Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century
Abstract
1. Antibiotic Resistance in the Global Healthcare Network: A Multifaceted Problem Involving Many Stakeholders of Disparate Sectors
1.1. The Modern Healthcare System: A Story of “Social Construction” with an Impact on AMR
1.2. The Understanding of AMR in the Healthcare Network: From Disease Ecology to Evolutionary Biology and Social Sciences
1.3. AMR at the Healthcare Network in the 21st Century: Novel Challenges in a Global World
2. Reframing AMR and Infectious Diseases in a Global Syndemic Scenario
2.1. Public Health to Control AMR in a Global Syndemic Scenario
2.2. AMR and Health, One-Health, and Global Health
2.3. Ecosystems and Their Feedback Loops: The Environmental Dimensions of AMR
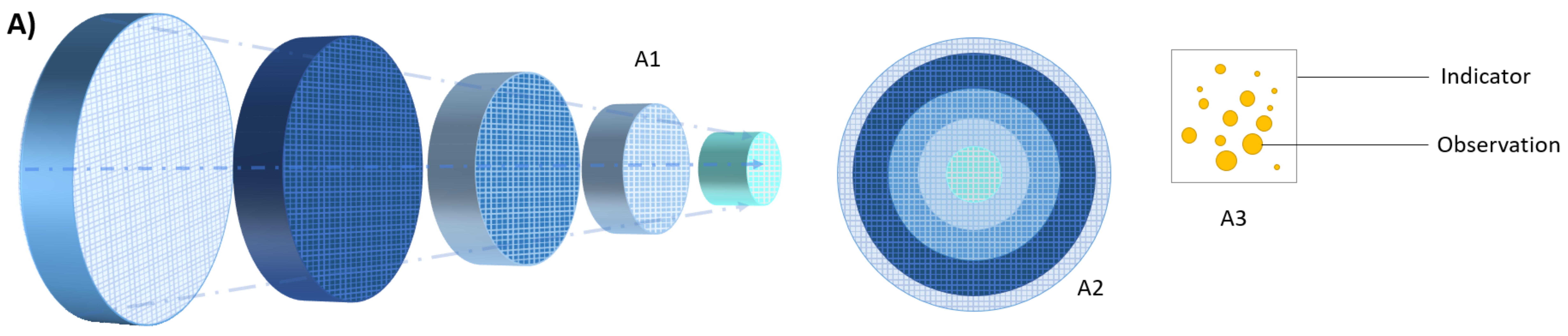
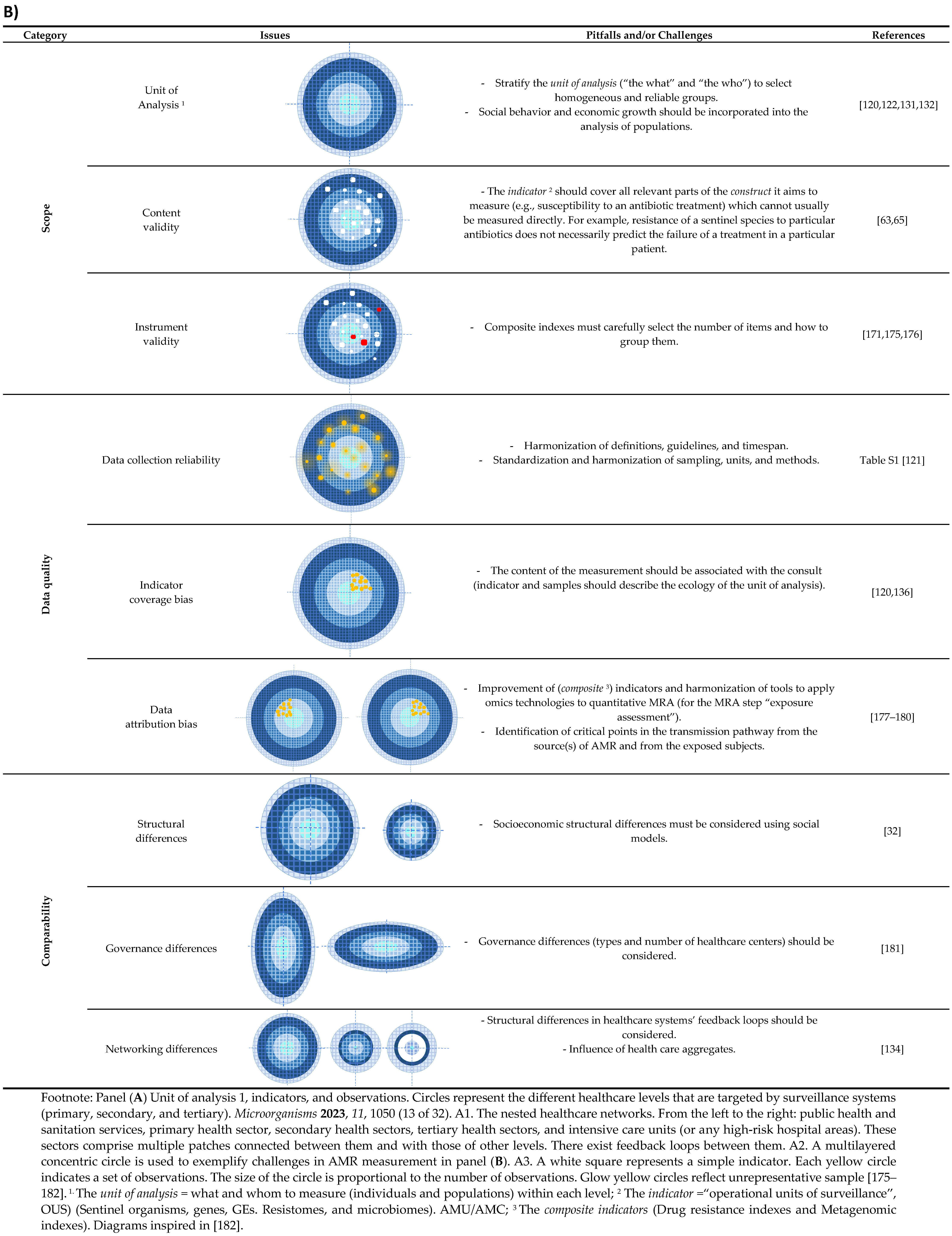
3. Challenges in Measuring AMR in the Heterogeneous Healthcare Network
3.1. The Sample (The Unit of Analysis)
3.2. The Indicators
3.3. Validity, Reliability, and Comparability of the Information
4. Heterogeneity of Stakeholders Involved in the Global Health Network: Activities, Objectives and Challenges
4.1. Patient-Centered (Addressing Clinical Demands)
4.2. Population-Centered (Addressing Policy Demands)
4.3. Microbial-Centered (Addressing Novel and Old Infection-Control Needs and Community Demands)
| Level | Stakeholders | Objectives | Required Information | Scope | Information Source | Gaps and Challenges |
|---|---|---|---|---|---|---|
| Patient-centered (addressing clinical demands) | Patients (and their families), doctors, clinical microbiologists, prescribers, and drug dispensers (pharmacists), and infection control practitioners. | Improving patient treatment. Design and implement standard treatment guidelines and essential drug lists. Expected burden of disease (BoD) at any geo-administrative level (individual setting). | Fine-scale information of individual risks for infection, colonization, and/or expected treatment outcome. Fine-scale information at setting level (identification of risks areas). | OUSs 1 Composite indexes (DRI, microbiome indexes) AMU | Real-time collection of local and stratified patient and AMC/AMU data. Lateral Public Health [208] | Develop criteria to define HAIs cases, still based on 48–72 h admission time [195,196]. Microbiome precision medicine [161,194]. Develop composite indexes [171,175]. Combine AMR and AMC/AMU trends in categorized patient populations [122,130,131,132,209,210]. Understand microbial transmission (plasmid, clones, and microbial consortiums). Plasmid surveillance: criteria, tools, and databases [156,160,211,212]. Evaluate the impact of the indoor microbiome on the health and safety of patients [164]. Implement AMU interventions based on behavioral models [13,213]. |
| Population-centered (addressing policy demands) | Health managers, infection control practitioners, public health experts, and health insurance companies. Politicians, policymakers, economists, and economic growth sectors. | To estimate the impact of AMR at national, regional, or international levels (trends and benchmarking). Identifying the leading causes for AMR emergence and spread. Quantifying AMC (pharmaco-epidemiology, sales). | Information aligned with validity, reliability, and comparability data from local, regional AMR surveillance networks. | Surveillance. OUS (list of priority pathogens). AMC (sales). Composite indexes. | Real-time collection of local and stratified patient data linked to local, regional, and/or national databases. Active population-based surveillance at local and regional levels | Identify population-level factors/groups linked to the emergence of AMR Granularity of the information to extrapolate estimates. Harmonize units of analysis and indicators (with appropriate corrector indexes. See Section 3 (data quality and comparability issues). Omic tools and infrastructures (capacity building, harmonized and standardized tools) [149,161]. Put AMR in the context of other Public Health threats (syndemics). Real-time surveillance only available in a few countries [121]. Community level surveillance adapted to healthcare loops (hospital wastewater, and insurance health networks,) [138,198,214]. |
| To design guidelines and policies. To monitor the effect of interventions. | Information aligned with validity, reliability and comparability data from regional AMR surveillance networks. | Aligned with National Antibiotic Plans (NAPs). | Active OneHealth sector-based surveillance aligned with NAPs | Harmonize NAPs according to local data and governance [181]. | ||
| Estimating the cost–benefit impact on public health, environment, and economy (BoD, biodiversity). | Return estimation ISOR. Social impact. | Public Health economics. | Public Health and Social data repositories. | Integrate the human, animal, and environmental policy outcomes with the economy markers. | ||
| Efficient awareness tools and campaigns between stakeholders and lay public to inform alerts or interventions (control, drug policies,…). | Awareness through feedback influencing consumers, policies, and investment. | Awareness Tailored according to social and cultural norms. | Consumers-public polls and enquiries. Data from patient’s associations (ITUs). | Develop efficient communication tools and channels (intra and inter-sectorial) [203]. Assure updating of messages [203]. Revise prescription models. | ||
| Revise market and marketing practices (pharma and food industry). | Evolution of sales and prescriptions. Control good practices. | Market and marketing. | National Health and Consumer services. | Strengthening governance, management, innovation, and financing [181]. | ||
| Pathogen-centered (addressing infection-control demands) 2 | Scientists and researchers, pharmaceutical industry, funding bodies, and global health donors. | Identification of transmission routes and microorganisms. Identify diagnostic biomarkers. Identification of reservoirs of MDR bacteria, plasmids, and hotspots for horizontal gene tranfer Identify PD parameters as minimal selective concentrations. Identify PD parameters as ecotoxicological concentrations. | Scientific publications, grey literature, workshop reports, international dossiers, white papers, and society reports. | Strains of microbial species (bacteria, fungi) of biomedical relevance from catalogued repositories and type strain collections. | Determine ethe effects of AMU (antibiotics and non-antibiotic antimicrobials) on dysbiosis patterns [164,215]. Understand microbial transmission (plasmid, clones, and microbial consortiums) Understand transmission pathways. (e.g., effects of particulate matters in the bacterial and fungal transmission, particularly in water–soil edges [216]. Plasmid surveillance: criteria, tools and databases [156,160,211,212]. Involve engineers in Public Health policies and guidelines. Environmental impact of sanitation measures [38]. Effects of interkingdom microbial interactions in the dynamics of MDR bacteria and ARGs [150]. Actions targeting the reduction of pharmaceuticals in the environment including the design, synthesis, and production of pharmaceuticals [38]. Determine the concentration of selective antibiotics for AMR in local environments [205,217,218]. Determine the impact of drugs in the environment to regulate microbial residue limits (MRLs) [218] 3. |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Terms | Definition/Comments |
|---|---|
| Abiotic reservoirs | The part of an ecosystem where a microorganism or a chemical accumulates or is stockpiled outside of living organisms. Abiotic surfaces are composed of a diversity of materials. The impact of environmental factors on the selection of bacteria on abiotic reservoirs is largely unexplored [38,217]. |
| AMR surveillance system | A structured and systematic procedure to measure the prevalence or incidence of AMR through continuous or periodical surveillance performed with a defined methodology and with specified indicators. Heterogeneities in the sampling, methods, or targets, for example, made current AMR surveillance systems inefficient for decision-making [85]. |
| Antibiotic resistance | See Table S1. |
| Bystander selection | The inadvertent pressure imposed by therapeutic treatments on microbes other than the targeted pathogen [215]. The effects of antibiotics on human microbiomes (at individual levels or at micro- and mesosystems) and on environmental microbiomes remain unexplored. |
| Disease ecology | The ecological study of host–pathogen interactions within the context of their environment and evolution as relating to the impact of diseases on populations. This field grows out of the awareness of the pervasive role of pathogens in ecosystems. While medical researchers, such as Theobald Smith, Frank Macfarlane Burnet, and Frank Fenner, approached ecological interactions at the level of human populations, René Dubos focused on the interface of human hosts and microbes in the physiological environments of individuals [59]. This view influenced the way to approach medical microbiology. |
| Community ecology | A subfield of ecology that investigates the factors that influence biodiversity, community structure, and the distribution and abundance of species (e.g., in the microbiosphere, interactions with the abiotic world, interactions between species, and between individuals of the same species (microbiome heterogeneity). Community ecology is the framework to analyze and interpret the human microbiome and resistome, including microbiomes of hospitalized patients, and hospital-built environment [73,169]. |
| Consumptogenic systems | The systems that promote the consumption of goods and services to the detriment of either population or environmental health. Excess consumption is arguably a product of societal pressures from cultural and economic actors encouraging market transactions to individual consumers that forge daily routines around consumption and trading money for goods and symbols to reflect their social status. An emphasis on the consumptogenic system attempts to shift the focus away from just individual choices to consume to the structural conditions that enable and promote excess consumption. |
| Environment, ecosystem, and assignation of these terms to the hospital space | The concept of “hospitals as environments” implies “hospitals as ecosystems”, comprising populations of individuals. Environment refers to “the sum total of all the conditions which surround man at a given space and time” [224], and, accordingly with the classic definition of ecosystem “the system resulting from the integration of all the living and non-living factors of the environment” [225,226]. |
| Exposome | The measure of all the exposures from our environment (diet, lifestyle, and professional) of an individual in a lifetime and how those exposures interact with our own unique characteristics (e.g., genetics, physiology, and epigenetics) relate to health [227]. |
| Evolutionary biology | A branch of biology focused on understanding the drivers’ (what/how) causal and stochastic processes and determined the evolutionary mechanisms (natural selection, common descent, and speciation-clonalization) that led to the current biodiversity of organisms on our planet [71]. |
| Globalization | The growing interdependence of the world’s economies, cultures, and populations brought about by cross-border trade in goods and services, technology, and flows of investment, people, and information [different articles/analysis by the World Bank, https://www.worldbank.org/, accessed on 28 March 2023 or World Economic Forum, https://www.weforum.org/, accessed on 28 March 2023]. |
| The Great Acceleration and the Anthropocene | The 1950s are the starting point of “The Great Acceleration” period which defines the sharp increase of the human activities (population, economy, resource use, and technologies) that drove rapid and unprecedented changes to the structure and functioning of the Earth System, such as the climate change. The “Great Acceleration” is the basis for a proposed new geologic epoch in Earth history, “the Anthropocene”. Both the Great Acceleration and the Anthropocene are often linked to the problem of AMR [14,228]. |
| Feedback loops | The part of a system in which some part (or all) of the system’s output is used as input for future actions. |
| Hybrid lineages | Biological lineages as bacterial species and clones, or mobile genetic elements that resulted from hybridization between two or more distinct lineages, frequently favored by DNA exchange [146]. |
| Health | The WHO defines it as “a state of complete physical, mental and social wellbeing and not merely the absence of disease or infirmity” in its Constitution of 1948 [29]. The definition implies to see health as a fundamental human right. However, the definition remains inadequately implemented at the level of national law and standardized codes of practice despite WHO requests, and with significant consequences (identify “health” with “wellbeing” and “health care” with “disease care [229]. Thus, it inadvertently contributes to the ‘over-medicalization’ of the population. It also allows a platform for industry, medical technologies, and professionals to redefine our health status. This results in unequal access to health care and ultimately social inequality by excluding most potential users from the development of standards and policy. |
| Health system | The WHO defines them as the totality of “organizations, people and actions whose primary intent is to promote, restore or maintain health”. The WHO health systems framework identifies the building blocks of a health system as related to governance, financial arrangements, medicines and technologies, health information systems, human resources, and health service delivery. This health system view is essential to approach the AMR problem [229]. |
| Health center or community-health centers (CHCs) | Community-based and patient-directed organizations that deliver comprehensive, culturally competent, high-quality primary healthcare services to the nation’s most vulnerable individuals and families, including people experiencing homelessness, agricultural workers, residents of public housing, and veterans. Health centers integrate access to pharmacy, mental health, substance use disorder, and oral health services in areas where economic, geographic, or cultural barriers limit access to affordable health care. |
| Hothouses | Closed and regulated environments with a high density and diversity of biological entities, favoring interactions between them. |
| Individual agency, agency relationship | Individual agency is the level of freedom and self-decision to act whereby people act as individual members of society (within cultures, countries) or given collective community (practitioners). Factors that determine or limit individual agency include social class, religion, gender, ethnicity, ability, customs, and professional sector. Individualism represents a cultural shift from and juxtaposition to collectivism. Agency relationship is a fiduciary relationship where one person (called the “principal”) allows an agent to act on his or her behalf (e.g., practitioner and patients). These terms are derived from sociology and are increasingly relevant in AMU/AMC policies [145,146]. |
| Koch’s postulates (or the Henle-Koch postulates) | Koch’s postulates (or Henle–Koch postulates) are four criteria designed to establish a causal relationship between a causative microbe and a disease. They were originally formulated by Friedrich Gustav Jacob Henle in the mid 1880s and refined and published by Robert Koch in 1890. Although they are still valid for a relatively small number of defined circumstances in which bacteria can be precisely tied to the cause of a particular clinical syndrome, they are revised under the light of available current knowledge [68,230]. |
| Miracle drugs | Sulfa drugs were coined as “magic bullets” and a “growing miracle” because they were credited with declines in mortality from childbirth, pneumonia, and other diseases by the late 1930s [11,201]. Shortly after, penicillin was hailed as a “miracle drug” when it proved its efficiency in curing untreatable infection episodes of septicemia [201]. These adjectives have been applied to drugs with antibiotic effect. |
| Risk assessment | A systematic and science-based approach to estimate the risks to the health and safety of persons arising out or in contact with AMR genes/pathogens, and also to support authorities in policymaking about the detection of critical points, and assessment of control and intervention strategies. Used in food safety [178] and infection control [180]. |
| Pandemics | A pandemic is “an epidemic occurring worldwide, or over a very wide area, crossing international boundaries, and usually affecting a large number of people” [231]. Although the definition has traditionally been associated with infectious diseases, it is increasingly applied to communicable and non-communicable diseases [77]. |
| Patches, landscape, and patches landscape | A patch is an area in a landscape that is different from surrounding areas. The patches generate what is called landscape heterogeneity, the diversity within a landscape. The diversity and uneven distribution that defines a landscape’s heterogeneity is based on many different features of an ecosystem (e.g., species and resources distribution and land-use patterns). The changes that occur in the separate spatial components of an ecosystem are described as patch dynamics. Patch dynamics is also a theoretical approach according to which the dynamics of an ecosystem can be understood through the analysis of its smaller spatial components (“patches”) that interact mutually. |
| Plasmid | The term “plasmid” was coined by Joshua Lederberg in 1952 to define any extrachromosomal determinant of heredity [232]. Outbreaks of MDR organisms in hospitals during 1950s or in farms in 1960s, demonstrated the transferability of single or combined (multiresistance) AMR phenotypes and represented the landmark discovery of non-Mendelian heredity. They are unique MGEs because of their ability to cross species barriers, to generate novel entities resulting from recombination events, and to increase the copy number (and thus the mutation rate) after gene acquisition [26,151,233]. |
| Population | The term “population” applies to the sets of individuals or evolutionary units with replication capacity that, at the subcellular (genes, plasmids, transposons, clones) and supracellular (bacterial communities, microbiomes, holobiome) level, are genetically and demographically connected. Thus, the ecological conditions (selection) in the system affect the adaptability of the units at each level (selective units) individually or cooperatively according to their degree of connectivity within a dynamic system (“evolution by association) [139,234]. |
| Resistome | Initially defined by D’Costa in 2006 as the set of genes in the pan genome which encode antibiotic resistance [235]. Other genes coding for resistance to antimicrobials that co-select AMR (heavy metals, antiseptics,…) could join this category [236]. |
| Social atomism | Atomism (or social atomism) is a sociological theory that refers to “the tendency for society to be made up of a collection of self-interested and largely self-sufficient individuals, operating as separate atoms”. Therefore, all social values, institutions, developments, and procedures evolve entirely out of the interests and actions of the individuals who inhabit any particular society. |
| Social behaviour components and layers | See Figure S1. |
| Syndemic or synergistic epidemic | The aggregation of two or more concurrent or sequential epidemics or diseases in a population with biological, psychological, or societal interactions and sharing common underlying societal drivers that exacerbate the prognosis and burden of disease. The syndemic approach contrasts with the biomedical approach to diseases that diagnostically isolate, study, and treat diseases as distinct entities separate from other diseases and independent of social contexts [75]. AMR is increasingly being approached as a syndemic threat (COVID, climate change, and emerging co-occurring infection). |
| SDGs | The Sustainable Development Goals (SDGs), also known as the Global Goals, were adopted by the United Nations in 2015 as a universal call to action to end poverty, protect the planet, and ensure that by 2030 all people enjoy peace and prosperity. The 17 SDGs are integrated—they recognize that action in one area will affect outcomes in others, and that development must balance social, economic, and environmental sustainability. |
| Therapeutic solutions to early major Public Health problems | Salvarsan represented the first effective treatment against syphilis; sulfonamides were the first effective options against infectious diseases of high mortality and morbidity, such as puerperal fever, pneumonia, scarlet fever, meningitis, gonorrhea, and erysipelas; and natural antibiotics (such as streptomycin and other aminoglycosides, penicillin, and tetracycline) were the first effective options for tuberculosis and safe alternatives to combat many infectious diseases. All were major public health problems in the early 20th century [15,16,18]. |
| “Watch lists” or “priority lists” of pathogens for which new antibiotics are urgently needed | They are catalogues of bacteria/fungi families that pose the greatest threat to human health. Their goal is to guide and promote research and development (R and D) of new antibiotics, to align R and D priorities with public health needs, and to coordinate the fight against AMR bacteria based on epidemiologic control of the infectious agent, its accurate surveillance and detection, and implementation of effective treatments [237]. In 2013, the Centers for Disease Control and Prevention (CDC) published the first “watch list of antibiotic threats” (AMR bacteria) which ranked them as urgent, serious, or concerning threats to humans [189,238]. In 2017, the WHO categorized the same pathogens into critical, high, and medium-priority groups in what they called priority pathogen lists [237,239]. The first watch list of fungal antimicrobial resistance threats has been available since late 2022 [239]. |
| World Antimicrobial Awareness Week (WAAW) | The WAAW is a global campaign that is celebrated annually to improve awareness and understanding of AMR and encourage best practices among the public, One Health stakeholders, and policymakers, who all play a critical role in reducing the further emergence and spread of AMR. |
References
- EClinicalMedicine Antimicrobial Resistance: A Top Ten Global Public Health Threat. EClinicalMedicine 2021, 41, 101221. [CrossRef] [PubMed]
- Gradmann, C. From Lighthouse to Hothouse: Hospital Hygiene, Antibiotics and the Evolution of Infectious Disease, 1950–1990. Hist. Philos. Life Sci. 2017, 40, 8. [Google Scholar] [CrossRef] [PubMed]
- Mughini-Gras, L.; Dorado-García, A.; van Duijkeren, E.; van den Bunt, G.; Dierikx, C.M.; Bonten, M.J.M.; Bootsma, M.C.J.; Schmitt, H.; Hald, T.; Evers, E.G.; et al. Attributable Sources of Community-Acquired Carriage of Escherichia Coli Containing β-Lactam Antibiotic Resistance Genes: A Population-Based Modelling Study. Lancet Planet Health 2019, 3, e357–e369. [Google Scholar] [CrossRef] [PubMed]
- Dorado-García, A.; Smid, J.H.; van Pelt, W.; Bonten, M.J.M.; Fluit, A.C.; van den Bunt, G.; Wagenaar, J.A.; Hordijk, J.; Dierikx, C.M.; Veldman, K.T.; et al. Molecular Relatedness of ESBL/AmpC-Producing Escherichia coli from Humans, Animals, Food and the Environment: A Pooled Analysis. J. Antimicrob. Chemother. 2018, 73, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Cantón, R.; Cornaglia, G. Public Health Microbiology, a Challenge for Europe. Clin. Microbiol. Infect. 2010, 16, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance-The Role of Public Health Organizations in Addressing Public Health Problems in Europe-NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536193/ (accessed on 28 March 2023).
- Baquero, F.; Lanza, V.F.; Cantón, R.; Coque, T.M. Public Health Evolutionary Biology of Antimicrobial Resistance: Priorities for Intervention. Evol. Appl. 2015, 8, 223–239. [Google Scholar] [CrossRef]
- World Bank. Drug-resistant infections: A threat to our economic future (Vol. 2): Final report (English). HNP/Agriculture Global Antimicrobial Resistance Initiative Washington, D.C.: World Bank Group. Available online: http://documents.worldbank.org/curated/en/323311493396993758/final-report (accessed on 28 March 2023).
- Roope, L.S.J.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Sarah Walker, A.; Robotham, J.V.; et al. The Challenge of Antimicrobial Resistance: What Economics Can Contribute. Science 2019, 364, eaau4679. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Review on Antimicrobial Resistance; Wellcome Trust: London, UK, 2014; Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 28 March 2023).
- Jayachandran, S.; Lleras-Muney, A.; Smith, K.V. Modern Medicine and the Twentieth Century Decline in Mortality: Evidence on the Impact of Sulfa Drugs. Am. Econ. J. Appl. Econ. 2010, 2, 118–146. [Google Scholar] [CrossRef]
- Dionisio, F.; Baquero, F.; Fuertes, M. Psychological and Cultural Factors Influencing Antibiotic Prescription. Trends Microbiol. 2023, 29. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0966842X22003468 (accessed on 28 March 2023). [CrossRef]
- Borek, A.J.; Santillo, M.; Wanat, M.; Butler, C.C.; Tonkin-Crine, S. How Can Behavioural Science Contribute to Qualitative Research on Antimicrobial Stewardship in Primary Care? JAC Antimicrob. Resist. 2022, 4, dlac007. [Google Scholar] [CrossRef]
- Jørgensen, P.S.; Aktipis, A.; Brown, Z.; Carrière, Y.; Downes, S.; Dunn, R.R.; Epstein, G.; Frisvold, G.B.; Hawthorne, D.; Gröhn, Y.T.; et al. Antibiotic and Pesticide Susceptibility and the Anthropocene Operating Space. Nat. Sustain. 2018, 1, 632–641. [Google Scholar]
- Landecker, H. Antibiotic Resistance and the Biology of History. Body Soc. 2016, 22, 19–52. [Google Scholar] [CrossRef] [PubMed]
- Landecker, H. Antimicrobials before Antibiotics: War, Peace, and Disinfectants. Palgrave Commun. 2019, 5, 45. [Google Scholar] [CrossRef]
- Podolsky, S.H. Historical Perspective on the Rise and Fall and Rise of Antibiotics and Human Weight Gain. Ann. Intern Med. 2017, 166, 133–138. [Google Scholar] [CrossRef]
- Podolsky, S.H. The Evolving Response to Antibiotic Resistance (1945–2018). Palgrave Commun. 2018, 4, 124. [Google Scholar] [CrossRef]
- Donzé, P.Y.; Fernández Pérez, P. Health Industries in the Twentieth Century. Bus. Hist. 2019, 61, 385–403. [Google Scholar] [CrossRef]
- American Chemical Society. Pfizer’s Work on Penicillin for World War II Becomes a National Historic Chemical Landmark. Available online: https://www.acs.org/pressroom/newsreleases/2008/june/pfizers-work-on-penicillin-for-world-war-ii-becomes-a-national-historic-chemical-landmark.html (accessed on 28 March 2023).
- Williams, K.J. The Introduction of ‘Chemotherapy’ Using Arsphenamine—the First Magic Bullet. J. R. Soc. Med. 2009, 102, 343. [Google Scholar] [CrossRef]
- Mohr, K.I. History of Antibiotics Research. Curr. Top Microbiol. Immunol. 2016, 398, 237–272. [Google Scholar]
- Tansey, E.M. Medicines and Men: Burroughs, Wellcome & Co, and the British Drug Industry before the Second World War. J. R. Soc. Med. 2002, 95, 411. [Google Scholar]
- UNDARK. “Big Chicken”: A 1948 Antibiotic Experiment That Shook the World. Available online: https://undark.org/2017/10/06/chicken-experiment-shook-world/ (accessed on 10 June 2017).
- Thoms, U. Between Promise and Threat: Antibiotics in Foods in West Germany 1950-1980. NTM Int. J. Hist. Ethics Nat. Sci. Technol. Med. 2012, 20, 181–214. [Google Scholar] [CrossRef]
- Watanabe, T. Infective Heredity of Multiple Drug Resistance in Bacteria. Bacteriol. Rev. 1963, 27, 87–115. [Google Scholar] [CrossRef] [PubMed]
- Farrar, W.E. Antibiotic Resistance in Developing Countries. J. Infect. Dis. 1985, 152, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Hone, T.; Macinko, J.; Millett, C. Revisiting Alma-Ata: What Is the Role of Primary Health Care in Achieving the Sustainable Development Goals? Lancet 2018, 392, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Constitution of the World Health Organization. Available online: https://www.who.int/about/governance/constitution (accessed on 28 March 2023).
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Gillings, M.; Simonet, P.; Stekel, D.; Banwart, S.; Penuelas, J. Microbial Mass Movements. Science 2017, 357, 1099–1100. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Antibiotic Resistance: Moving From Individual Health Norms to Social Norms in One Health and Global Health. Front. Microbiol. 2020, 11, 1914. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Emerging Microbial Threats to Health in the 21st Century. Microbial Threats to Health: Emergence, Detection, and Response; Smolinski, M.S., Hamburg, M.A., Lederberg, J., Eds.; National Academies Press (US): Washington, DC, USA, 2003. [Google Scholar]
- Cecchini, M.; Langer, J.; Slawomirski, L. Antimicrobial Resistance in G7 Countries and beyond: Economic Issues, Policies and Options for Action; Cecchini, M., Langer, J., Slawomirski, L., Eds.; Organization for Economic Co-operation and Development (OECD), 2015; pp. 1–75. Available online: https://www.oecd.org/els/health-systems/Antimicrobial-Resistance-in-G7-Countries-and-Beyond.pdf (accessed on 28 March 2023).
- O’Neill, J. Tackling a Crisis for the Health and Wealth of nations_1.pdf. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2016. [Google Scholar]
- Minh Pham, T.; Kondor, I.; Hanel, R.; Thurner, S. The Effect of Social Balance on Social Fragmentation. J. R. Soc. Interface 2020, 17, 20200752. [Google Scholar] [CrossRef]
- Antimicrobial Resistance and the United Nations Sustainable Development Cooperation Framework: Guidance for United Nations Country Teams. World Health Organization (WHO), Food and Agriculture Organization (FAO), World Organisation for Animal Health (OIE), UN Environment Programme (UNEP) eds. 2021. Available online: https://sdg.iisd.org/news/antimicrobial-resistance-threatens-development-sdgs-tripartite-report (accessed on 28 March 2023).
- United Nations Environment Programme. Bracing for Superbugs: Strengthening Environmental Action in the One Health Response to Antimicrobial Resistance. 2023. Available online: https://www.unep.org/resources/superbugs/environmental-action (accessed on 28 March 2023).
- Culturico. The Spreading Process of Social Atomization. Available online: https://culturico.com/2019/03/01/the-spreading-process-of-social-atomization/ (accessed on 28 March 2023).
- Lewontin, R.C. Biology as Ideology: The Doctrine of DNA; Harper Perennial Press: New York, NY, USA, 1991; Volume 148, pp. 148–162. [Google Scholar]
- Dubos, R.J. Microbiology. Annu. Rev. Biochem. 1942, 11, 659–678. [Google Scholar] [CrossRef]
- Moberg, C.L. René Dubos, a Harbinger of Microbial Resistance to Antibiotics. Perspect Biol. Med. 1999, 42, 559–580. [Google Scholar] [CrossRef]
- Dubos, R. Mirage of Health: Utopias, Progress, and Biological Change; Anchor Books: Garden City, NY, USA, 1959. [Google Scholar]
- Rogers, D.E. The Changing Pattern of Life-Threatening Microbial Disease. N. Engl. J. Med. 1959, 261, 677–683. [Google Scholar] [CrossRef]
- Williams, R.E.O.; Shooter, R.A. Infection in Hospitals. Epidemiology and Control. A Symposium; Williams, R.E.O., Shooter, R.A., Eds.; Blackwell Scientific Publications Ltd.: Oxford, UK, 1963. [Google Scholar]
- McDermott, W. The Problem of Staphylococcal Infection. Br. Med. J. 1956, 2, 837. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R. Stalking Microbes: A Relentless Pursuit of Infection Control; AuthorHouse Ltd.: Bloomington, IN, USA, 2005. [Google Scholar]
- Schaffner, W. Priorities in Infection Control: The Impact of New Technology. J. Hosp. Infect. 1984, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.E.O.; Blowers, R.; Garrod, L.P.; Shooter, R.A. Hospital Infection, Causes and Prevention; Lloyd-Luke (Medical Books) Ltd.: London, UK, 1960. [Google Scholar]
- Freeman, J.; McGowan, J.E.; Freeman, J.; McGowan, J.E. Risk Factors for Nosocomial Infection. J. Infect. Dis. 1978, 138, 811–819. [Google Scholar] [CrossRef]
- Barber, M.; Dutton, A.A.C.; Beard, M.A.; Elmes, P.C.; Williams, R. Reversal of Antibiotic Resistance in Hospital Staphylococcal Infection. Br. Med. J. 1960, 1, 11. [Google Scholar] [CrossRef]
- Dixon, R.E. Control of Health-Care--Associated Infections, 1961–2011. Morbility Mortal. Wkly. Rep. MMWR Suppl. 2011, 60, 58–63. [Google Scholar]
- World Health Organization (WHO). Surveillance for the Prevention and Control of Health Hazards due to Antibiotic-Resistant Enterobacteria: Report of a WHO Meeting. 1978. Available online: https://apps.who.int/iris/handle/10665/41319 (accessed on 28 March 2023).
- World Health Organization (WHO). Public health aspects of antibiotic-resistant bacteria in the environment: Report on a consultation meeting, Brussels, Belgium, 9–12 December 1975. Available online: https://agris.fao.org/agris-search/search.do?recordID=XF2015030555 (accessed on 28 March 2023).
- Levy, S.B.; Clowes, R.C.; Koenig, E.L. Statement Regarding Worldwide Antibiotic Misuse. In Molecular Biology, Pathogenicity, and Ecology of Bacterial Plasmids; Plenum: New York, NY, USA, 1981; pp. 679–681. [Google Scholar]
- Alliance for the Prudent Use of Antibiotics (APUA). Available online: https://apua.org/ourhistory (accessed on 28 March 2023).
- Anderson, R.M. The Pandemic of Antibiotic Resistance. Nature Med. 1999, 5, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B. The Antibiotic Paradox; Springer: New York, NY, USA, 1992. [Google Scholar]
- Honigsbaum, M. Antibiotic Antagonist: The Curious Career of René Dubos. Lancet 2016, 387, 118. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Levin, B.R. Population Dynamics of Tuberculosis Treatment: Mathematical Models of the Roles of Non-Compliance and Bacterial Heterogeneity in the Evolution of Drug Resistance. Int. Union Against Tuberc. Lung Dis. 1998, 2, 187–199. [Google Scholar]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and Combating Antibiotic Resistance from One Health and Global Health Perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 1 January 2016).
- Perry, J.A.; Westman, E.L.; Wright, G.D. The Antibiotic Resistome: What’s New? Curr. Opin. Microbiol. 2014, 21, 45–50. [Google Scholar] [CrossRef]
- Perry, J.A.; Wright, G.D. The Antibiotic Resistance “Mobilome”: Searching for the Link between Environment and Clinic. Front. Microbiol. 2013, 4, 138. [Google Scholar] [CrossRef]
- Ruppé, E.; Ghozlane, A.; Tap, J.; Pons, N.; Alvarez, A.S.; Maziers, N.; Cuesta, T.; Hernando-Amado, S.; Clares, I.; Martínez, J.L.; et al. Prediction of the Intestinal Resistome by a Three-Dimensional Structure-Based Method. Nat. Microbiol. 2019, 4, 112–123. [Google Scholar] [CrossRef]
- Lax, S.; Gilbert, J.A. Hospital-Associated Microbiota and Implications for Nosocomial Infections. Trends Mol. Med. 2015, 21, 427–432. [Google Scholar] [CrossRef]
- Miller, E.T.; Svanbäck, R.; Bohannan, B.J.M. Microbiomes as Metacommunities: Understanding Host-Associated Microbes through Metacommunity Ecology. Trends Ecol. Evol. 2018, 33, 926–935. [Google Scholar] [CrossRef]
- Byrd, A.L.; Segre, J.A. Adapting Koch’s Postulates. Science 2016, 351, 224–226. [Google Scholar] [CrossRef]
- Koskella, B.; Hall, L.J.; Metcalf, C.J.E. The Microbiome beyond the Horizon of Ecological and Evolutionary Theory. Nat. Ecol. Evol. 2017, 1, 1606–1615. [Google Scholar] [CrossRef]
- Tibayrenc, M. The Golden Age of Genetics and the Dark Age of Infectious Diseases. Infect. Genet. Evol. 2001, 1, 1–2. [Google Scholar] [CrossRef]
- Nesse, R.M.; Bergstrom, C.T.; Ellison, P.T.; Flier, J.S.; Gluckman, P.; Govindaraju, D.R.; Niethammer, D.; Omenn, G.S.; Perlman, R.L.; Schwartz, M.D.; et al. Making Evolutionary Biology a Basic Science for Medicine. Proc. Natl. Acad. Sci. USA 2010, 107, 1800–1807. [Google Scholar] [CrossRef]
- Schlechte, J.; Zucoloto, A.Z.; Yu, I.L.; Doig, C.J.; Dunbar, M.J.; McCoy, K.D.; McDonald, B. Dysbiosis of a Microbiota–Immune Metasystem in Critical Illness Is Associated with Nosocomial Infections. Nat. Med. 2023, 1–11. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Lynch, S.V. Community Ecology as a Framework for Human Microbiome Research. Nat. Med. 2019, 25, 884–889. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; De Roode, J.C.; Fenton, A. Why Infectious Disease Research Needs Community Ecology. Science 2015, 349, 1259504. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Clair, S. Syndemics and Public Health: Reconceptualizing Disease in Bio-Social Context. Med. Anthropol. Q. 2003, 17, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.M.; Akinyi, M.Y.; DeWitte, S.N.; Stone, A.C. Ancient Pathogens Provide a Window into Health and Well-Being. Proc. Natl. Acad. Sci. USA 2023, 120, e2209476119. [Google Scholar] [CrossRef] [PubMed]
- Stephen, C. Rethinking Pandemic Preparedness in the Anthropocene. Healthc. Manag. Forum 2020, 33, 153–157. [Google Scholar] [CrossRef]
- Baquero, F.; Coque, T.M.; Martínez, J.L.; Aracil-Gisbert, S.; Lanza, V.F. Gene Transmission in the One Health Microbiosphere and the Channels of Antimicrobial Resistance. Front. Microbiol. 2019, 10, 2892. [Google Scholar] [CrossRef] [PubMed]
- Eimer, J.; Patimeteeporn, C.; Jensenius, M.; Gkrania-Klotsas, E.; Duvignaud, A.; Barnett, E.D.; Hochberg, N.S.; Chen, L.H.; Trigo-Esteban, E.; Gertler, M.; et al. Multidrug-Resistant Tuberculosis Imported into Low-Incidence Countries—a GeoSentinel Analysis, 2008–2020. J. Travel. Med. 2021, 28, taab069. [Google Scholar] [CrossRef]
- Chatham-Stephens, K.; Medalla, F.; Hughes, M.; Appiah, G.D.; Aubert, R.D.; Caidi, H.; Angelo, K.M.; Walker, A.T.; Hatley, N.; Masani, S.; et al. Emergence of Extensively Drug-Resistant Salmonella Typhi Infections Among Travelers to or from Pakistan — United States, 2016–2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 11–13. [Google Scholar] [CrossRef]
- Voor In ’T Holt, A.F.; Mourik, K.; Beishuizen, B.; van der Schoor, A.S.; Verbon, A.; Vos, M.C.; Severin, J.A. Acquisition of Multidrug-Resistant Enterobacterales during International Travel: A Systematic Review of Clinical and Microbiological Characteristics and Meta-Analyses of Risk Factors. Antimicrob. Resist. Infect. Control 2020, 9, 71. [Google Scholar] [CrossRef]
- Nielsen, R.T.; Köse, G.; Sloth, L.; Andersen, C.Ø.; Petersen, J.H.; Norredam, M. Pathogen Distribution and Antimicrobial Resistance in Infections in Migrants and Non-migrants in Denmark, a Cross-Sectional Study. Trop. Med. Int. Health 2022, 27, 999–1008. [Google Scholar] [CrossRef]
- Rodríguez-Molina, D.; Berglund, F.; Blaak, H.; Flach, C.F.; Kemper, M.; Marutescu, L.; Gradisteanu, G.P.; Popa, M.; Spießberger, B.; Wengenroth, L.; et al. International Travel as a Risk Factor for Carriage of Extended-Spectrum β-Lactamase-Producing Escherichia coli in a Large Sample of European Individuals—The AWARE Study. Int. J. Environ. Res. Public Health 2022, 19, 4758. [Google Scholar] [CrossRef]
- Mcneil, C.J.; Kirkcaldy, R.D.; Workowski, K. Enteric Infections in Men Who Have Sex With Men. Clin. Infect. Dis. 2022, 74, S169–S178. [Google Scholar] [CrossRef] [PubMed]
- Charles, H.; Prochazka, M.; Thorley, K.; Crewdson, A.; Greig, D.R.; Jenkins, C.; Painset, A.; Fifer, H.; Browning, L.; Cabrey, P.; et al. Outbreak of Sexually Transmitted, Extensively Drug-Resistant Shigella sonnei in the UK, 2021–2022: A Descriptive Epidemiological Study. Lancet Infect. Dis. 2022, 22, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Ngaruka, G.B.; Neema, B.B.; Mitima, T.K.; Kishabongo, A.S.; Kashongwe, O.B. Animal Source Food Eating Habits of Outpatients with Antimicrobial Resistance in Bukavu, D.R. Congo. Antimicrob. Resist. Infect. Control 2021, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Yamaichi, Y.; Chao, M.C.; Sasabe, J.; Clark, L.; Davis, B.M.; Yamamoto, N.; Mori, H.; Kurokawa, K.; Waldor, M.K. High-Resolution Genetic Analysis of the Requirements for Horizontal Transmission of the ESBL Plasmid from Escherichia coli O104:H4. Nucleic Acids Res. 2014, 43, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.T. Antimicrobial Resistance and Aging: Beginning of the End of the Antibiotic Era? J. Am. Geriatr. Soc. 2002, 50, 226–229. [Google Scholar] [CrossRef]
- Mendenhall, E.; Kohrt, B.A.; Logie, C.H.; Tsai, A.C. Syndemics and Clinical Science. Nat. Med. 2022, 28, 1359–1362. [Google Scholar] [CrossRef]
- REAct. Tracking Antimicrobial Resistance in the Sustainable Development Goals. 2019. Available online: https://www.reactgroup.org/news-and-views/news-and-opinions/year-2019/tracking-antimicrobial-resistance-in-the-sustainable-development-goals/ (accessed on 28 March 2023).
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; de Schutter, O.; Devarajan, R.; et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission Report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef]
- Allen, L. Are We Facing a Noncommunicable Disease Pandemic? J. Epidemiol. Glob. Health 2017, 7, 5–9. [Google Scholar] [CrossRef]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial Resistance and Covid-19: Intersections and Implications. Elife 2021, 10, 1–27. [Google Scholar] [CrossRef]
- Klein, E.Y.; van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Kenyon, C.; Kenyon, C.; Manoharan-Basil, S.S.; van Dijck, C. Gonococcal Resistance Can Be Viewed Productively as Part of a Syndemic of Antimicrobial Resistance: An Ecological Analysis of 30 European Countries. Antimicrob. Resist. Infect. Control 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Goldberg, J.; Uyen-Cateriano, A.; Van Gassen, G. Making Matters Worse: Antimicrobial Resistance in Conflict Settings. Available online: https://revive.gardp.org/making-matters-worse-antimicrobial-resistance-in-conflict-settings/ (accessed on 23 June 2022).
- Organization for Economic Co-Operation and Development (OECD). Stemming the Superbug Tide: Just A Few Dollars More. Available online: https://www.oecd-ilibrary.org/social-issues-migration-health/stemming-the-superbug-tide_9789264307599-en?itemId=/content/publication/9789264307599-en&_csp_=33f7c05cefd0845031c4db9085aa0f90&itemIGO=oecd&itemContentType=book (accessed on 7 November 2018).
- Burnham, J.P. Climate Change and Antibiotic Resistance: A Deadly Combination. Ther. Adv. Infect. Dis. 2021, 8, 2049936121991374. [Google Scholar] [CrossRef]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic Resistance Increases with Local Temperature. Nature Climate Chang. 2018, 8, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ Warning to Humanity: Microorganisms and Climate Change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Paull, S.H.; Song, S.; McClure, K.M.; Sackett, L.C.; Kilpatrick, A.M.; Johnson, P.T.J. From Superspreaders to Disease Hotspots: Linking Transmission across Hosts and Space. Front. Ecol. Environ. 2012, 10, 75. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). Antimicrobial Resistance, Fueled by the COVID-19 Pandemic. Policy Brief November 2021. 2022. Available online: https://iris.paho.org/handle/10665.2/55864 (accessed on 28 March 2023).
- Liebana, E.; Carattoli, A.; Coque, T.M.; Hasman, H.; Magiorakos, A.-P.; Mevius, D.; Peixe, L.; Poirel, L.; Schuepbach-Regula, G.; Torneke, K.; et al. Public Health Risks of Enterobacterial Isolates Producing Extended-Spectrum β-Lactamases or AmpC β-Lactamases in Food and Food-Producing Animals: An EU Perspective of Epidemiology, Analytical Methods, Risk Factors, and Control Options. Clin. Infect. Dis. 2013, 56, 1030–1037. [Google Scholar] [CrossRef]
- Gaygısız, Ü.; Lajunen, T.; Gaygısız, E. Socio-Economic Factors, Cultural Values, National Personality and Antibiotics Use: A Cross-Cultural Study among European Countries. J. Infect. Public Health 2017, 10, 755–760. [Google Scholar] [CrossRef]
- Park, S.; Kang, J.E.; Choi, H.J.; Kim, C.J.; Chung, E.K.; Kim, S.A.; Rhie, S.J. Antimicrobial Stewardship Programs in Community Health Systems Perceived by Physicians and Pharmacists: A Qualitative Study with Gap Analysis. Antibiotics 2019, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Cooke, P.; Ahorlu, C.; Arjyal, A.; Baral, S.; Carter, L.; Dasgupta, R.; Fieroze, F.; Fonseca-Braga, M.; Huque, R.; et al. Community Engagement: The Key to Tackling Antimicrobial Resistance (AMR) across a One Health Context? Glob. Public Health 2022, 17, 2647–2664. [Google Scholar] [CrossRef]
- Rutter, H.; Savona, N.; Glonti, K.; Bibby, J.; Cummins, S.; Finegood, D.T.; Greaves, F.; Harper, L.; Hawe, P.; Moore, L.; et al. The Need for a Complex Systems Model of Evidence for Public Health. Lancet 2017, 390, 2602–2604. [Google Scholar] [CrossRef] [PubMed]
- Van den Bunt, G.; Fluit, A.C.; Spaninks, M.P.; Timmerman, A.J.; Geurts, Y.; Kant, A.; Scharringa, J.; Mevius, D.; Wagenaar, J.A.; Bonten, M.J.M.; et al. Faecal Carriage, Risk Factors, Acquisition and Persistence of ESBL-Producing Enterobacteriaceae in Dogs and Cats and Co-Carriage with Humans Belonging to the Same Household. J. Antimicrob. Chemother. 2020, 75, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.K.; Guttman, D.S.; Fitzgerald, J.R. Population Genomics of Bacterial Host Adaptation. Nat. Rev. Gen. 2018, 19, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.J.M.; Mevius, D. Less Evidence for an Important Role of Food-Producing Animals as Source of Antibiotic Resistance in Humans. Clin. Infect. Dis. 2015, 60, 1867. [Google Scholar] [CrossRef]
- Pruden, A.; Larsson, D.G.J.; Amézquita, A.; Collignon, P.; Brandt, K.K.; Graham, D.W.; Lazorchak, J.M.; Suzuki, S.; Silley, P.; Snape, J.R.; et al. Management Options for Reducing the Release of Antibiotics and Antibiotic Resistance Genes to the Environment. Environ. Health Perspect. 2013, 121, 878–885. [Google Scholar] [CrossRef]
- Mounier-Jack, S.; Griffiths, U.K.; Closser, S.; Burchett, H.; Marchal, B. Measuring the Health Systems Impact of Disease Control Programmes: A Critical Reflection on the WHO Building Blocks Framework. BMC Public Health 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Sunsern, R.; Lawang, W. Bronfenbrenner’s Ecological Model: Theoretical Lens for a Community-Based Research. J. Health Sci. Altern. Med. 2019, 1, 4–7. [Google Scholar]
- Bronfenbrenner, U.; Morris, P.A. The Bioecological Model of Human Development. In Handbook of Child Psychology. Theoretical Models of Human Development; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Volume 1, p. 793. [Google Scholar]
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year Due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Jernigan, J.A.; Hatfield, K.M.; Wolford, H.; Nelson, R.E.; Olubajo, B.; Reddy, S.C.; McCarthy, N.; Paul, P.; McDonald, L.C.; Kallen, A.; et al. Multidrug-Resistant Bacterial Infections in U.S. Hospitalized Patients, 2012–2017. N. Engl. J. Med. 2022, 382, 1309–1319. [Google Scholar] [CrossRef]
- Infectious Diseases Society of America (IDSA) Drug-Resistant Infections Led to $1.9 Billion in Health Care Costs, More Than 10,000 Deaths Among Older Adults in One Year. Available online: https://www.idsociety.org/news--publications-new/articles/2021/drug-resistant-infections-led-to-$1.9-billion-in-health-care-costs-more-than-10000-deaths-among-older-adults-in-one-year/ (accessed on 21 January 2023).
- Grimshaw, A.; Ferrari, A.; Lindahl, G.; Holcomb, K. Metasystems. Commun. ACM 1998, 41, 46–47. [Google Scholar] [CrossRef]
- Campos, M.; Capilla, R.; Naya, F.; Futami, R.; Coque, T.; Moya, A.; Fernandez-Lanza, V.; Cantón, R.; Sempere, J.M.; Llorens, C.; et al. Simulating Multilevel Dynamics of Antimicrobial Resistance in a Membrane Computing Model. mBio 2019, 10, e02460-18. [Google Scholar] [CrossRef]
- Grundmann, H. Towards a Global Antibiotic Resistance Surveillance System: A Primer for a Roadmap. Ups J. Med. Sci. 2014, 119, 87–95. [Google Scholar] [CrossRef]
- Diallo, O.O.; Baron, S.A.; Abat, C.; Colson, P.; Chaudet, H.; Rolain, J.M. Antibiotic Resistance Surveillance Systems: A Review. J. Glob. Antimicrob. Resist. 2020, 23, 430–438. [Google Scholar] [CrossRef]
- Tacconelli, E.; Sifakis, F.; Harbarth, S.; Schrijver, R.; van Mourik, M.; Voss, A.; Sharland, M.; Rajendran, N.B.; Rodríguez-Baño, J.; Bielicki, J.; et al. Surveillance for Control of Antimicrobial Resistance. Lancet Infect Dis. 2018, 18, e99–e106. [Google Scholar] [CrossRef]
- Johnson, A.P. Surveillance of Antibiotic Resistance. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140080. [Google Scholar] [CrossRef] [PubMed]
- Frost, I.; Kapoor, G.; Craig, J.; Liu, D.; Laxminarayan, R. Status, Challenges and Gaps in Antimicrobial Resistance Surveillance around the World. J. Glob. Antimicrob. Resist. 2021, 25, 222–226. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Dye, C.; Etard, J.F.; Smith, T.; Charlwood, J.D.; Garnett, G.P.; Hagan, P.; Hii, J.L.K.; Ndhlovu, P.D.; Quinnell, R.J.; et al. Heterogeneities in the Transmission of Infectious Agents: Implications for the Design of Control Programs. Proc. Natl. Acad. Sci. USA 1997, 94, 338. [Google Scholar] [CrossRef]
- Gough, E.K. The Impact of Mass Drug Administration of Antibiotics on the Gut Microbiota of Target Populations. Infect Dis. Poverty 2022, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Nellums, L.B.; Thompson, H.; Holmes, A.; Castro-Sánchez, E.; Otter, J.A.; Norredam, M.; Friedland, J.S.; Hargreaves, S. Antimicrobial Resistance among Migrants in Europe: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2018, 18, 796–811. [Google Scholar] [CrossRef]
- Lloyd-Smith, J.O.; Schreiber, S.J.; Kopp, P.E.; Getz, W.M. Superspreading and the Effect of Individual Variation on Disease Emergence. Nature 2005, 438, 355. [Google Scholar] [CrossRef]
- Baquero, F.; Saralegui, C.; Marcos-Mencía, D.; Ballestero, L.; Vañó-Galván, S.; Moreno-Arrones, Ó.M.; del Campo, R. Epidermis as a Platform for Bacterial Transmission. Front. Immunol. 2021, 12, 4935. [Google Scholar] [CrossRef]
- Sibani, M.; Mazzaferri, F.; Carrara, E.; Pezzani, M.D.; Arieti, F.; Göpel, S.; Paul, M.; Tacconelli, E.; Mutters, N.T.; Voss, A. White Paper: Bridging the Gap between Surveillance Data and Antimicrobial Stewardship in Long-Term Care Facilities—Practical Guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net Networks. J. Antimicrob. Chemother. 2020, 75, ii33. [Google Scholar] [CrossRef]
- Pezzani, M.D.; Carrara, E.; Sibani, M.; Presterl, E.; Gastmeier, P.; Renk, H.; Kanj, S.S.; Velavan, T.P.; Song, L.H.; Leibovici, L.; et al. White Paper: Bridging the Gap between Human and Animal Surveillance Data, Antibiotic Policy and Stewardship in the Hospital Sector-Practical Guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net Networks. J. Antimicrob. Chemother. 2020, 75, II20–II32. [Google Scholar] [CrossRef]
- Arieti, F.; Göpel, S.; Sibani, M.; Carrara, E.; Pezzani, M.D.; Murri, R.; Mutters, N.T.; Lòpez-Cerero, L.; Voss, A.; Cauda, R.; et al. White Paper: Bridging the Gap between Surveillance Data and Antimicrobial Stewardship in the Outpatient Sector-Practical Guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net Networks. J. Antimicrob. Chemother. 2020, 75, II42–II51. [Google Scholar] [CrossRef]
- Feng, J.; Guo, Z.; Ai, L.; Liu, J.; Zhang, X.; Cao, C.; Xu, J.; Xia, S.; Zhou, X.N.; Chen, J.; et al. Establishment of an Indicator Framework for Global One Health Intrinsic Drivers Index Based on the Grounded Theory and Fuzzy Analytical Hierarchy-Entropy Weight Method. Infect. Dis. Poverty 2022, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Naylor, N.R.; Lines, J.; Waage, J.; Wieland, B.; Knight, G.M. Quantitatively Evaluating the Cross-Sectoral and One Health Impact of Interventions: A Scoping Review and Case Study of Antimicrobial Resistance. One Health 2020, 11, 100194. [Google Scholar] [CrossRef]
- Ashley, E.A.; Recht, J.; Chua, A.; Dance, D.; Dhorda, M.; Thomas, N.V.; Ranganathan, N.; Turner, P.; Guerin, P.J.; White, N.J.; et al. An Inventory of Supranational Antimicrobial Resistance Surveillance Networks Involving Low- and Middle-Income Countries since 2000. J. Antimicrob. Chemother. 2018, 73, 1737–1749. [Google Scholar] [CrossRef]
- Perkins, K.M.; Reddy, S.C.; Fagan, R.; Arduino, M.J.; Perz, J.F. Investigation of Healthcare Infection Risks from Water-Related Organisms: Summary of CDC Consultations, 2014–2017. Infect. Control Hosp. Epidemiol. 2019, 40, 621–626. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, L.R.; Stapleton, P.J.; Eshaghi, A.; Soares, D.; Brisse, S.; Patel, S.N.; Kus, J.V. Application of Whole Genome Sequencing to Query a Potential Outbreak of Elizabethkingia Anophelis in Ontario, Canada. Access Microbiol. 2019, 1, e000017. [Google Scholar] [CrossRef]
- Liguori, K.; Keenum, I.; Davis, B.C.; Calarco, J.; Milligan, E.; Harwood, V.J.; Pruden, A. Antimicrobial Resistance Monitoring of Water Environments: A Framework for Standardized Methods and Quality Control. Environ. Sci. Technol. 2022, 56, 9149–9160. [Google Scholar] [CrossRef]
- Fernando, B.; Coque, T.M. Multilevel Population Genetics in Antibiotic Resistance. FEMS Microbiol. Rev. 2011, 35, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Willems, R.J.L.; Hanage, W.P.; Bessen, D.E.; Feil, E.J. Population Biology of Gram-Positive Pathogens: High-Risk Clones for Dissemination of Antibiotic Resistance. FEMS Microbiol. Rev. 2011, 35, 872–900. [Google Scholar] [CrossRef]
- Woodford, N.; Turton, J.F.; Livermore, D.M. Multiresistant Gram-Negative Bacteria: The Role of High-Risk Clones in the Dissemination of Antibiotic Resistance. FEMS Microbiol. Rev. 2011, 35, 736–755. [Google Scholar] [CrossRef]
- Riley, L.W. Pandemic Lineages of Extraintestinal Pathogenic Escherichia coli. Clin. Microbiol. Infect. 2014, 20, 380–390. [Google Scholar] [CrossRef]
- Tedim, A.P.; Ruiz-Garbajosa, P.; Corander, J.; Rodríguez, C.M.; Cantón, R.; Willems, R.; Baquero, F.; Coque, T.M. Population Biology of Enterococcus from Intestinal Colonization in Hospitalized and Non-Hospitalized Individuals in Different Age Groups. Appl. Environ. Microbiol. 2014, 81, AEM-03661. [Google Scholar]
- Valverde, A.; Grill, F.; Coque, T.M.; Pintado, V.; Baquero, F.; Canton, R.; Cobo, J. High Rate of Intestinal Colonization with Extended-Spectrum- -Lactamase-Producing Organisms in Household Contacts of Infected Community Patients. J. Clin. Microbiol. 2008, 46, 2796–2799. [Google Scholar] [CrossRef]
- Faith, J.J.; Colombel, J.F.; Gordon, J.I. Identifying Strains That Contribute to Complex Diseases through the Study of Microbial Inheritance. Proc. Natl. Acad. Sci. USA 2015, 112, 633–640. [Google Scholar] [CrossRef]
- Croucher, N.J.; Klugman, K.P. The Emergence of Bacterial “Hopeful Monsters”. MBio 2014, 5, e01550-14. [Google Scholar] [CrossRef]
- Cointe, A.; Birgy, A.; Mariani-Kurkdjian, P.; Liguori, S.; Courroux, C.; Blanco, J.; Delannoy, S.; Fach, P.; Loukiadis, E.; Bidet, P.; et al. Emerging Multidrug-Resistant Hybrid Pathotype Shiga Toxin–Producing Escherichia coli O80 and Related Strains of Clonal Complex 165, Europe. Emerg. Infect. Dis. 2018, 24, 2262. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). ECDC strategic framework for the integration of molecular and genomic typing into European surveillance and multi-country outbreak investigations 2019–2021. Stockholm: ECDC; 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/framework-for-genomic-surveillance.pdf (accessed on 28 March 2023).
- Hendriksen, R.S.; Bortolaia, V.; Tate, H.; Tyson, G.H.; Aarestrup, F.M.; McDermott, P.F. Using Genomics to Track Global Antimicrobial Resistance. Front. Public Health 2019, 7, 242. [Google Scholar] [CrossRef]
- Amaro, F.; Martín-González, A. Microbial Warfare in the Wild-the Impact of Protists on the Evolution and Virulence of Bacterial Pathogens. Int. Microbiol. 2021, 24, 559–571. [Google Scholar] [CrossRef]
- Baquero, F.; Martínez, J.L.; Lanza, V.F.; Rodríguez-Beltrán, J.; Galán, J.C.; San Millán, A.; Cantón, R.; Coque, T.M. Evolutionary Pathways and Trajectories in Antibiotic Resistance. Clin. Microbiol. Rev. 2021, 34, e00050-19. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, B.; Chau, K.K.; Phuong Quan, T.; Rodger, G.; Andersson, M.I.; Jeffery, K.; Lipworth, S.; Gweon, H.S.; Peniket, A.; Pike, G.; et al. Genomic Surveillance of Escherichia coli and Klebsiella spp. in Hospital Sink Drains and Patients. Microb. Genom. 2020, 6, 4–16. [Google Scholar] [CrossRef]
- Lipworth, S.; Matlock, W.; Shaw, L.; Rodger, G.; Chau, K.; Barker, L.; George, S.; Kavanagh, J.; Davies, T.; Vaughan, A.; et al. The Mobilome Associated with Gram-Negative Bloodstream Infections: A Large-Scale Observational Hybrid Sequencing Based Study. medRxiv 2022. [CrossRef]
- Rodrigues, C.; Lanza, V.F.; Peixe, L.; Coque, T.M.; Novais, Â.; the Epidemiological Markers Study Group (ESGEM). Phylogenomics of Globally Spread Clonal Groups 14 and 15 of Klebsiella pneumoniae. bioRxiv 2022. [CrossRef]
- Johnson, T.J.; Danzeisen, J.L.; Youmans, B.; Case, K.; Llop, K.; Munoz-Aguayo, J.; Flores-Figueroa, C.; Aziz, M.; Stoesser, N.; Sokurenko, E.; et al. Separate F-Type Plasmids Have Shaped the Evolution of the H30 Subclone of Escherichia coli Sequence Type 131. MSphere 2016, 1, e00121-16. [Google Scholar] [CrossRef] [PubMed]
- Lanza, V.F.; Tedim, A.P.; Martínez, J.L.; Baquero, F.; Coque, T.M. The Plasmidome of Firmicutes: Impact on the Emergence and the Spread of Resistance to Antimicrobials. Microbiol. Spectr. 2015, 3, 379–419. [Google Scholar] [CrossRef]
- León-Sampedro, R.; DelaFuente, J.; Díaz-Agero, C.; Crellen, T.; Musicha, P.; Rodríguez-Beltrán, J.; de la Vega, C.; Hernández-García, M.; López-Fresneña, N.; Ruiz-Garbajosa, P.; et al. Pervasive Transmission of a Carbapenem Resistance Plasmid in the Gut Microbiota of Hospitalized Patients. Nat. Microbiol. 2021, 6, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Tato, M.; Coque, T.M.; Ruíz-Garbajosa, P.; Pintado, V.; Cobo, J.; Sader, H.S.; Jones, R.N.; Baquero, F.; Cantón, R. Complex Clonal and Plasmid Epidemiology in the First Outbreak of Enterobacteriaceae Infection Involving VIM-1 Metallo-Beta-Lactamase in Spain: Toward Endemicity? Clin. Infect. Dis. 2007, 45, 1171–1178. [Google Scholar] [CrossRef]
- David, S.; Cohen, V.; Reuter, S.; Sheppard, A.E.; Giani, T.; Parkhill, J.; Rossolini, G.M.; Feil, E.J.; Grundmann, H.; Aanensen, D.M. Integrated Chromosomal and Plasmid Sequence Analyses Reveal Diverse Modes of Carbapenemase Gene Spread among Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA 2020, 117, 25043–25054. [Google Scholar] [CrossRef]
- Joint Programming Initiative on Antimicrobial Resistance (JPIAMR). TEAPOTS (Tools for the Epidemiology of AMR Plasmids, One-Health Transmission and Surveillance). Available online: https://www.jpiamr.eu/api-projects/teapots/ (accessed on 28 March 2023).
- Ko, K.K.K.; Chng, K.R.; Nagarajan, N. Metagenomics-Enabled Microbial Surveillance. Nat. Microbiol. 2022, 7, 486–496. [Google Scholar] [CrossRef]
- Pérez-Cobas, A.E.; Baquero, F.; de Pablo, R.; Soriano, M.C.; Coque, T.M. Altered Ecology of the Respiratory Tract Microbiome and Nosocomial Pneumonia. Front. Microbiol. 2022, 12, 4295. [Google Scholar] [CrossRef]
- Lee, K.; Raguideau, S.; Sirén, K.; Asnicar, F.; Cumbo, F.; Hildebrand, F.; Segata, N.; Cha, C.J.; Quince, C. Population-Level Impacts of Antibiotic Usage on the Human Gut Microbiome. Nature Commun. 2023, 14, 1–19. [Google Scholar] [CrossRef]
- Fierer, N.; Ferrenberg, S.; Flores, G.E.; González, A.; Kueneman, J.; Legg, T.; Lynch, R.C.; McDonald, D.; Mihaljevic, J.R.; O’Neill, S.P.; et al. From Animalcules to an Ecosystem: Application of Ecological Concepts to the Human Microbiome. Ann. Rev. Ecol. Evol Syst 2012, 43, 137–155. [Google Scholar] [CrossRef]
- Kang, J.T.L.; Teo, J.J.Y.; Bertrand, D.; Ng, A.; Ravikrishnan, A.; Yong, M.; Ng, O.T.; Marimuthu, K.; Chen, S.L.; Chng, K.R.; et al. Long-Term Ecological and Evolutionary Dynamics in the Gut Microbiomes of Carbapenemase-Producing Enterobacteriaceae Colonized Subjects. Nature Microbiol. 2022, 7, 1516–1524. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Microbiomes of the Built Environment: A Research Agenda for Indoor Microbiology, Human Health, and Buildings; The National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Mahnert, A.; Moissl-Eichinger, C.; Zojer, M.; Bogumil, D.; Mizrahi, I.; Rattei, T.; Martinez, J.L.; Berg, G. Man-Made Microbial Resistances in Built Environments. Nat. Commun. 2019, 10, 968. [Google Scholar] [CrossRef]
- Gibbons, S.M. The Built Environment Is a Microbial Wasteland. mSystems 2016, 1, e00033-16. [Google Scholar] [CrossRef]
- Gilbert, J.A. Ecological Medicine. Environ. Microbiol. 2018, 20, 1917–1919. [Google Scholar] [CrossRef]
- Jeffery, I.B.; Claesson, M.J.; O’Toole, P.W.; Shanahan, F. Categorization of the Gut Microbiota: Enterotypes or Gradients? Nat. Rev. Microbiol. 2012, 10, 591–592. [Google Scholar] [CrossRef]
- Santiago, M.; Eysenbach, L.; Allegretti, J.; Aroniadis, O.; Brandt, L.J.; Fischer, M.; Grinspan, A.; Kelly, C.; Morrow, C.; Rodriguez, M.; et al. Microbiome Predictors of Dysbiosis and VRE Decolonization in Patients with Recurrent Clostridium difficile Infections in a Multi-Center Retrospective Study. AIMS Microbiol. 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Popoola, O.O.; Madhur, G.; Mehrim, M.M.; Omondi, M.O.; Owusu-Mensah, P.; Mamtani, S.A.; Etukakpan, A.U. A Literature Review on the Global Burden and Impact of Substandard and Falsified Medicine. Ann. Public Health Issues 2022, 2, 16–31. [Google Scholar] [CrossRef]
- Tedijanto, C.; Grad, Y.H.; Lipsitch, M. Potential Impact of Outpatient Stewardship Interventions on Antibiotic Exposures of Common Bacterial Pathogens. Elife 2020, 9, e52307. [Google Scholar] [CrossRef]
- Tedijanto, C.; Olesen, S.W.; Grad, Y.H.; Lipsitch, M. Estimating the Proportion of Bystander Selection for Antibiotic Resistance among Potentially Pathogenic Bacterial Flora. Proc. Natl. Acad. Sci. USA 2018, 115, E11988–E11995. [Google Scholar] [CrossRef]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Hvas, C.L.; Licht, T.R. Determining Gut Microbial Dysbiosis: A Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl. Environ. Microbiol. 2021, 87, 1–13. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Klugman, K.P. Communicating Trends in Resistance Using a Drug Resistance Index. BMJ Open 2011, 1, e000135. [Google Scholar] [CrossRef]
- Ashbolt, N.J.; Amézquita, A.; Backhaus, T.; Borriello, P.; Brandt, K.K.; Collignon, P.; Coors, A.; Finley, R.; Gaze, W.H.; Heberer, T.; et al. Human Health Risk Assessment (HHRA) for Environmental Development and Transfer of Antibiotic Resistance. Environ. Health Perspect. 2013, 121, 993–1001. [Google Scholar] [CrossRef]
- Pires, S.M.; Duarte, A.S.; Hald, T. Source Attribution and Risk Assessment of Antimicrobial Resistance. Microbiol. Spectr. 2018, 6, 3. [Google Scholar] [CrossRef]
- Brul, S.; Bassett, J.; Cook, P.; Kathariou, S.; McClure, P.; Jasti, P.R.; Betts, R. ‘Omics’ Technologies in Quantitative Microbial Risk Assessment. Trends Food Sci. Technol. 2012, 27, 12–24. [Google Scholar] [CrossRef]
- Quality Compliance Systems. Infection Control Policy and the Importance of Risk Assessment. Available online: https://www.qcs.co.uk/infection-control-policy-importance-risk-assessment/ (accessed on 28 March 2023).
- Anderson, M.; Schulze, K.; Cassini, A.; Plachouras, D.; Mossialos, E. A Governance Framework for Development and Assessment of National Action Plans on Antimicrobial Resistance. Lancet Infect. Dis. 2019, 19, e371–e384. [Google Scholar] [CrossRef]
- Der Hertog, P.; Jager, C.-J.; Vankan, A.; te Velde, R.; Veldkamp, J.; Aksnes, D.W.; Sivertsen, G.; van Leuween, T.; van Wijk, E. Science, Technology and Innovation Indicators. Thematic paper 1. Challenges in Measuring the Efficiency of National Science, Technology & Innovation Systems. 2014. Available online: www.sti2.nl (accessed on 28 March 2023).
- Berryhill, B.A.; Gil-Gil, T.; Manuel, J.A.; Smith, A.P.; Margollis, E.; Baquero, F.; Levin, B.R. What’s the Matter with MICs: The Contribution of Nutrients and Limiting Resources to the Pharmacodynamics of Antibiotics and Bacteria. bioRxiv 2022. [CrossRef]
- Aschbacher, R.; Pagani, L.; Migliavacca, R.; Pagani, L.; Confalonieri, M.; Farina, C.; Fazii, P.; Luzzaro, F.; Rigoli, R.; Spalla, M. Recommendations for the Surveillance of Multidrug-Resistant Bacteria in Italian Long-Term Care Facilities by the GLISTer Working Group of the Italian Association of Clinical Microbiologists (AMCLI). Antimicrob. Resist. Infect. Control 2020, 9, 106. [Google Scholar] [CrossRef]
- The BMJ Opinion. Antimicrobial Resistance: More Quick Action Is Needed in BRICS and MINT Economic Transition Countries. Available online: https://blogs.bmj.com/bmj/2019/10/02/antimicrobial-resistance-more-quick-action-is-needed-in-brics-and-mint-economic-transition-countries/ (accessed on 2 October 2019).
- Scherer, L.; de Koning, A.; Tukker, A. BRIC and MINT Countries’ Environmental Impacts Rising despite Alleviative Consumption Patterns. Sci. Total Environ. 2019, 665, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Malik, B.; Bhattacharyya, S. Antibiotic Drug-Resistance as a Complex System Driven by Socio-Economic Growth and Antibiotic Misuse. Sci. Rep. 2019, 9, 9788. [Google Scholar] [CrossRef]
- Avershina, E.; Shapovalova, V.; Shipulin, G. Fighting Antibiotic Resistance in Hospital-Acquired Infections: Current State and Emerging Technologies in Disease Prevention, Diagnostics and Therapy. Front. Microbiol. 2021, 12, 2044. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC), Antibiotic Resistance Threats Report|CDC. 2019. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 28 March 2023).
- Abat, C.; Fournier, P.E.; Jimeno, M.T.; Rolain, J.M.; Raoult, D. Extremely and Pandrug-Resistant Bacteria Extra-Deaths: Myth or Reality? Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1687–1697. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. The Indoor Microbiome. Available online: https://www.epa.gov/indoor-air-quality-iaq/indoor-microbiome#microbiome (accessed on 28 March 2023).
- Dalton, K.R.; Rock, C.; Carroll, K.C.; Davis, M.F. One Health in Hospitals: How Understanding the Dynamics of People, Animals, and the Hospital Built-Environment Can Be Used to Better Inform Interventions for Antimicrobial-Resistant Gram-Positive Infections. Antimicrob. Resist. Infect. Control 2020, 9, 78. [Google Scholar] [CrossRef]
- Christoff, A.P.; Sereia, A.F.R.; Hernandes, C.; de Oliveira, L.F.V. Uncovering the Hidden Microbiota in Hospital and Built Environments: New Approaches and Solutions. Exp. Biol. Med. 2019, 244, 534. [Google Scholar] [CrossRef]
- Kuntz, T.M.; Gilbert, J.A. Introducing the Microbiome into Precision Medicine. Trends Pharmacol. Sci. 2017, 38, 81–91. [Google Scholar] [CrossRef]
- Rodríguez, I.; Figueiredo, A.S.; Sousa, M.; Lanza, V.; Rodríguez, C.; Mingo, P.; Zamora, J.; Loza, E.; Brooks, C.; Cantón, R.; et al. A 21-Year Survey of Escherichia coli from Bloodstream Infections (BSIs) in a Tertiary Hospital Reveals How Community-Hospital Dynamics, Influence Local BSI Rates, the Trends of the B2 Phylogroup and the STc131 Pandemic Clone. mSphere 2021, 6, e0086821. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Shaw, E.; Padilla, B.; Pintado, V.; Calbo, E.; Benito, N.; Gamallo, R.; Gozalo, M.; Rodríguez-Baño, J. Healthcare-Associated, Community-Acquired and Hospital-Acquired Bacteraemic Urinary Tract Infections in Hospitalized Patients: A Prospective Multicentre Cohort Study in the Era of Antimicrobial Resistance. Clin. Microbiol. Infect. 2013, 19, 962–968. [Google Scholar] [CrossRef]
- Laupland, K.B.; Church, D.L. Population-Based Epidemiology and Microbiology of Community-Onset Bloodstream Infections. Clin. Microbiol. Rev. 2014, 27, 647–664. [Google Scholar] [CrossRef]
- Chau, K.K.; Barker, L.; Budgell, E.P.; Vihta, K.D.; Sims, N.; Kasprzyk-Hordern, B.; Harriss, E.; Crook, D.W.; Read, D.S.; Walker, A.S.; et al. Systematic Review of Wastewater Surveillance of Antimicrobial Resistance in Human Populations. Environ. Int. 2022, 162, 107171. [Google Scholar] [CrossRef]
- Ecosystems and Human Well-being: Synthesis. In Millennium Ecosystem Assessment, 2005; Island Press: Washington, DC, USA, 2005.
- The New York Times. Young Roosevelt Saved By New Drug. Available online: https://www.nytimes.com/1936/12/17/archives/young-roosevelt-saved-by-new-drug-doctor-used-prontylin-in-fight-on.html (accessed on 28 March 2023).
- TIME. Mold for Infections. Available online: https://time.com/vault/issue/1941-09-15/page/57/ (accessed on 28 March 2023).
- Report Joint Committee on the Use of Antibiotics in Animal Husbandry and Veterinary Medicine-Google Libros. Available online: https://books.google.es/books/about/Report_Joint_Committee_on_the_Use_of_Ant.html?id=JOtFtAEACAAJ&redir_esc=y (accessed on 28 March 2023).
- Huttner, B.; Saam, M.; Moja, L.; Mah, K.; Sprenger, M.; Harbarth, S.; Magrini, N. How to Improve Antibiotic Awareness Campaigns: Findings of a WHO Global Survey. BMJ Glob. Health 2019, 4, 1239. [Google Scholar] [CrossRef]
- Joint Programming Initiative on Antimicrobial Resistance (JPIAMR). Available online: https://www.jpiamr.eu/ (accessed on 28 March 2023).
- Larsson, D.G.J.; Andremont, A.; Bengtsson-Palme, J.; Brandt, K.K.; de Roda Husman, A.M.; Fagerstedt, P.; Fick, J.; Flach, C.F.; Gaze, W.H.; Kuroda, M.; et al. Critical Knowledge Gaps and Research Needs Related to the Environmental Dimensions of Antibiotic Resistance. Environ. Int. 2018, 117, 132–138. [Google Scholar] [CrossRef]
- United Nations Environmental Programme (UNEP). Summary for Policymakers-Environmental Dimensions of Antimicrobial Resistance. Available online: https://www.unep.org/resources/report/summary-policymakers-environmental-dimensions-antimicrobial-resistance (accessed on 28 February 2022).
- Murray, A.K.; Stanton, I.; Gaze, W.H.; Snape, J. Dawning of a New ERA: Environmental Risk Assessment of Antibiotics and Their Potential to Select for Antimicrobial Resistance. Water Res. 2021, 200, 117233. [Google Scholar] [CrossRef]
- Semenza, J.C. Lateral Public Health: Advancing Systemic Resilience to Climate Change. Lancet Reg. Health Eur. 2021, 9, 100231. [Google Scholar] [CrossRef]
- Pollack, L.A.; Srinivasan, A. Core Elements of Hospital Antibiotic Stewardship Programs from the Centers for Disease Control and Prevention. Clin. Infect. Dis. 2014, 59 (Suppl. S3), S97–S100. [Google Scholar] [CrossRef]
- Schuts, E.C.; Hulscher, M.E.J.L.; Mouton, J.W.; Verduin, C.M.; Stuart, J.W.T.C.; Overdiek, H.W.P.M.; van der Linden, P.D.; Natsch, S.; Hertogh, C.M.P.M.; Wolfs, T.F.W.; et al. Current Evidence on Hospital Antimicrobial Stewardship Objectives: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2016, 16, 847–856. [Google Scholar] [CrossRef]
- Villa, L.; Carattoli, A. Plasmid Typing and Classification. In Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, 2020; Volume 2075, pp. 309–321. [Google Scholar] [CrossRef]
- Redondo-Salvo, S.; Fernández-López, R.; Ruiz, R.; Vielva, L.; de Toro, M.; Rocha, E.P.C.; Garcillán-Barcia, M.P.; de la Cruz, F. Pathways for Horizontal Gene Transfer in Bacteria Revealed by a Global Map of Their Plasmids. Nat. Commun. 2020, 11, 3602. [Google Scholar] [CrossRef]
- Workman, C.L. Syndemics and Global Health. Nature Hum. Behav. 2021, 6, 25–26. [Google Scholar] [CrossRef]
- Virginia, M.; Riquelme, P.; Garner, E.; Gupta, S.; Metch, J.; Zhu, N.; Blair, M.F.; Arango-Argoty, G.; Maile-Moskowitz, A.; Li, A.-D.; et al. Demonstrating a Comprehensive Wastewater-Based Surveillance Approach That Differentiates Globally Sourced Resistomes. Environ. Sci. Technol. 2022, 56, 14982–14993. [Google Scholar]
- Morley, V.J.; Woods, R.J.; Read, A.F. Bystander Selection for Antimicrobial Resistance: Implications for Patient Health. Trends Microbiol. 2019, 27, 864–877. [Google Scholar] [CrossRef]
- Baquero, F.; Coque, T.M.; Guerra-Pinto, N.; Galán, J.C.; Jiménez-Lalana, D.; Tamames, J.; Pedrós-Alió, C. The Influence of Coalescent Microbiotic Particles From Water and Soil on the Evolution and Spread of Antimicrobial Resistance. Front. Environ. Sci. 2022, 10, 385. [Google Scholar] [CrossRef]
- Baquero, F.; Coque, T.M.; Martínez, J.L. Natural Detoxification of Antibiotics in the Environment: A One Health Perspective. Front. Microbiol. 2022, 13, 1062399. [Google Scholar] [CrossRef]
- Murray, A.K.; Zhang, L.; Yin, X.; Zhang, T.; Buckling, A.; Snape, J.; Gaze, W.H. Novel Insights into Selection for Antibiotic Resistance in Complex Microbial Communities. mBio 2018, 9, e00969-18. [Google Scholar] [CrossRef]
- Levy, S.B.; Fitzgerald, G.B.; Macone, A.B. Spread of Antibiotic-Resistant Plasmids from Chicken to Chicken and from Chicken to Man. Nature 1976, 260, 40–42. [Google Scholar] [CrossRef]
- Martínez, J.L.; Coque, T.M.; Baquero, F. What Is a Resistance Gene? Ranking Risk in Resistomes. Nat. Rev. Microbiol. 2015, 13, 116–123. [Google Scholar] [CrossRef]
- EUCAST: New S, I and R Definitions. Available online: https://www.eucast.org/newsiandr/ (accessed on 4 September 2022).
- Davison, H.C.; Woolhouse, M.E.J.; Low, J.C. What Is Antibiotic Resistance and How Can We Measure It? Trends Microbiol. 2000, 8, 554–559. [Google Scholar] [CrossRef]
- Cantón, R. Antibiotic Resistance Genes from the Environment: A Perspective through Newly Identified Antibiotic Resistance Mechanisms in the Clinical Setting. Clin. Microbiol. Infect. 2009, 15 (Suppl. S1), 20–25. [Google Scholar] [CrossRef]
- Park, C. The Environment: Principles and Applications; Gregory, J., Walling, E.K., Eds.; Routledge: London, UK, 2001. [Google Scholar]
- Tansley, A.G. “The Use and Abuse of Vegetational Concepts and Terms” (1935). In The Future of Nature: Documents of Global Change; Robin, L., Sörlin, S., Warde, P., Eds.; Yale University Press: New Haven, CT, USA, 2013; pp. 220–232. [Google Scholar] [CrossRef]
- Willis, A.J. The Ecosystem: An Evolving Concept Viewed Historically. Funct. Ecol. 1997, 11, 268–271. [Google Scholar] [CrossRef]
- Exposome and Exposomics|NIOSH|CDC. Available online: https://www.cdc.gov/niosh/topics/exposome/default.html (accessed on 11 September 2022).
- Gillings, M.R. Lateral Gene Transfer, Bacterial Genome Evolution, and the Anthropocene. Ann. N. Y. Acad. Sci. 2017, 1389, 20–36. [Google Scholar] [CrossRef]
- Tomson, G.; Vlad, I. The Need to Look at Antibiotic Resistance from a Health Systems Perspective. Ups J. Med. Sci. 2014, 119, 117. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.J. Changing How We Think about Infectious Diseases. Taxon. Guide Infect. Dis. 2019, 321–365. [Google Scholar] [CrossRef]
- Last, J.M. Dictionary of Epidemiology. CMAJ Can. Med. Assoc. J. 1993, 149, 400. [Google Scholar] [CrossRef]
- Lederberg, J. Plasmid (1952–1997). Plasmid 1998, 39, 1–9. [Google Scholar] [CrossRef]
- Lederberg, J.; Lederberg, E. Infection and Heredity. In Cellular Mechanisms in Differentiation and Growth; Rudnick, D., Ed.; Princeton University Press: Princeton, NJ, USA, 1956; pp. 101–124. [Google Scholar]
- Baquero, F. From Pieces to Patterns: Evolutionary Engineering in Bacterial Pathogens. Nat. Rev. Microbiol. 2004, 2, 510–518. [Google Scholar] [CrossRef]
- D’Costa, V.M.; McGrann, K.M.; Hughes, D.W.; Wright, G.D. Sampling the Antibiotic Resistome. Science (1979) 2006, 311, 374–377. [Google Scholar] [CrossRef]
- Lanza, V.F.; Baquero, F.; Martínez, J.L.; Ramos-Ruíz, R.; González-Zorn, B.; Andremont, A.; Sánchez-Valenzuela, A.; Ehrlich, S.D.; Kennedy, S.; Ruppé, E.; et al. In-Depth Resistome Analysis by Targeted Metagenomics. Microbiome 2018, 6, 11. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Frieden, T. Antibiotic Resistance Threats. CDC 2013, 22–50. [Google Scholar]
- World Health Organization (WHO). WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 25 October 2022).
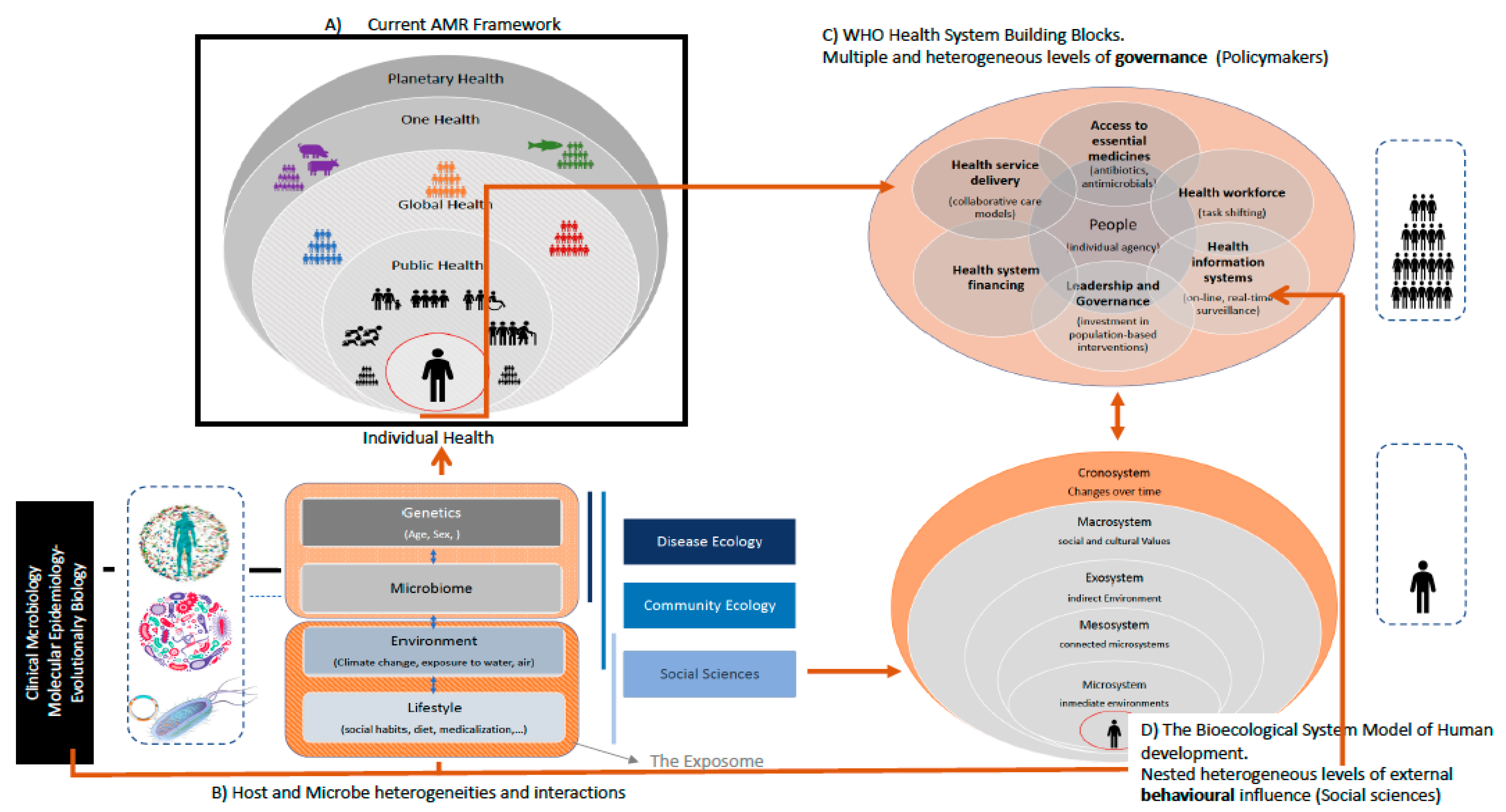
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coque, T.M.; Cantón, R.; Pérez-Cobas, A.E.; Fernández-de-Bobadilla, M.D.; Baquero, F. Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century. Microorganisms 2023, 11, 1050. https://doi.org/10.3390/microorganisms11041050
Coque TM, Cantón R, Pérez-Cobas AE, Fernández-de-Bobadilla MD, Baquero F. Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century. Microorganisms. 2023; 11(4):1050. https://doi.org/10.3390/microorganisms11041050
Chicago/Turabian StyleCoque, Teresa M., Rafael Cantón, Ana Elena Pérez-Cobas, Miguel D. Fernández-de-Bobadilla, and Fernando Baquero. 2023. "Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century" Microorganisms 11, no. 4: 1050. https://doi.org/10.3390/microorganisms11041050
APA StyleCoque, T. M., Cantón, R., Pérez-Cobas, A. E., Fernández-de-Bobadilla, M. D., & Baquero, F. (2023). Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century. Microorganisms, 11(4), 1050. https://doi.org/10.3390/microorganisms11041050