Probiotic Bifidobacterium breve MCC1274 Protects against Oxidative Stress and Neuronal Lipid Droplet Formation via PLIN4 Gene Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Live Bacterial Cells
2.2. Chemicals
2.3. Preparation of Bacterial Cell Extracts
2.3.1. Preparation of Heat-Killed Cell Extracts
2.3.2. Preparation of Sonicated Cell Extracts
2.4. Cell Culture
2.5. RNA Extraction
2.6. PCR
2.7. Measurement of NA and Related Metabolites by Mass Spectrometry
2.8. FACS and LD Assay
2.9. Microscopy
2.10. Statistical Analysis
3. Results
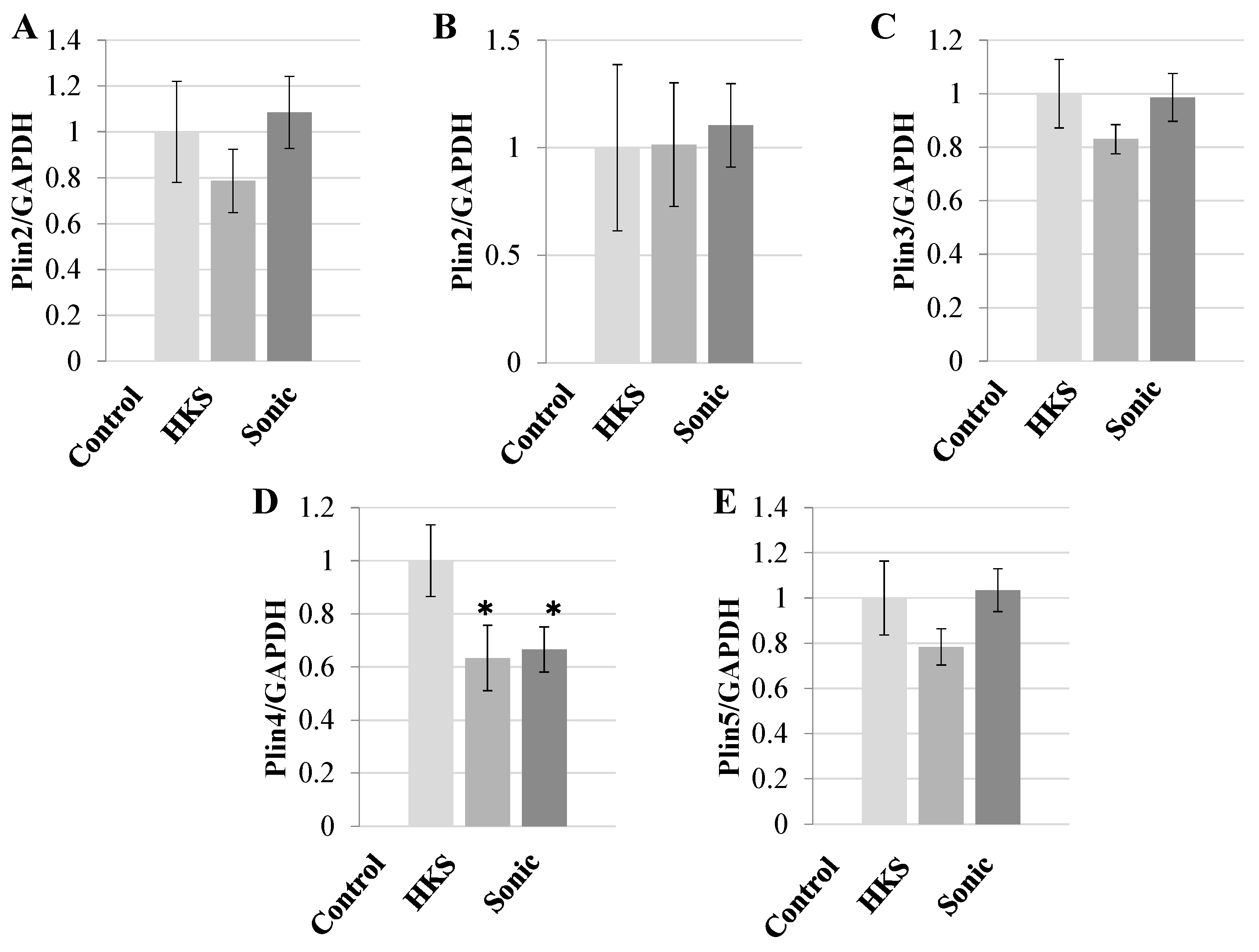
3.1. Metabolites in B. breve MCC1274 Extracts Specifically Reduce PLIN4 Expression in Human Neuroblastoma Cells
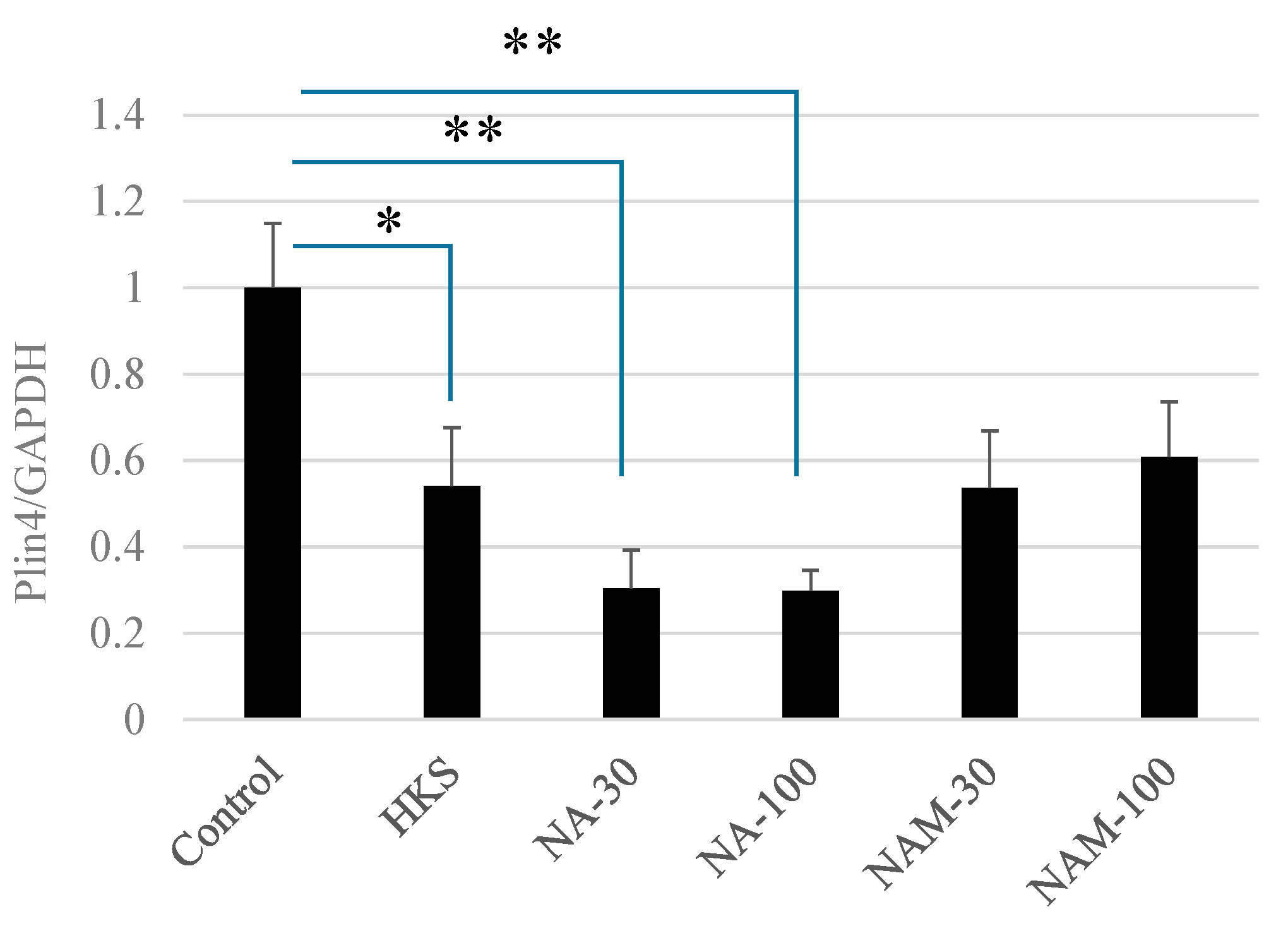
3.2. NA and B. breve MCC1274 Extracts Reduce PLIN4 mRNA Expression in SH-SY5Y Neuroblastoma Cells
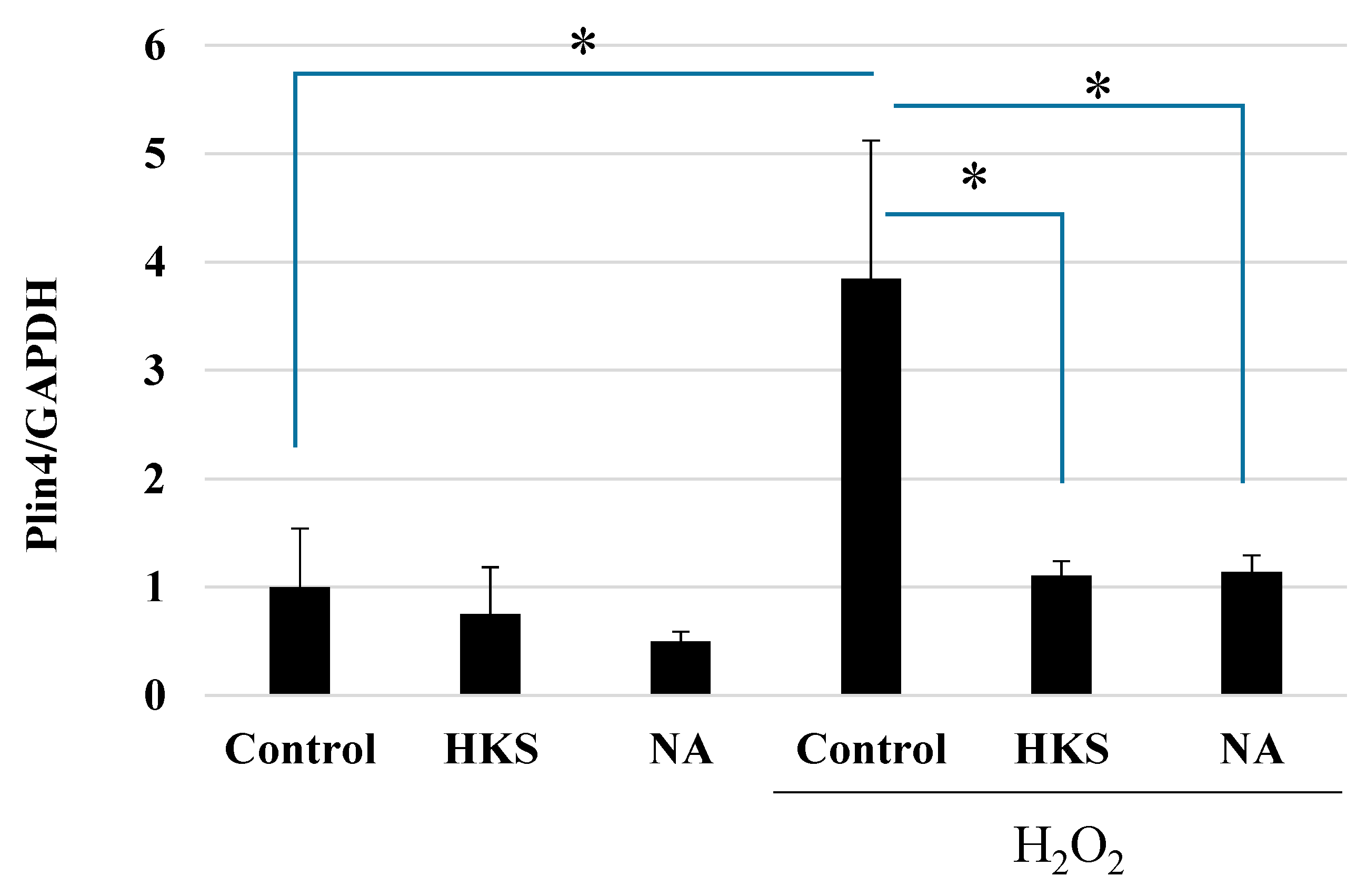
3.3. NA and B. breve MCC1274 Extracts Protect against Oxidative Stress Caused by Exposure to H2O2
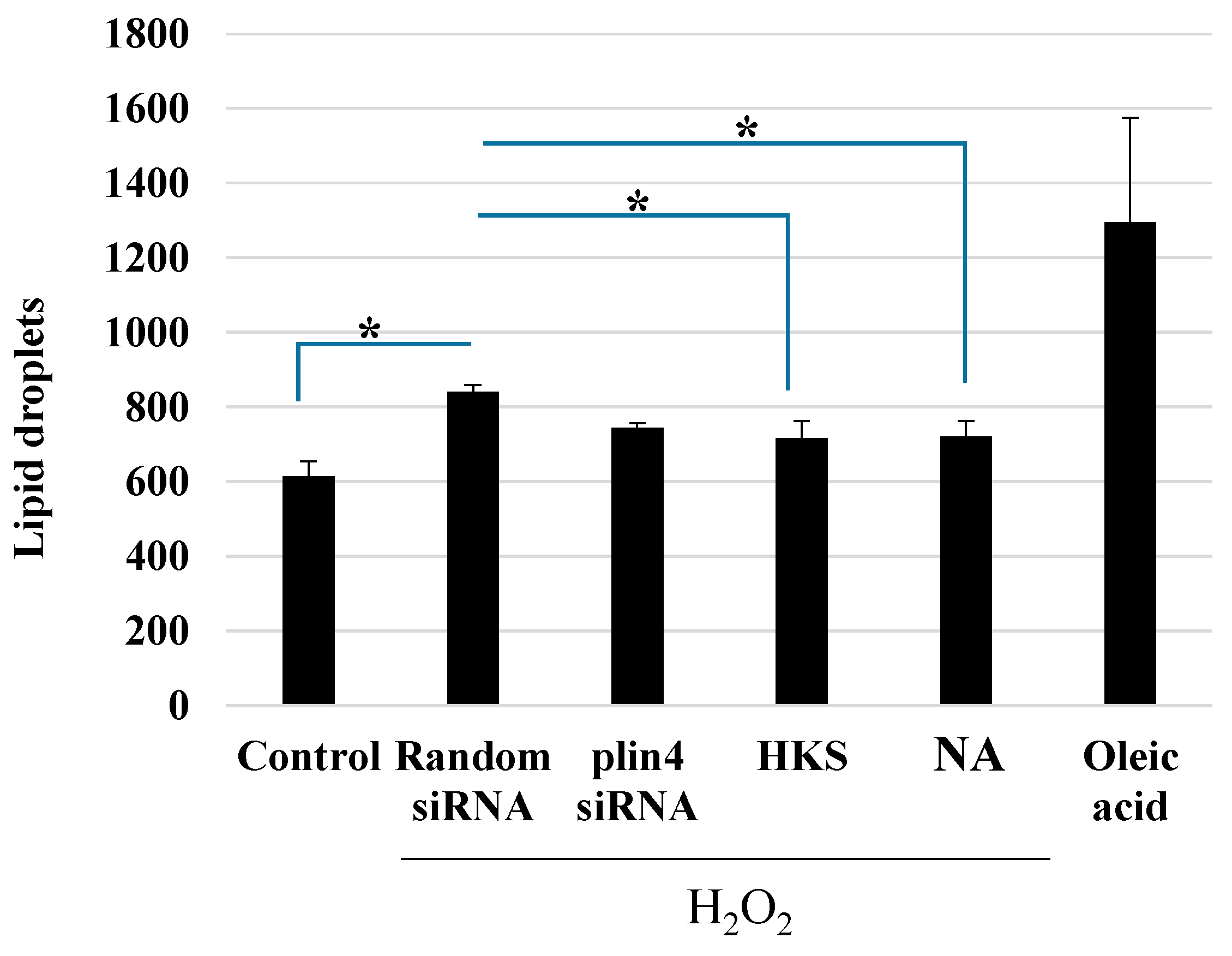
3.4. NA and B. breve MCC1274 Extracts Reduce LD Formation after Exposure to H2O2
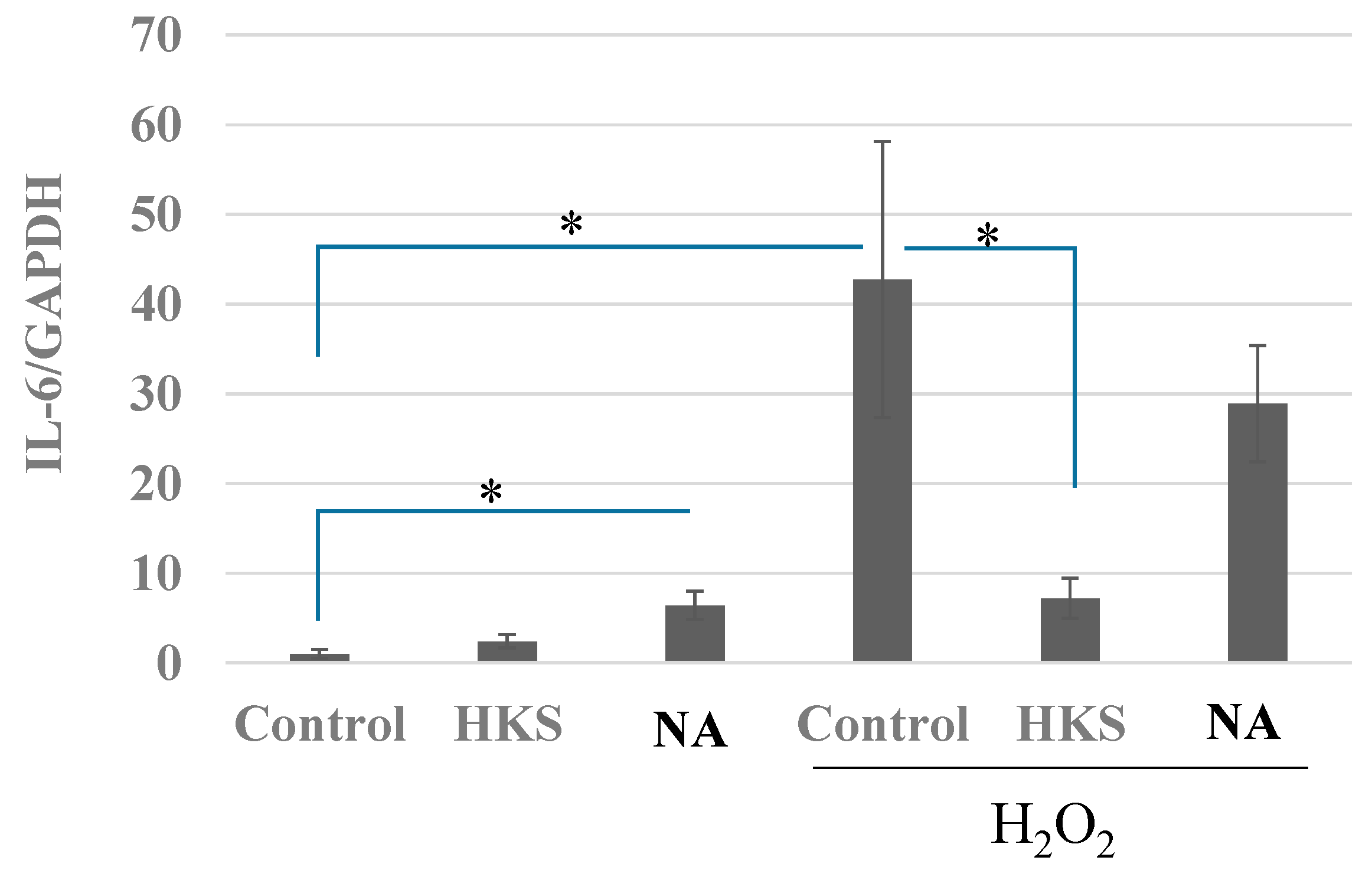
3.5. NA and B. breve MCC1274 Extracts Prevent the Induction of IL-6 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorszewska, J.; Prendecki, M.; Oczkowska, A.; Dezor, M.; Kozubski, W. Molecular Basis of Familial and Sporadic Alz-heimer’s Disease. Curr. Alzheimer Res. 2016, 13, 952–963. [Google Scholar] [CrossRef]
- Asher, S.; Priefer, R. Alzheimer’s disease failed clinical trials. Life Sci. 2022, 4, 120861. [Google Scholar] [CrossRef]
- Moir, R.D.; Lathe, R.; Tanzi, R.E. The antimicrobial protection hypothesis of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 1602–1614. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Munawara, U.; Larbi, A.; Desroches, M.; Rodrigues, S.; Catanzaro, M.; Guidolin, A.; Khalil, A.; Bernier, F.; Barron, A.E.; et al. Targeting Infectious Agents as a Therapeutic Strategy in Alzheimer’s Disease. CNS Drugs 2020, 34, 673–695. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroin-flammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 1046. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, K.; Sandoval, H.; Yamamoto, S.; Jaiswal, M.; Sanz, E.; Li, Z.; Hui, J.; Graham, B.H.; Quintana, A.; et al. Glial Lipid Droplets and ROS Induced by Mitochondrial Defects Promote Neurodegeneration. Cell 2015, 160, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Claes, C.; Danhash, E.P.; Hasselmann, J.; Chadarevian, J.P.; Shabestari, S.K.; England, W.E.; Lim, T.E.; Hidalgo, J.L.S.; Spitale, R.C.; Davtyan, H.; et al. Plaque-associated human microglia accumulate lipid droplets in a chimeric model of Alzheimer’s disease. Mol. Neurodegener. 2021, 16, 50. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef]
- Khasawneh, J.; Schulz, M.D.; Walch, A.; Rozman, J.; De Angelis, M.H.; Klingenspor, M.; Buck, A.; Schwaiger, M.; Saur, D.; Schmid, R.M.; et al. Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proc. Natl. Acad. Sci. USA 2009, 106, 3354–3359. [Google Scholar] [CrossRef]
- Yang, M.; Luo, S.; Yang, J.; Chen, W.; He, L.; Liu, D.; Zhao, L.; Wang, X. Lipid droplet—Mitochondria coupling: A novel lipid metabolism regulatory hub in diabetic nephropathy. Front. Endocrinol. 2022, 13, 2642. [Google Scholar] [CrossRef] [PubMed]
- Najt, C.P.; Devarajan, M.; Mashek, D.G. Perilipins at a glance. J. Cell Sci. 2022, 135, jcs259501. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhu, J.; Zhang, X.; Song, Q.; Ding, J.; Lu, M.; Sun, S.; Hu, G. PLIN4-dependent lipid droplets hamper neuronal mitophagy in the MPTP/p-induced mouse model of Par-kinson’s disease. Front. Neurosci. 2018, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Reiser, G. Why does Brain Metabolism not Favor Burning of Fatty Acids to Provide Energy?—Reflections on Disadvantages of the Use of Free Fatty Acids as Fuel for Brain. J. Cereb. Blood Flow Metab. 2013, 33, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Pourjafar, H.; Tabrizi, A.; Homayouni, A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Re-view on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Bingöl, F.G.; Çelik, E.; Cemali, Ö.; Özenir, Ç.; Özoğul, F.; Capasso, R. Recent developments in the probiotics as live biotherapeutic products (LBPs) as modulators of gut brain axis related neurological conditions. J. Transl. Med. 2022, 20, 460. [Google Scholar] [CrossRef]
- Xu, L.; Yang, C.S.; Liu, Y.; Zhang, X. Effective Regulation of Gut Microbiota With Probiotics and Prebiotics May Prevent or Alleviate COVID-19 Through the Gut-Lung Axis. Front. Pharmacol. 2022, 13, 895193. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3, 858. [Google Scholar] [CrossRef]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate Production by Probiotic Bacteria. Nutrients 2011, 3, 118. [Google Scholar] [CrossRef]
- Deguchi, Y.; Morishita, T.; Mutai, M. Comparative Studies on Synthesis of Water-soluble Vitamins among HumanSpecies of Bifidobacteria. Biol. Chern. 1985, 49, 13–19. [Google Scholar]
- Giri, B.; Belanger, K.; Seamon, M.; Bradley, E.; Purohit, S.; Chong, R.; Morgan, J.C.; Baban, B.; Wakade, C. Niacin Ameliorates Neuro-Inflammation in Parkinson’s Disease via GPR109A. Int. J. Mol. Sci. 2019, 20, 4559. [Google Scholar] [CrossRef]
- Lipszyc, P.S.; Cremaschi, G.A.; Zubilete, M.Z.; Bertolino, M.L.; Capani, F.; Genaro, A.M.; Wald, M.R. Niacin Modulates Pro-inflammatory Cytokine Secretion. A Potential Mechanism Involved in its Anti-atherosclerotic Effect. Open Cardiovasc. Med. J. 2013, 7, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, D.; Xiao, J.; Takeda, T.; Yanagisawa, N.; Yamazaki, T.; Matsubara, Y.; Sugiyama, H.; Endo, N.; Higa, M.; Kasanuki, K.; et al. Effect of Probiotic Bifidobacterium breve in Improving Cognitive Function and Preventing Brain Atrophy in Older Patients with Suspected Mild Cognitive Impairment: Results of a 24-Week Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimer’s Dis. 2022, 88, 75–95. [Google Scholar] [CrossRef]
- Xiao, J.; Katsumata, N.; Bernier, F.; Ohno, K.; Yamauchi, Y.; Odamaki, T.; Yoshikawa, K.; Ito, K.; Kaneko, T. Probiotic Bifidobacterium breve in Improving Cognitive Functions of Older Adults with Suspected Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2020, 77, 139–147. [Google Scholar] [CrossRef]
- Abdelhamid, M.; Zhou, C.; Ohno, K.; Kuhara, T.; Taslima, F.; Abdullah, M.; Jung, C.G.; Michikawa, M. Probiotic Bifidobacterium breve Prevents Memory Impairment Through the Reduction of Both Amy-loid-β Production and Microglia Activation in APP Knock-In Mouse. J. Alzheimers Dis. 2022, 85, 1555–1571. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Abdelhamid, M.; Zhou, C.; Jung, C.G.; Michikawa, M. Bifidobacterium breve MCC1274 Supplementation In-creased the Plasma Levels of Metabolites with Potential Anti-Oxidative Activity in APP Knock-In Mice. J. Alzheimer’s Dis. 2022, 89, 1413–1425. [Google Scholar] [CrossRef]
- Moutinho, M.; Puntambekar, S.S.; Tsai, A.P.; Coronel, I.; Lin, P.B.; Casali, B.T.; Martinez, P.; Oblak, A.L.; Lasagna-Reeves, C.A.; Lamb, B.T.; et al. The Niacin receptor HCAR2 modulates microglial response and limits disease progression in a mouse model of Alzheimer’s disease. Sci. Transl. Med. 2022, 14, eabl7634. [Google Scholar] [CrossRef]
- Farmer, B.C.; Walsh, A.E.; Kluemper, J.C.; Johnson, L.A. Lipid Droplets in Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 742. [Google Scholar] [CrossRef]
- Jin, Y.; Tan, Y.; Chen, L.; Liu, Y.; Ren, Z. Reactive Oxygen Species Induces Lipid Droplet Accumulation in HepG2 Cells by Increasing Perilipin 2 Expression. Int. J. Mol. Sci. 2018, 19, 3445. [Google Scholar] [CrossRef]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a Major Cytokine in the Central Nervous System. Int. J. Biol. Sci. 2012, 8, 1254. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.A. Nicotinic acid: The broad-spectrum lipid drug. A 50th anniversary review. J. Intern. Med. 2005, 258, 94–114. [Google Scholar] [CrossRef]
- Digby, J.E.; Martinez, F.; Jefferson, A.; Ruparelia, N.; Chai, J.; Wamil, M.; Greaves, D.R.; Choudhury, R.P. Anti-inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.M.; Adams, M.H.; González, M.A.; Tolbert, D.S.; Leu, J.H.; Cefali, E.A. Plasma and urine pharmacokinetics of Niacin and its metabolites from an extended-release Niacin formulation. Int. J. Clin. Pharmacol. Ther. 2007, 45, 448–454. [Google Scholar] [CrossRef]
- Chellappa, K.; McReynolds, M.R.; Lu, W.; Zeng, X.; Makarov, M.; Hayat, F.; Mukherjee, S.; Bhat, Y.R.; Lingala, S.R.; Shima, R.T.; et al. NAD precursors cycle between host tissues and the gut microbiome. Cell Metab. 2022, 34, 1947–1959.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Meng, L.; Song, L.; Du, J.; Du, S.; Cui, W.; Liu, C.; Li, F. Roles of Perilipins in Diseases and Cancers. Curr. Genom. 2018, 19, 247. [Google Scholar] [CrossRef]
- Toson, B.; Simon, C.; Moreno, I. The Endometrial Microbiome and Its Impact on Human Conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef]
- Boutriq, S.; González-González, A.; Plaza-Andrades, I.; Laborda-Illanes, A.; Sánchez-Alcoholado, L.; Peralta-Linero, J.; Domínguez-Recio, M.E.; Bermejo-Pérez, M.J.; Lavado-Valenzuela, R.; Alba, E.; et al. Gut and Endometrial Microbiome Dysbiosis: A New Emergent Risk Factor for Endometrial Cancer. J. Pers. Med. 2021, 11, 659. [Google Scholar] [CrossRef]
- Rebersek, M. Gut microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef]
- Drake, J.C.; Wilson, R.J.; Laker, R.C.; Guan, Y.; Spaulding, H.R.; Nichenko, A.S.; Shen, W.; Shang, H.; Dorn, M.V.; Huang, K.; et al. Mitochondria-localized AMPK responds to local energetics and contributes to exercise and energetic stress-induced mitophagy. Proc. Natl. Acad. Sci. USA 2021, 118, e2025932118. [Google Scholar] [CrossRef]
- Balan, E.; Schwalm, C.; Naslain, D.; Nielens, H.; Francaux, M.; Deldicque, L. Regular Endurance Exercise Promotes Fission, Mitophagy, and Oxidative Phosphorylation in Human Skeletal Muscle Independently of Age. Front. Physiol. 2019, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, A.; García-Aguilar, A.; Asahara, S.I.; Kido, Y.; Guillén, C.; Pajvani, U.B.; Benito, M. MTORC1 Regulates both General Autophagy and Mitophagy Induction after Oxidative Phosphorylation Uncoupling. Mol. Cell Biol. 2017, 37, e00441-17. [Google Scholar] [CrossRef] [PubMed]
- Divakaruni, S.S.; Van Dyke, A.M.; Chandra, R.; LeGates, T.A.; Contreras, M.; Dharmasri, P.A.; Higgs, H.N.; Lobo, M.K.; Thompson, S.M.; Blanpied, T.A. Long-Term Potentiation Requires a Rapid Burst of Dendritic Mitochondrial Fission during Induction. Neuron 2018, 100, 860–875.e7. [Google Scholar] [CrossRef] [PubMed]
| HKS | Sonic | |
|---|---|---|
| NA | 1.43 | 1.12 |
| NAM | 7.01 | 14.8 |
| NR | 0.25 | 0.2 |
| NAD+ | 53.35 | 23.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernier, F.; Kuhara, T.; Xiao, J. Probiotic Bifidobacterium breve MCC1274 Protects against Oxidative Stress and Neuronal Lipid Droplet Formation via PLIN4 Gene Regulation. Microorganisms 2023, 11, 791. https://doi.org/10.3390/microorganisms11030791
Bernier F, Kuhara T, Xiao J. Probiotic Bifidobacterium breve MCC1274 Protects against Oxidative Stress and Neuronal Lipid Droplet Formation via PLIN4 Gene Regulation. Microorganisms. 2023; 11(3):791. https://doi.org/10.3390/microorganisms11030791
Chicago/Turabian StyleBernier, François, Tatsuya Kuhara, and Jinzhong Xiao. 2023. "Probiotic Bifidobacterium breve MCC1274 Protects against Oxidative Stress and Neuronal Lipid Droplet Formation via PLIN4 Gene Regulation" Microorganisms 11, no. 3: 791. https://doi.org/10.3390/microorganisms11030791
APA StyleBernier, F., Kuhara, T., & Xiao, J. (2023). Probiotic Bifidobacterium breve MCC1274 Protects against Oxidative Stress and Neuronal Lipid Droplet Formation via PLIN4 Gene Regulation. Microorganisms, 11(3), 791. https://doi.org/10.3390/microorganisms11030791





