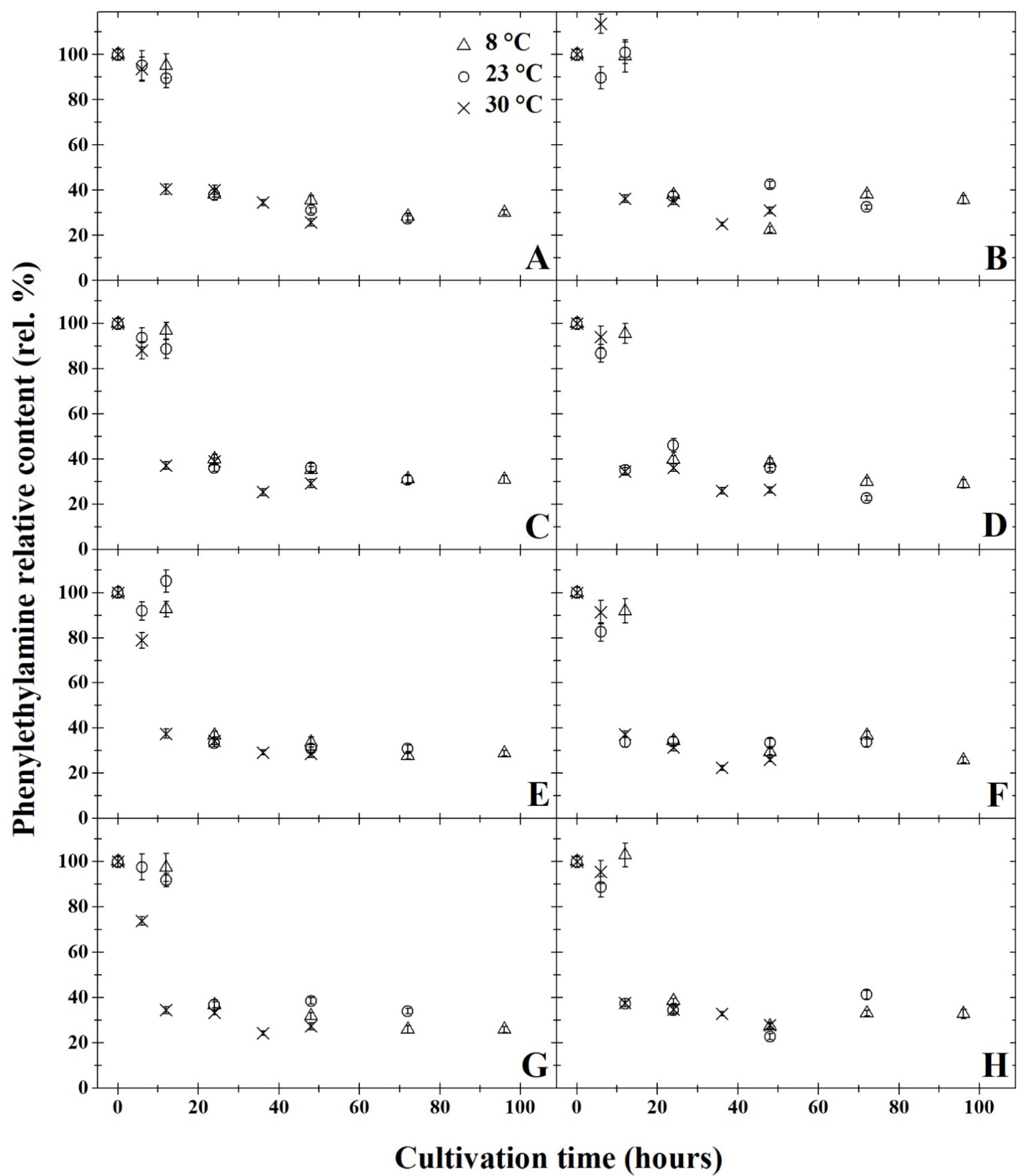
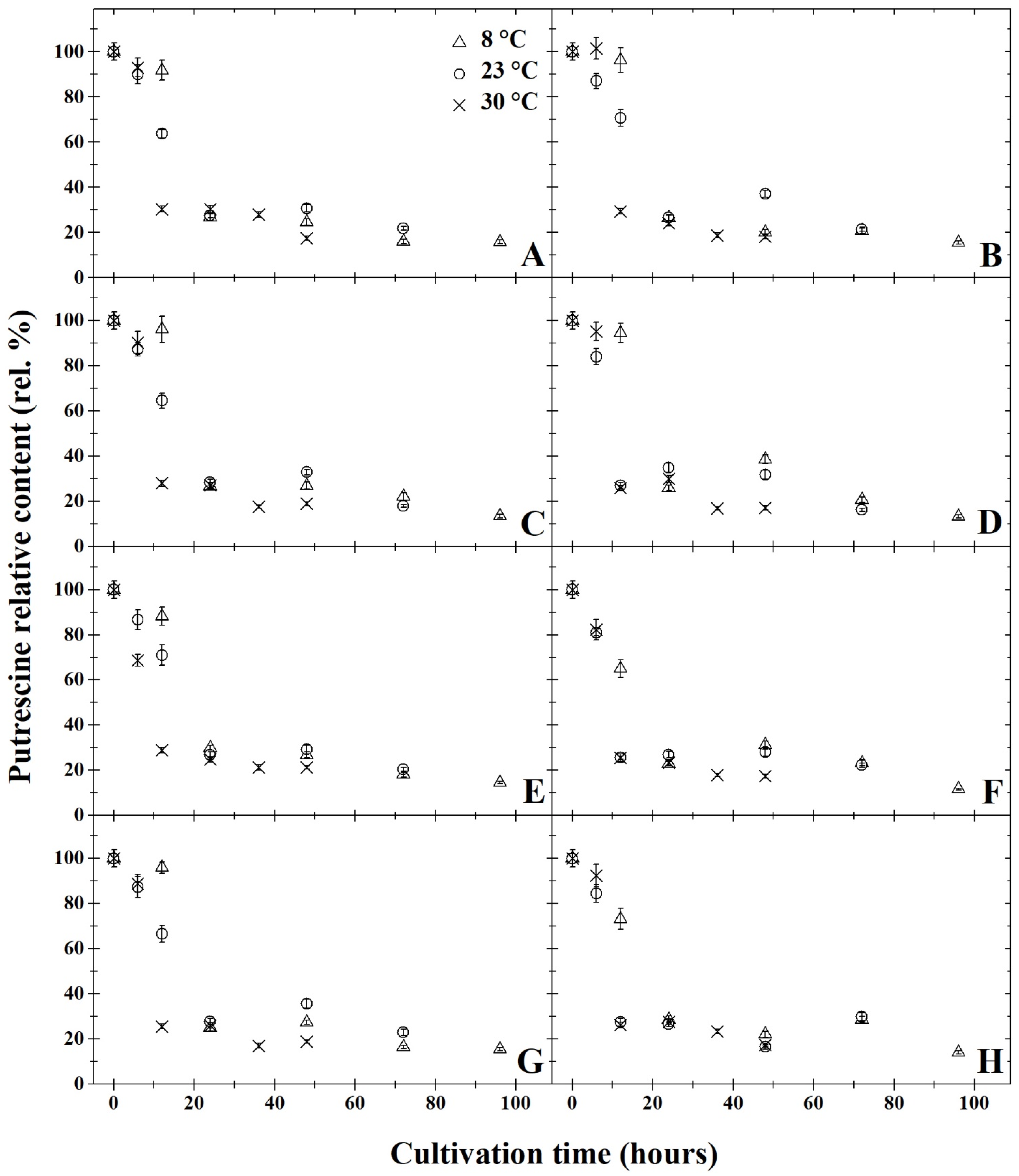
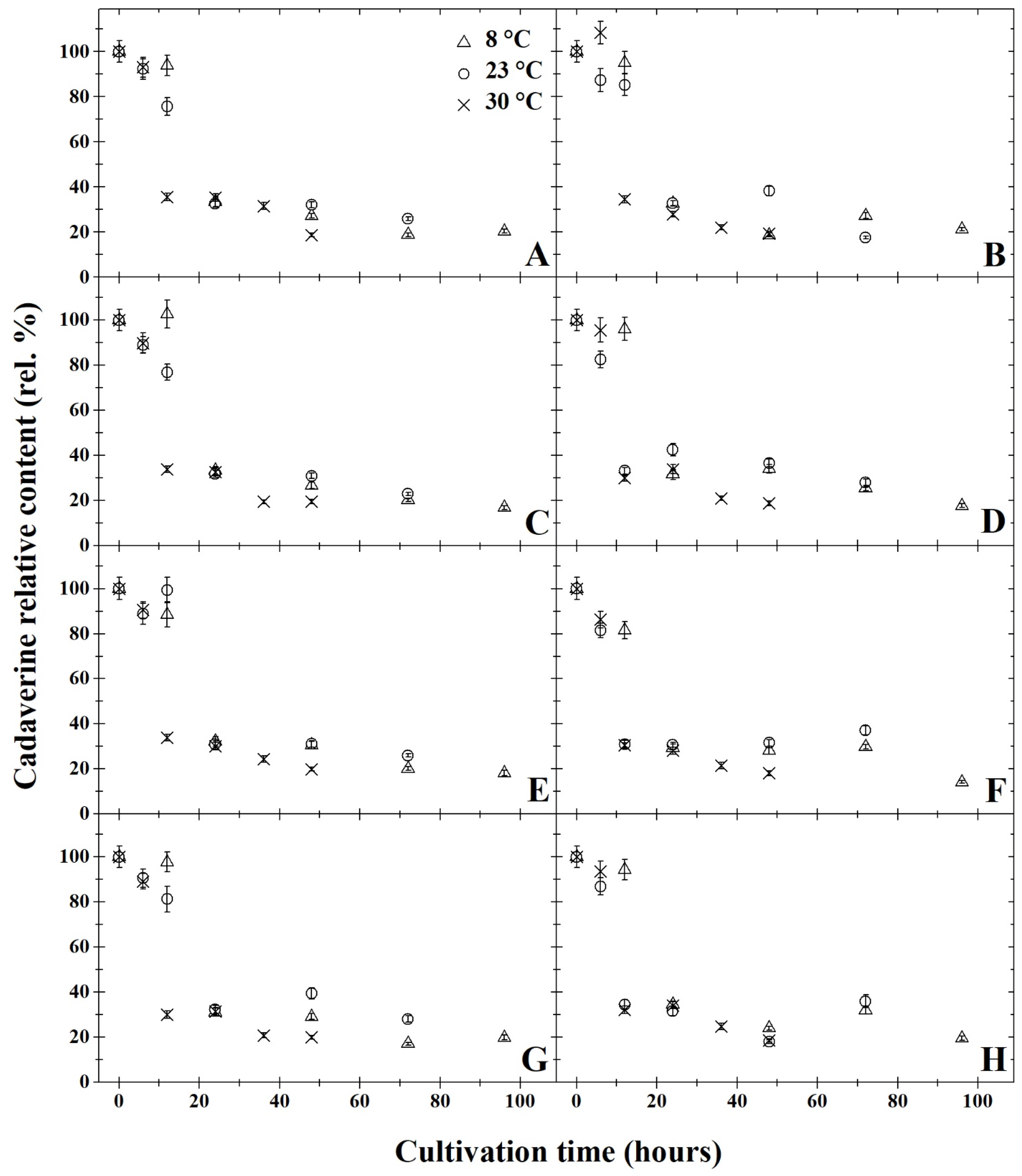
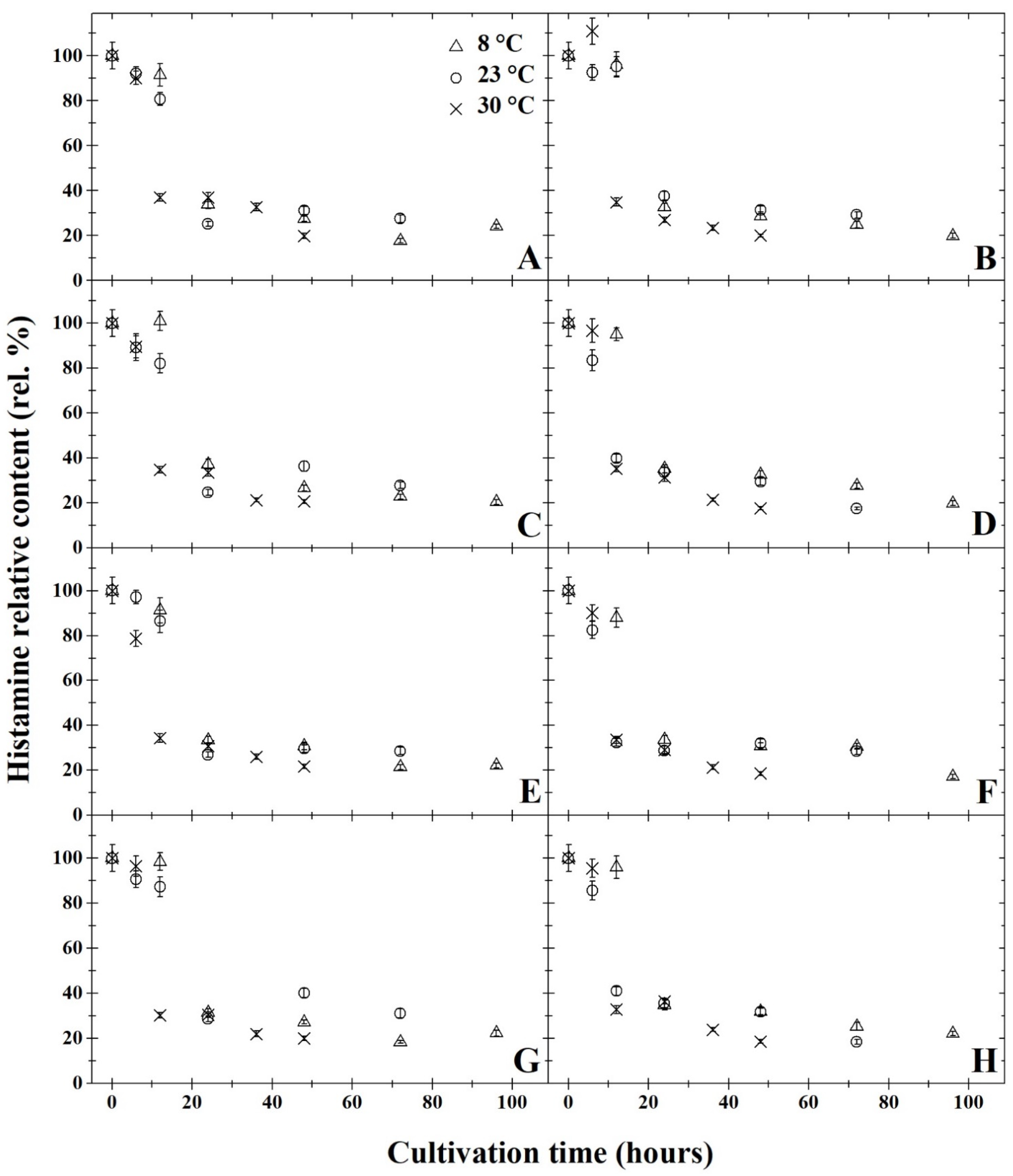
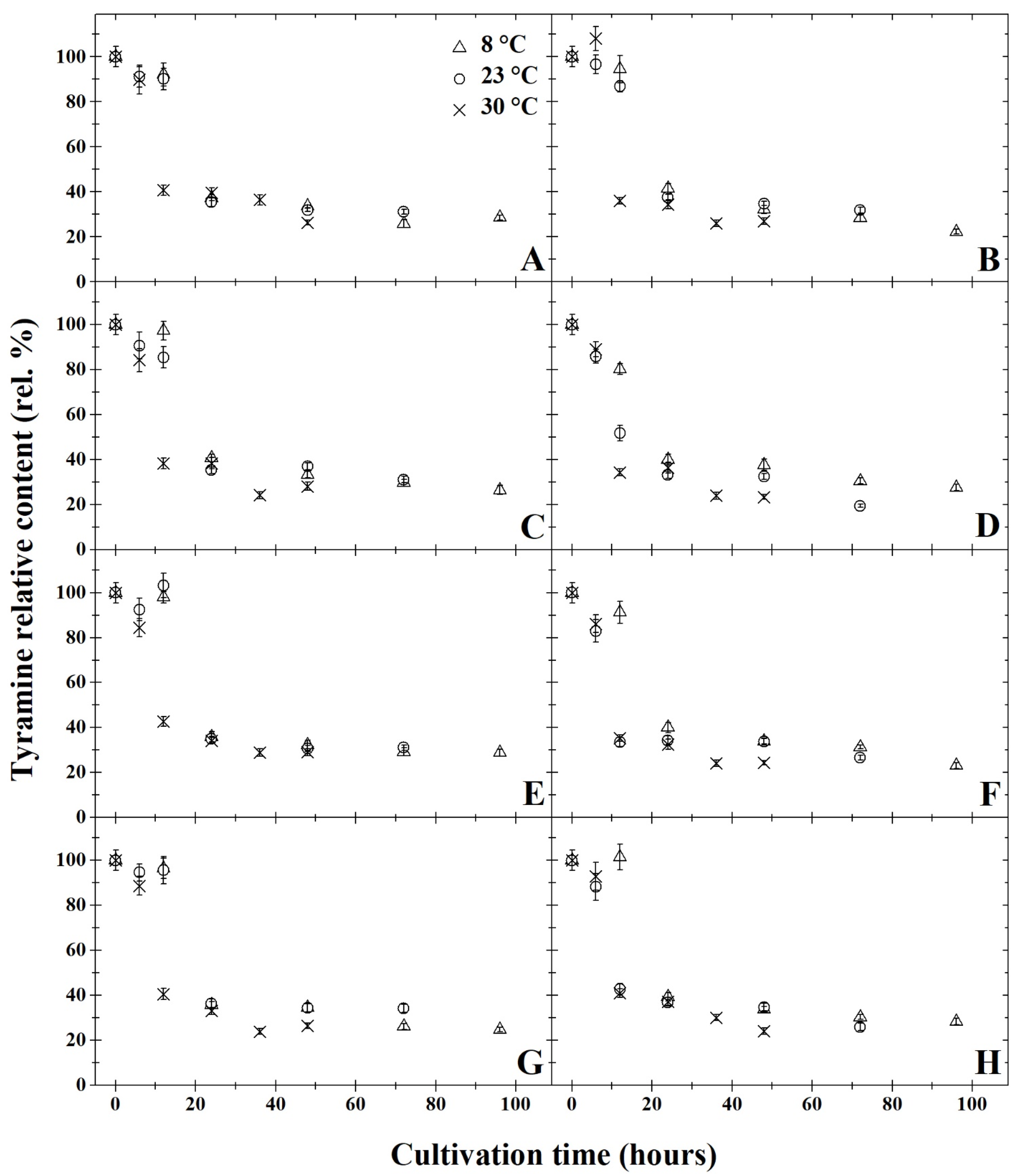
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silla Santos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Bardócz, S.; Grant, G.; Brow, D.S.; Ralph, A.; Pusztai, A. Polyamines in food—Implications for growth and health. J. Nutr. Biochem. 1993, 4, 66–71. [Google Scholar] [CrossRef]
- Shalaby, A.R. Significance of biogenic amines in food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Ladero, V.; Calles-Enríquez, M.; Fernández, M.; Alvarez, M.A. Toxicological effects of dietary biogenic amines. Curr. Res. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Gücügl, A.; Küpülü, Ö. The effect of different starter cultures and ripening temperatures on formation of biogenic amine in Turkish fermented sausages. Eur. Food Res. Technol. 2010, 230, 875–884. [Google Scholar] [CrossRef]
- Pachlová, V.; Buňková, L.; Flasarová, R.; Salek, R.-N.; Dlabajová, A.; Butor, I.; Buňka, F. Biogenic amine production by nonstarter strains of Lactobacillus curvatus and Lactobacillus paracasei in the model system of Dutch-type cheese. LWT—Food Sci. Technol. 2018, 97, 730–735. [Google Scholar] [CrossRef]
- Weremfo, A.; Kodjo Eduafo, M.; Agyei Gyimah, H.; Abassah-Oppong, S. Monitoring the Levels of Biogenic Amines in Canned Fish Products Marketed in Ghana. J. Food Qual. 2020, 1–6. [Google Scholar] [CrossRef]
- Fusek, M.; Michálek, J.; Buňková, L.; Buňka, F. Modelling biogenic amines in fish meat in Central Europe using censored distributions. Chemosphere 2020, 251, 126390. [Google Scholar] [CrossRef]
- Halász, A.; Baráth, A.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994, 5, 42–49. [Google Scholar] [CrossRef]
- Zhernov, Y.V.; Simanduyev, M.Y.; Zaostrovtseva, O.K.; Semeniako, E.E.; Kolykhalova, K.I.; Fadeeva, I.A.; Kashutina, M.I.; Vysokanschya, S.O.; Belova, E.V.; Scherbakov, D.V.; et al. Molecular Mechaisms of Scromboid Food Poisoning. Int. J. Mol. Sci. 2023, 24, 809. [Google Scholar] [CrossRef]
- Buňková, L.; Adamcová, G.; Hudcová, K.; Velichová, H.; Pachlová, V.; Lorencová, E.; Buňka, F. Monitoring of biogenic amines in cheeses manufactured at small-scale farms in fermented dairy products in the Czech Republic. Food Chem. 2013, 141, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Ladero, V.; del Rio, B.; Redruello, B.; Fernández, M.; Cruz Martin, M.; Alvarez, M.A. Biofilm-forming capacity of biogenic amine-producing bacteria isolated from dairy products. Front. Microbiol. 2016, 7, 591. [Google Scholar] [CrossRef] [PubMed]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef]
- Fernández, M.; Linares, D.M.; del Río, B.; Ladero, V.; Alvarez, M.A. HPLC quantification of biogenic amines in cheeses: Correlation with PCR-detection of tyramineproducing microorganisms. J. Dairy Res. 2007, 74, 276–282. [Google Scholar] [CrossRef]
- Kim, J.M.; Ku, S.; Kim, S.J.; Lee, H.H.; Jin, H.; Kang, S.; Li, R.; Johnston, T.V.; Park, M.S.; Ji, G.E. Safety Evaluations of Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI. Int. J. Mol. Sci. 2018, 19, 1422. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Pištěková, H.; Jančová, P.; Berčíková, L.; Buňka, F.; Sokolová, I.; Šopík, T.; Maršálková, K.; Pacheco De Amaral, O.M.R.; Buňková, L. Application of qPCR for multicopper oxidase gene (MCO) in biogenic amines degradation by Lactobacillus Casei. Food Microbiol. 2020, 91, 103550. [Google Scholar] [CrossRef]
- Laranjo, M.; Elias, M.; Fraqueza, M.J. The Use of Starter Cultures in Traditional Meat Products. J. Food Qual. 2017, 42, 1–18. [Google Scholar] [CrossRef]
- Sun, X.; Sun, E.; Sun, L.; Su, L.; Jin, Y.; Ren, L.; Zhao, L. Effect of Biogenic Amine-Degrading Lactobacillus on the Biogenic Amines and Quality in Fermented Lamb Jerky. Foods 2022, 11, 2057. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Zhang, S.; Lin, X.; Liang, H.; Chen, Y.; Ji, C. Heterologous Expression of the Lactobacillus sakei Multiple Copper Oxidase to Degrade Histamine and Tyramine at Different Environmental Conditions. Foods 2022, 11, 3306. [Google Scholar] [CrossRef]
- Alvarez, M.; Moreno-Arribas, M. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Li, B.; Lu, S. The Importance of Amine-degrading Enzymes on the Biogenic Amine Degradation in Fermented Foods: A review. Process Biochem. 2020, 99, 331–339. [Google Scholar] [CrossRef]
- Zaman, M.Z.; Abu Bakar, F.; Selamat, J.; Bakar, J. Occurrence of biogenic amines and amines degrading bacteria in fish sauce. Czech J. Food Sci. 2010, 28, 440–449. [Google Scholar] [CrossRef]
- Adámek, R.; Pachlová, V.; Salek, R.N.; Němečková, I.; Buňka, F.; Buňková, L. Reduction of biogenic amine content in Dutch-type cheese as affected by the applied adjunct culture. LWT—Food Sci. Technol. 2021, 152, 112397. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, C.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef]
- Herrero-Fresno, A.; Martínez, N.; Sánchez-Llana, E.; Díaz, M.; Fernández, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. Lactobacillus casei strains isolated from cheese reduce biogenic amine accumulation in an experimental model. Int. J. Food Microbiol. 2012, 157, 297–304. [Google Scholar] [CrossRef]
- Postollec, F.; Falentin, H.; Pavan, S.; Combrisson, J.; Sohier, D. Recent advances in quantitative PCR (qPCR) applications in food mikrobiology. Food Microbiol. 2011, 157, 848–861. [Google Scholar] [CrossRef]
- Dadáková, E.; Křížek, M.; Pelikánová, T. Determination of biogenic amines in foods using ultra-performance liquid chromatography (UPLC). Food Chem. 2009, 116, 365–370. [Google Scholar] [CrossRef]
- Jančová, P.; Pachlová, V.; Čechová, E.; Cedidlová, K.; Šerá, J.; Pištěková, H.; Buňka, F.; Buňková, L. Occurrence of Biogenic Amines Producers in the Wastewater of the Dairy Industry. Molecules 2020, 25, 5143. [Google Scholar] [CrossRef]
- Purevdorj, K.; Buňková, L.; Dlabajová, A.; Čechová, E.; Pachlová, V.; Buňka, F. The impact of cell-free supernatants of Lactococcus lactis subsp. lactis strains on the tyramine formation of Lactobacillus and Lactiplantibacillus strains isolated from cheese and beer. Food Microbiol. 2021, 99, 103813. [Google Scholar] [CrossRef]
- Hoffmann, T.; Bremer, E. Protection of Bacillus subtilis against cold stress via compatible-solute acquisition. J. Bacteriol. 2011, 193, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, R.G.K.; Heidel, M.; Hammes, W.P. Histamine and tyramine degradation by food fermenting microorganisms. Int. J. Food Microbiol. 1998, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jin, Y.H.; Pawluk, A.M.; Mah, J.-H. Reduction in Biogenic Amine Content in Baechu (Napa Cabbage) Kimchi by Biogenic Amine-Degrading Lactic Acid Bacteria. Microorganisms 2021, 9, 2570. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, W.; Zhai, Y.; Zhao, L.; Liu, T.; Yang, L.; Jin, Y.; Duan, Y. Screening and the ability of biogenic amine-degrading strains from traditional meat products in Inner Mongolia. LWT—Food Sci. Technol. 2023, 176, 114533. [Google Scholar] [CrossRef]
- Leuschner, R.G.K.; Hammes, W.P. Tyramine Degradation by Micro-cocci During Ripening of Fermented Sausage. Meat Sci. 1998, 49, 289–296. [Google Scholar] [CrossRef]
- Eom, J.S.; Seo, B.Y.; Choi, H.S. Biogenic Amine Degradation by Bacillus Species Isolated from Traditional Fermented Soybean Food and Detection of Decarboxylase-Related Genes. J. Microbiol. Biotechnol. 2015, 25, 1519–1527. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lin, C.S.; Liu, F.L.; Huang, T.C.; Tsai, Y.H. Degradation of histamine by Bacillus polymyxa isolated from salted fish products. J. Food Drug Anal. 2015, 23, 836–844. [Google Scholar] [CrossRef]
- Saad, M.A.; Abd-Rabou, H.S.; Elkhtab, E.; Rayan, A.M.; Abdeen, A.; Abdelkader, A.; Ibrahim, S.F.; Hussien, H. Occurrence of Toxic Biogenic Amines in Various Types of Soft and Hard Cheeses and Their Control by Bacillus polymyxa D05-1. Fermentation 2022, 8, 327. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, W.; Fu, M.; Yu, Y.; Wu, J.; Xu, Y.; Li, L. Effects of selected Bacillus strains on the biogenic amines, bioactive ingredients and antioxidant capacity of shuidouchi. Int. J. Food Microbiol. 2023, 388, 110084. [Google Scholar] [CrossRef]
- Tepkasikul, P.; Santiyanont, P.; Boocharoen, A.; Abhisingha, M.; Mhuantong, W.; Chantarasakha, K.; Pitaksutheepong, C.; Visessanguan, W.; Tepaamorndech, S. The functional starter and its genomic insight for histamine degradation in fish sauce. Food Microbiol. 2022, 104, 103988. [Google Scholar] [CrossRef]
- Kim, S.-H.; Yehuala, G.A.; Bang, W.Y.; Yang, J.; Jung, Y.H.; Park, M.-K. Safety Evaluation of Bacillus subtilis IDCC1101, Newly Isolated from Cheonggukjang, for Industrial Applications. Microorganisms 2022, 10, 2494. [Google Scholar] [CrossRef] [PubMed]
- Harirchi, S.; Sar, T.; Ramezani, M.; Aliyu, H.; Etemadifar, Z.; Nojoumi, S.A.; Yazdian, F.; Awasthi, M.K.; Taherzadeh, M.J. Bacillales: From Taxonomy to Biotechnological and Industrial Perspectives. Microorganisms 2022, 10, 2355. [Google Scholar] [CrossRef] [PubMed]
- Ouoba, L.I.; Diawara, B.; Amoa-Awua, W.K.; Traoré, A.S.; Møller, P.L. Genotyping of starter cultures of Bacillus subtilis and Bacillus pumilus for fermentation of African locust bean (Parkia biglobosa) to produce Soumbala. Int. J. Food Microbiol. 2004, 90, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wu, J.; Chen, H.; Ni, S. Review on biological degradation of biogenic amines in food. Int. J. Agric. Sci. Food Technol. 2021, 7, 331–334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butor, I.; Jančová, P.; Purevdorj, K.; Klementová, L.; Kluz, M.; Huňová, I.; Pištěková, H.; Buňka, F.; Buňková, L. Effect of Selected Factors Influencing Biogenic Amines Degradation by Bacillus subtilis Isolated from Food. Microorganisms 2023, 11, 1091. https://doi.org/10.3390/microorganisms11041091
Butor I, Jančová P, Purevdorj K, Klementová L, Kluz M, Huňová I, Pištěková H, Buňka F, Buňková L. Effect of Selected Factors Influencing Biogenic Amines Degradation by Bacillus subtilis Isolated from Food. Microorganisms. 2023; 11(4):1091. https://doi.org/10.3390/microorganisms11041091
Chicago/Turabian StyleButor, Irena, Petra Jančová, Khatantuul Purevdorj, Lucie Klementová, Maciej Kluz, Ivana Huňová, Hana Pištěková, František Buňka, and Leona Buňková. 2023. "Effect of Selected Factors Influencing Biogenic Amines Degradation by Bacillus subtilis Isolated from Food" Microorganisms 11, no. 4: 1091. https://doi.org/10.3390/microorganisms11041091
APA StyleButor, I., Jančová, P., Purevdorj, K., Klementová, L., Kluz, M., Huňová, I., Pištěková, H., Buňka, F., & Buňková, L. (2023). Effect of Selected Factors Influencing Biogenic Amines Degradation by Bacillus subtilis Isolated from Food. Microorganisms, 11(4), 1091. https://doi.org/10.3390/microorganisms11041091






