Characterization of a Riboflavin-Producing Mutant of Bacillus subtilis Isolated by Droplet-Based Microfluidics Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Medium, Reagents, and Instruments
2.2. Gene Recombination Method
- The upstream homologous arm (UP) and downstream homologous arm (DN) of the target gene were amplified using the B. subtilis 168 (BS168) genome as the template, and the fragment containing the screening marker cat-araR (CR) and the direct repeats (DR) was amplified using the strain with CR as the template, and the corresponding mutation site was introduced in the design of the primer.
- Upstream, CR, and downstream fragments could be overlapped to generate the UP-CR-DN fragment. The product was transferred into the B. subtilis by the Spizizen transformation method. The cultures were spread on the LB solid plate containing 10 μg/mL chloramphenicol.
- Based on the principle of homologous recombination, the homologous sequence was integrated into the genome of B. subtilis. The positive clones can be screened through chloramphenicol resistance, and the colonies can be verified.
- The positive clones were inoculated into the test tube containing 10 μg/mL chloramphenicol 5 mL LB medium, incubated at 37 °C, 200 r/min for 12 h. The strain with CR fragment as a positive screen mark was stored at −80 °C.
- The cultures from the previous test tube were inoculated into the test tube containing 5 mL of LB medium without resistance and incubated at 37 °C for 8 h. Because of the existence of the same short sequence DR, there will be secondary homologous recombination in the cells, and the screening marker CR will be discarded. Then the cultures were spread on the LB solid plate containing 60 μg/mL neomycin, and the positive clones could be screened.
- PCR verification was carried out, and the single colony with the correct band size was inoculated into the test tube containing 5 mL LB medium with 20 μg/mL neomycin at 37 °C, 200 r/min for 12 h, and the strain was stored at −80 °C.
2.3. Droplet Preparation and Mutagenesis
2.4. Droplet Separation
2.5. Flask Fermentation Conditions, Measurement of Cell Density, Riboflavin Titers, and Concentration of Glucose and Sucrose
2.6. Fed-Batch Fermentation
2.7. Biofilm Determination
3. Results
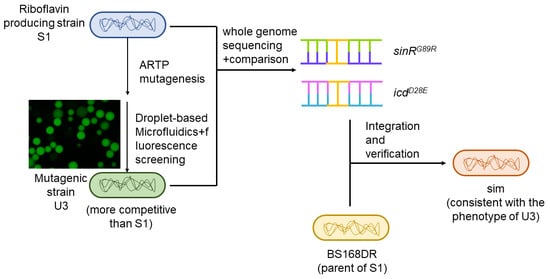
3.1. Strain Screening
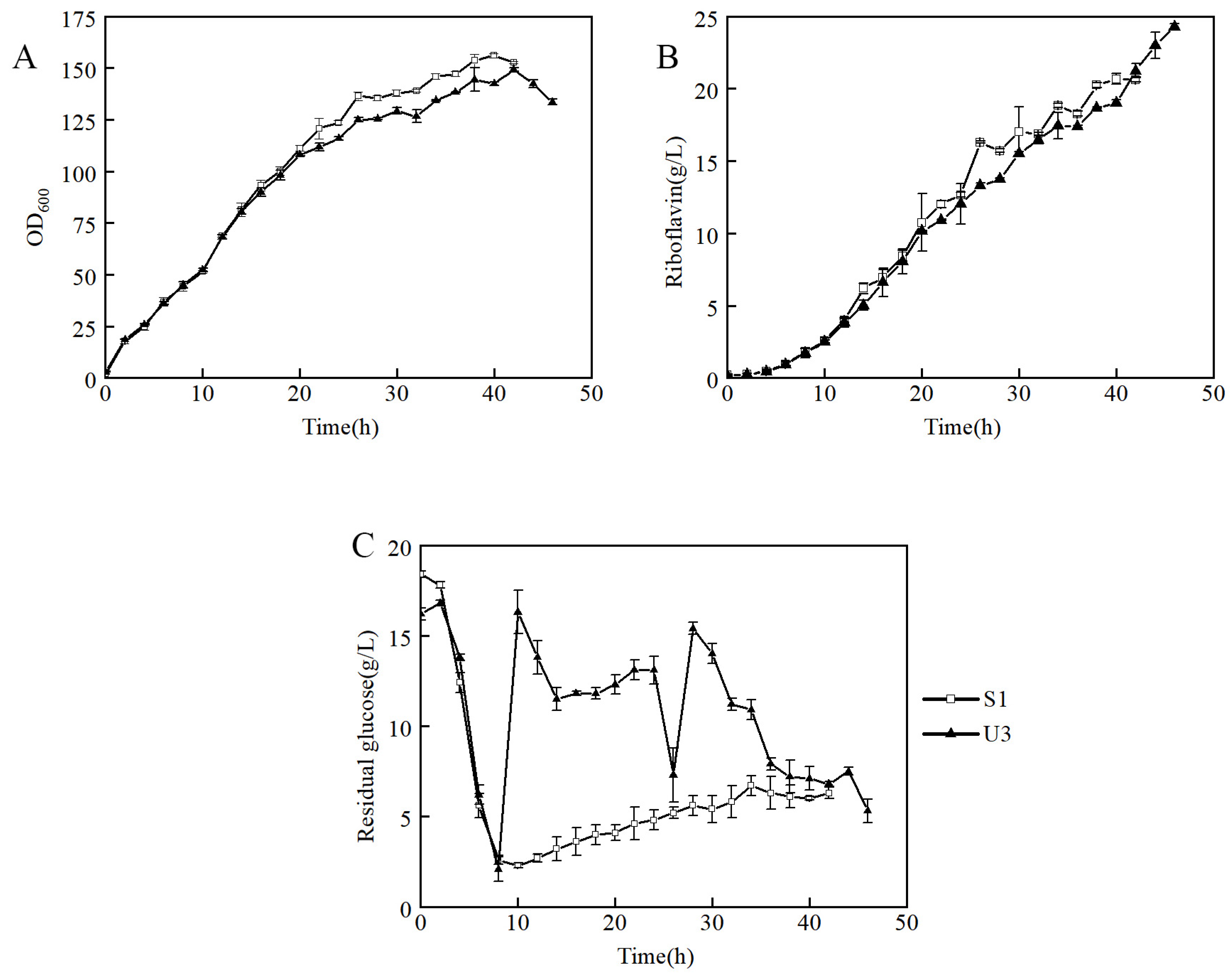
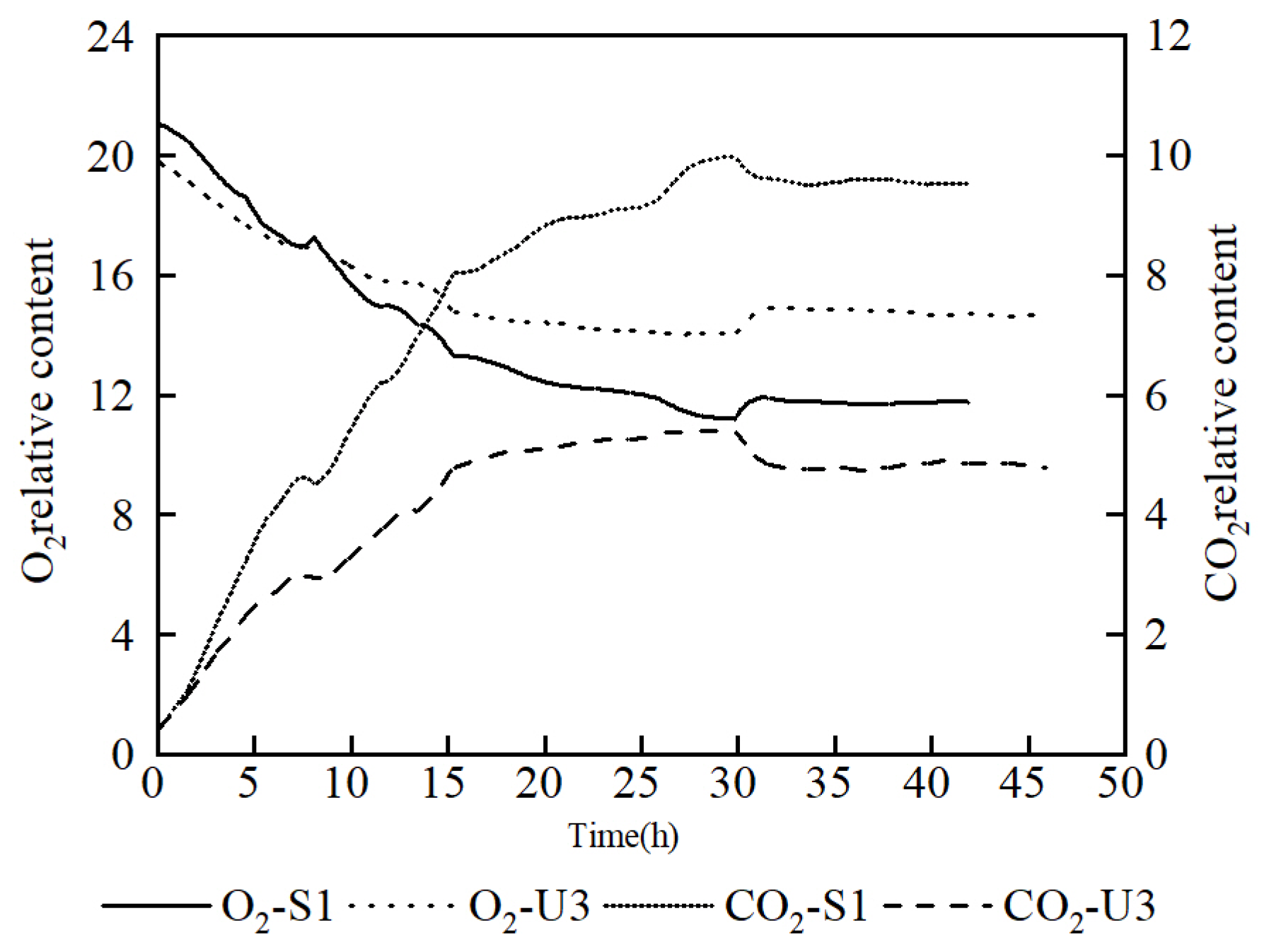
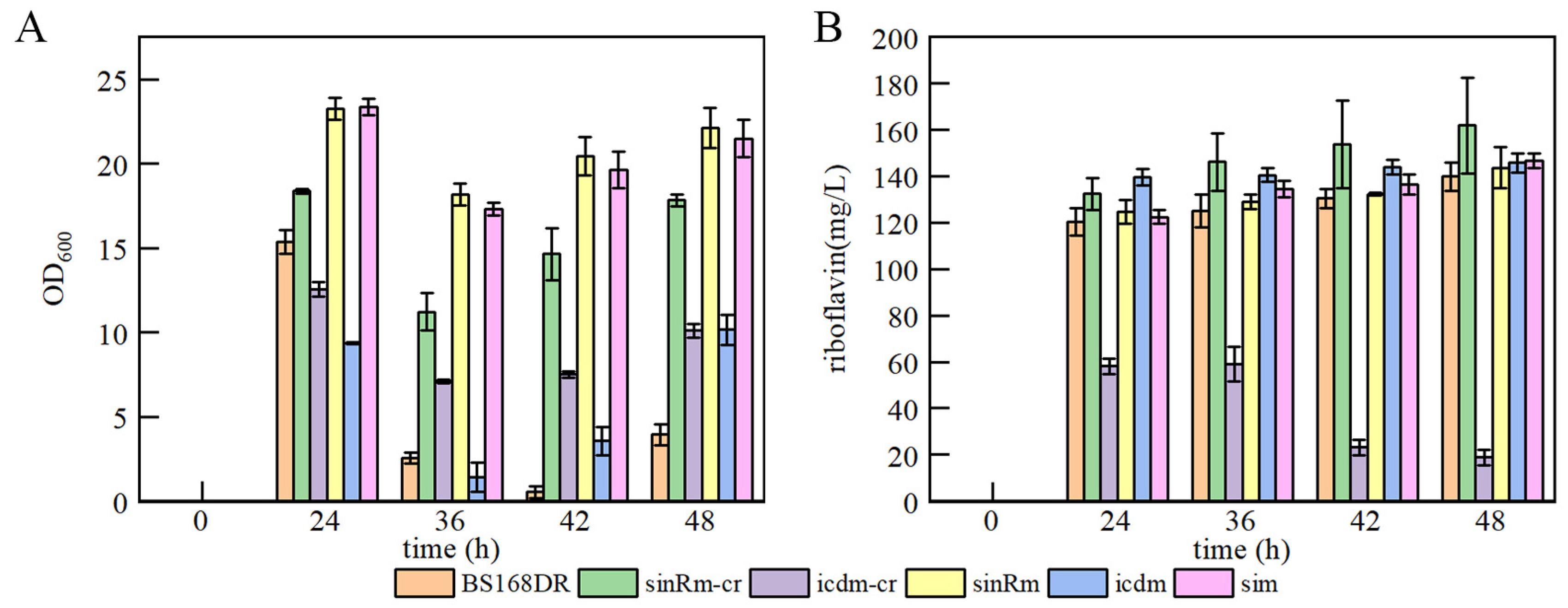
3.2. Production of Riboflavin of Mutants in Flask and Fed-Batch Fermentation
3.3. Comparison and Analysis of the Genome Sequence of U3 with S1
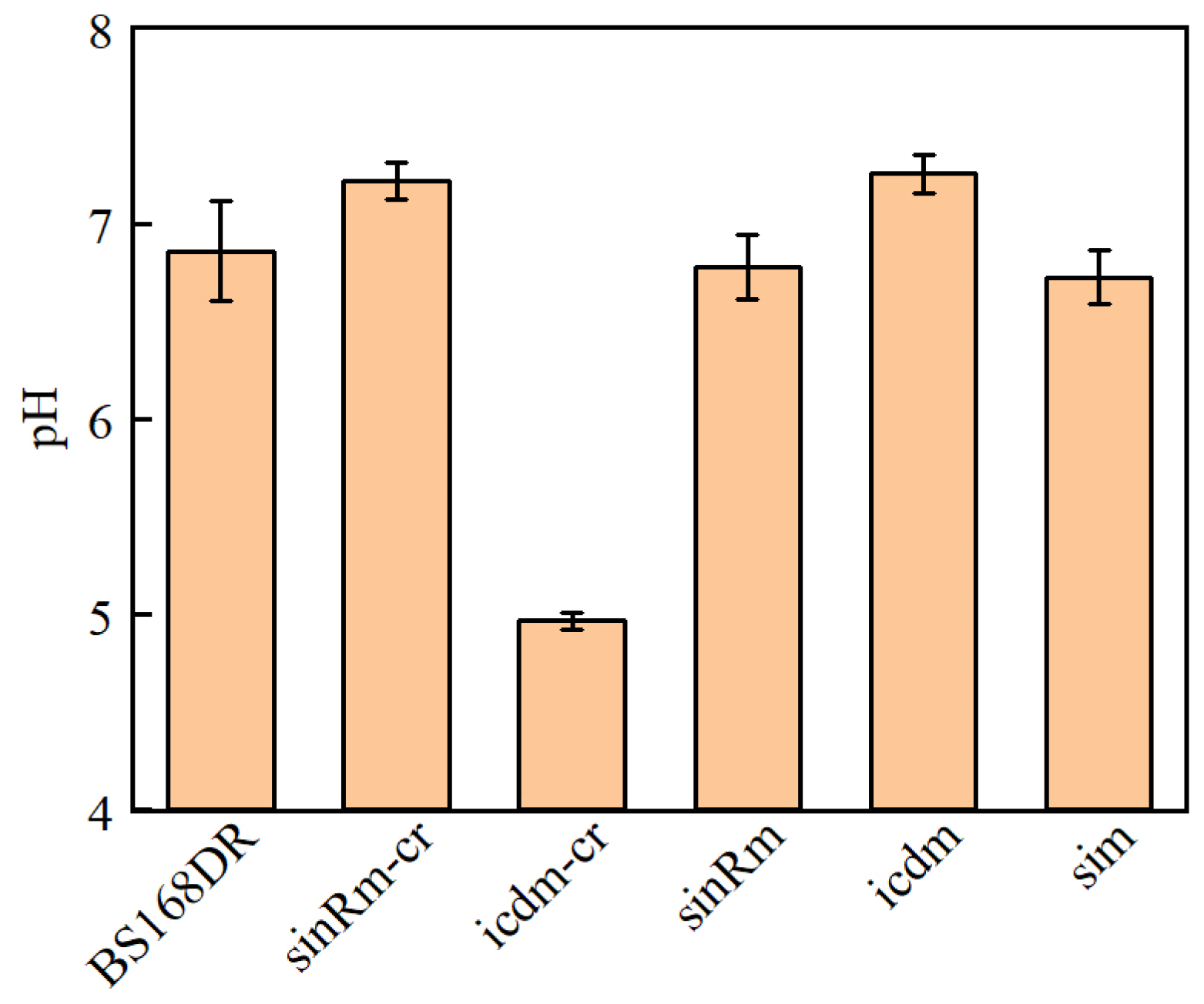
3.4. Reverse Verification of Mutation Site and Fermentation Results
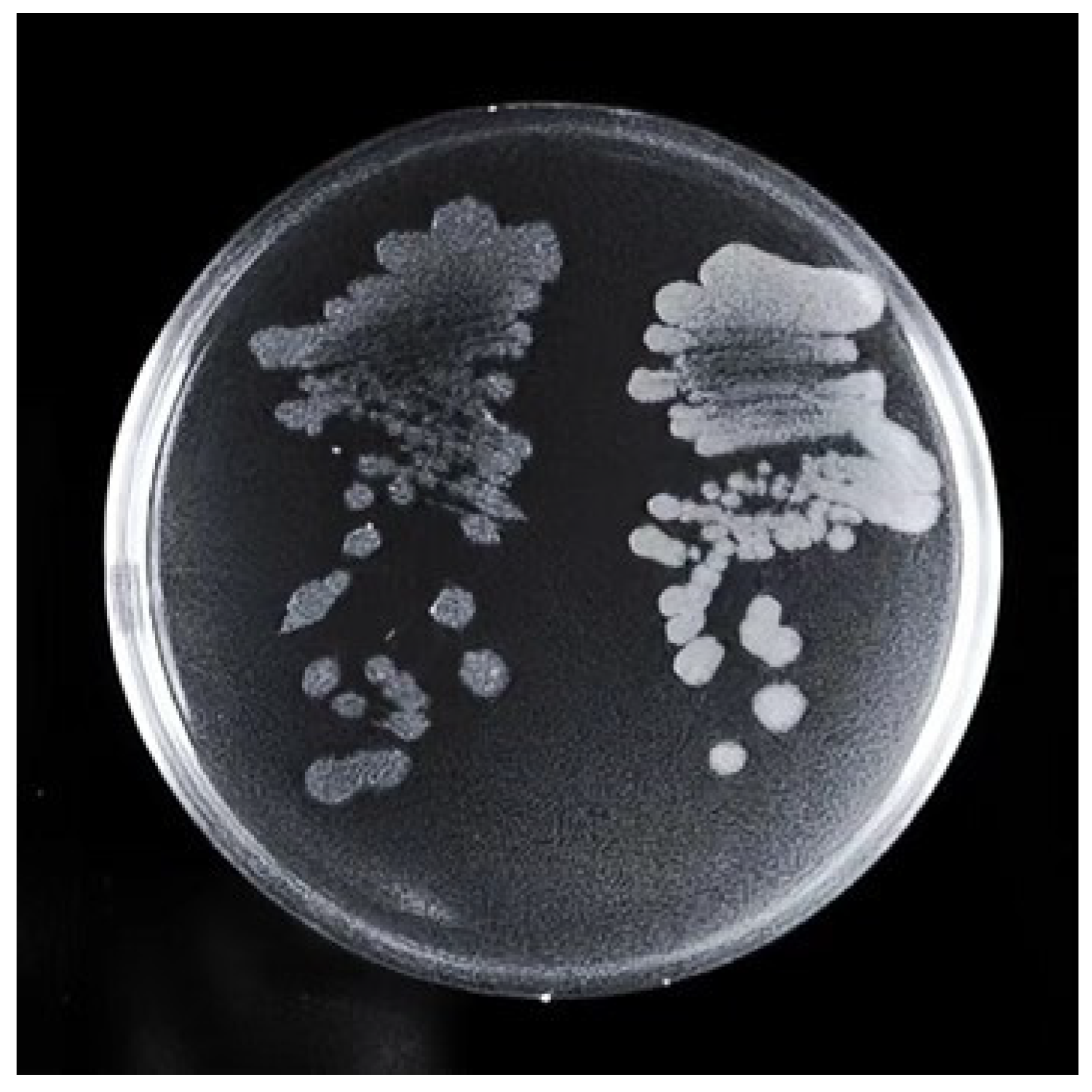
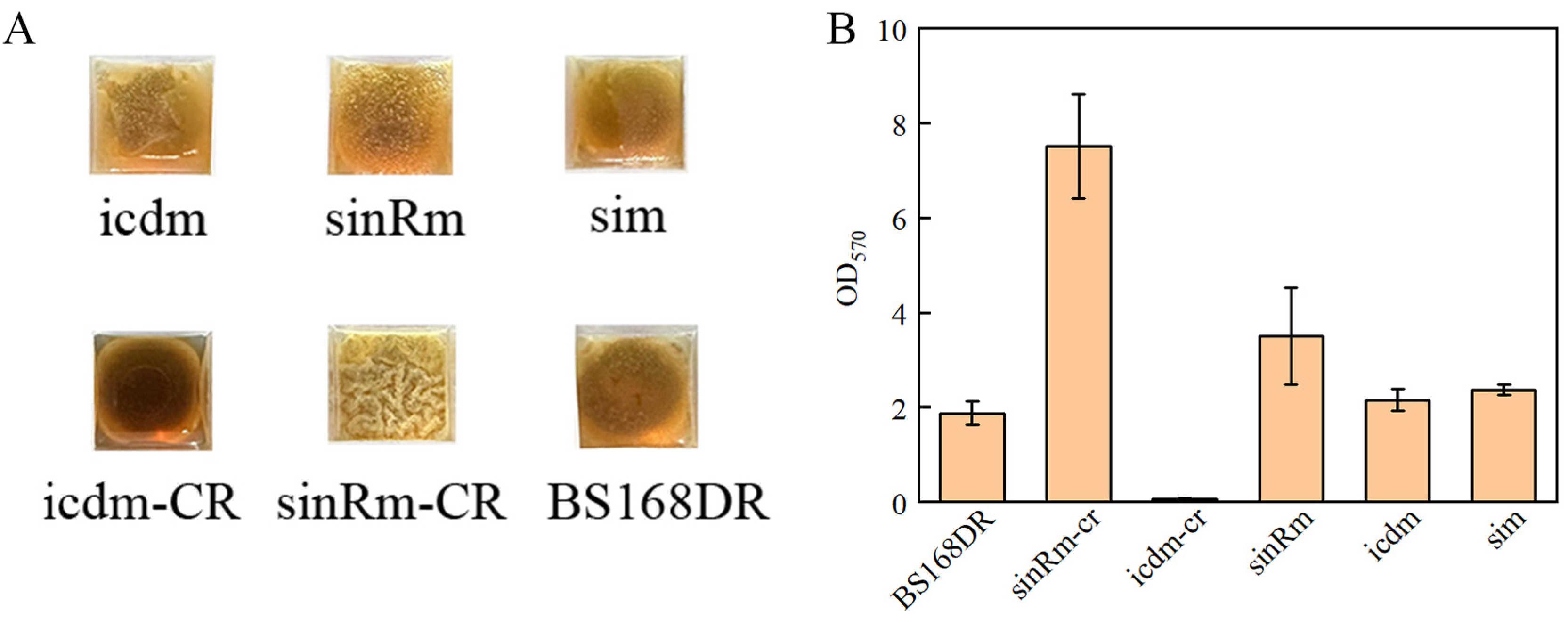
3.5. Biofilm Determination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suwannasom, N.; Kao, I.; Pruss, A.; Georgieva, R.; Baeumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef] [PubMed]
- Luis Revuelta, J.; Ledesma-Amaro, R.; Lozano-Martinez, P.; Diaz-Fernandez, D.; Buey, R.M.; Jimenez, A. Bioproduction of riboflavin: A bright yellow history. J. Ind. Microbiol. Biotechnol. 2017, 44, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Darguzyte, M.; Drude, N.; Lammers, T.; Kiessling, F. Riboflavin-Targeted Drug Delivery. Cancers 2020, 12, 295. [Google Scholar] [CrossRef]
- Shuang, L.; Wenya, H.; Zhiwen, W.; Tao, C. Production of riboflavin and related cofactors by biotechnological processes. Microb. Cell Factories 2020, 19, 31. [Google Scholar] [CrossRef]
- Förster, C.; Santos, M.A.; Ruffert, S.; Krämer, R.; Revuelta, J.L. Physiological Consequence of Disruption of the VMA1Gene in the Riboflavin Overproducer Ashbya gossypii. J. Biol. Chem. 1999, 274, 9442–9448. [Google Scholar] [PubMed]
- Kasler, B.; Sahm, H.; Stahmann, K.-P.; Schmidt, G.; Boddecker, T.; Seulberger, H. Riboflavin-Production Process by Means of Micro-Organisms with Modified Isocitratlyase Activity. U.S. Patent 5,976,844, 2 November 1999. [Google Scholar]
- Jianhua, H.; Zhaokun, Z.; Yongli, L.; Hui, L.; Zhanying, L. Screening of high-yield riboflavin Bacillus subtilis strain by heavy-ion beam irradiation. J. Biol. 2022, 39, 33. [Google Scholar]
- Jinshan, Z.; Yefei, W.; Jianwei, L. Mutagenesis of Bacillus subtilis using the atmospheric and room temperature plasma technology for improved riboflavin production. Bull. Ferment. Sci. Technolgy 2020, 49, 58–62. [Google Scholar]
- Leamon, J.H.; Link, D.R.; Egholm, M.; Rothberg, J.M. Overview: Methods and applications for droplet compartmentalization of biology. Nat. Methods 2006, 3, 541–543. [Google Scholar]
- Yang, J.; Tu, R.; Yuan, H.; Wang, Q.; Zhu, L. Recent advances in droplet microfluidics for enzyme and cell factory engineering. Crit. Rev. Biotechnol. 2021, 41, 1023–1045. [Google Scholar] [CrossRef]
- Shouying, F.; Miaomiao, X.; Yining, Z.; Chuan, L.; Ran, T.; Dawei, Z. Estabilishment and Application of High-throughput Screening Method of Riboflavin Industrial Strain. Biotechnol. Bull. 2020, 36, 47. [Google Scholar]
- Dawei, Z.; Yuan, S.; Bin, Y.; Chuan, L. Engineering Strain of Bacillus subtilis with High Vitamin B2 Production, Its Construction and Application. CN Patent 113025550B, 10 September 2021. [Google Scholar]
- Bin, Y.; Yiwen, S.; Shouying, F.; Miaomiao, X.; Yuan, S.; Chuan, L.; Chunzhi, Z.; Dawei, Z. Improving the Production of Riboflavin by Introducing a Mutant Ribulose 5-Phosphate 3-Epimerase Gene in Bacillus subtilis. Front. Bioeng. Biotechnol. 2021, 9, 704650. [Google Scholar] [CrossRef]
- Yingfang, M.; Haiquan, Y.; Xianzhong, C.; Bo, S.; Guocheng, D.; Zhemin, Z.; Jiangning, S.; You, F.; Wei, S. Significantly improving the yield of recombinant proteins in Bacillus subtilis by a novel powerful mutagenesis tool (ARTP): Alkaline α-amylase as a case study. Protein Expr. Purif. 2015, 114, 82–88. [Google Scholar]
- Yuan, H.; Tu, R.; Tong, X.; Lin, Y.; Zhang, Y.; Wang, Q. Ultrahigh-throughput screening of industrial enzyme-producing strains by droplet-based microfluidic system. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac007. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Hung, K.-C.; Mitra, A.; Ung, L.W.; Lightwood, D.; Tu, R.; Starkie, D.; Cai, L.; Mazutis, L.; Chong, S. Rapid isolation of antigen-specific B-cells using droplet microfluidics. RSC Adv. 2020, 10, 27006–27013. [Google Scholar] [CrossRef]
- Yongcheng, W. Metabolic Engineering of Bacillus subtilis for Riboflavin Production; Tianjin University: Tianjin, China, 2015. [Google Scholar]
- Zhenyu, G.; Miaomiao, X.; Yuan, S.; Huajun, L. Metabolic engineering modifies purine synthesis pathway to increase riboflavin production. Food Ferment. Ind. 2022, 48, 10–15. [Google Scholar]
- Moyano, A.J.; Mas, C.R.; Colque, C.A.; Smania, A.M. Dealing with biofilms of Pseudomonas aeruginosa and Staphylococcus aureus: In vitro evaluation of a novel aerosol formulation of silver sulfadiazine. Burns 2020, 46, 128–135. [Google Scholar] [CrossRef]
- Cairns, L.S.; Hobley, L.; Stanley-Wall, N.R. Biofilm formation by Bacillus subtilis: New insights into regulatory strategies and assembly mechanisms. Mol. Microbiol. 2014, 93, 587–598. [Google Scholar] [CrossRef]
- Diehl, A.; Roske, Y.; Ball, L.; Chowdhury, A.; Hiller, M.; Molière, N.; Kramer, R.; Stöppler, D.; Worth, C.L.; Schlegel, B. Structural changes of TasA in biofilm formation of Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2018, 115, 3237–3242. [Google Scholar] [CrossRef]
- Romero, D.; Aguilar, C.; Losick, R.; Kolter, R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. USA 2010, 107, 2230–2234. [Google Scholar] [CrossRef]
- Shixiu, C.; Hongzhi, X.; Taichi, C.; Yang, G.; Xueqin, L.; Yanfeng, L.; Jianghua, L.; Guocheng, D.; Long, L. Cell membrane and electron transfer engineering for improved synthesis of menaquinone-7 in Bacillus subtilis. Iscience 2020, 23, 100918. [Google Scholar]
- Matsuno, K.; Blais, T.; Serio, A.W.; Conway, T.; Henkin, T.M.; Sonenshein, A.L. Metabolic imbalance and sporulation in an isocitrate dehydrogenase mutant of Bacillus subtilis. J. Bacteriol. 1999, 181, 3382–3391. [Google Scholar] [CrossRef] [PubMed]
- Shengfang, J.; Levin, P.A.; Matsuno, K.; Grossman, A.D.; Sonenshein, A.L. Deletion of the Bacillus subtilis isocitrate dehydrogenase gene causes a block at stage I of sporulation. J. Bacteriol. 1997, 179, 4725–4732. [Google Scholar]
- Vidwans, S.J.; Ireton, K.; Grossman, A.D. Possible role for the essential GTP-binding protein Obg in regulating the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 1995, 177, 3308–3311. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Wei, L.; Shiguang, Z.; Senhe, Q.; Zhou, W.; Mengjie, Z.; Wensong, H.; Jian, W.; Liuxiu, H.; Yan, L. Site-directed mutagenesis of the quorum-sensing transcriptional regulator SinR affects the biosynthesis of menaquinone in Bacillus subtilis. Microb. Cell Factories 2021, 20, 113. [Google Scholar]
- Yunrong, C.; Beauregard, P.B.; Vlamakis, H.; Losick, R.; Kolter, R. Galactose metabolism plays a crucial role in biofilm formation by Bacillus subtilis. MBio 2012, 3, e00184-12. [Google Scholar]
- Gaur, N.K.; Cabane, K.; Smith, I. Structure and expression of the Bacillus subtilis sin operon. J. Bacteriol. 1988, 170, 1046–1053. [Google Scholar] [CrossRef]
- Kobayashi, K.; Iwano, M. BslA (YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms. Mol. Microbiol. 2012, 85, 51–66. [Google Scholar] [CrossRef]
- Ostrowski, A.; Mehert, A.; Prescott, A.; Kiley, T.B.; Stanley-Wall, N.R. YuaB functions synergistically with the exopolysaccharide and TasA amyloid fibers to allow biofilm formation by Bacillus subtilis. J. Bacteriol. 2011, 193, 4821–4831. [Google Scholar] [CrossRef]
- Yepes, A.; Schneider, J.; Mielich, B.; Koch, G.; García-Betancur, J.C.; Ramamurthi, K.S.; Vlamakis, H.; López, D. The biofilm formation defect of a Bacillus subtilis flotillin-defective mutant involves the protease FtsH. Mol. Microbiol. 2012, 86, 457–471. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Liu, C.; Xia, M.; Li, S.; Tu, R.; Wang, S.; Jin, H.; Zhang, D. Characterization of a Riboflavin-Producing Mutant of Bacillus subtilis Isolated by Droplet-Based Microfluidics Screening. Microorganisms 2023, 11, 1070. https://doi.org/10.3390/microorganisms11041070
Xu F, Liu C, Xia M, Li S, Tu R, Wang S, Jin H, Zhang D. Characterization of a Riboflavin-Producing Mutant of Bacillus subtilis Isolated by Droplet-Based Microfluidics Screening. Microorganisms. 2023; 11(4):1070. https://doi.org/10.3390/microorganisms11041070
Chicago/Turabian StyleXu, Fan, Chuan Liu, Miaomiao Xia, Shixin Li, Ran Tu, Sijia Wang, Hongxing Jin, and Dawei Zhang. 2023. "Characterization of a Riboflavin-Producing Mutant of Bacillus subtilis Isolated by Droplet-Based Microfluidics Screening" Microorganisms 11, no. 4: 1070. https://doi.org/10.3390/microorganisms11041070
APA StyleXu, F., Liu, C., Xia, M., Li, S., Tu, R., Wang, S., Jin, H., & Zhang, D. (2023). Characterization of a Riboflavin-Producing Mutant of Bacillus subtilis Isolated by Droplet-Based Microfluidics Screening. Microorganisms, 11(4), 1070. https://doi.org/10.3390/microorganisms11041070