Fire and Rhizosphere Effects on Bacterial Co-Occurrence Patterns
Abstract
1. Introduction
2. Materials and Methods
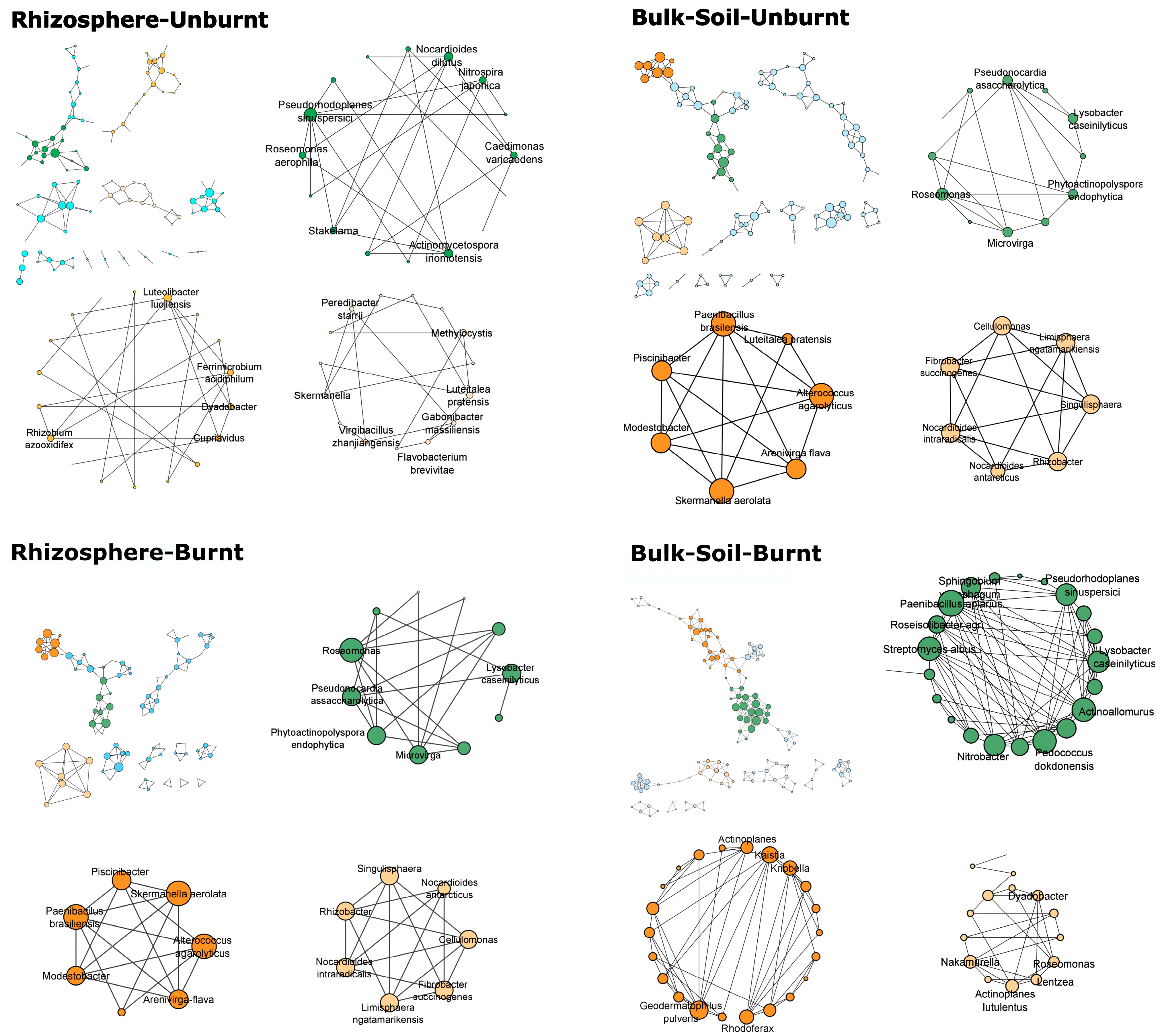
3. Results
4. Discussion
4.1. Differences in Network Parameters between Burnt and Unburnt Soil Networks
4.2. Regulation of Bacterial Communities in Mediterranean Soil Ecosystems: Stochasticity vs. Determinism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stamou, G.P. Arthropods of Mediterranean-Type Ecosystems; Springer: Berlin/Heidelberg, Germany, 1998; p. 140. [Google Scholar] [CrossRef]
- Mooney, H.A. Convergent Evolution of Chile and California Mediterranean Climate Ecosystems; Dowden, Hutchinson and Ross: Stroudsburg, PA, USA, 1977; p. 224. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Haichar, F.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Rodríguez, J.; González-Pérez, J.A.; Turmero, A.; Hernández, M.; Ball, A.S.; González-Vila, F.J.; Arias, M.E. Wildfire effects on the microbial activity and diversity in a Mediterranean forest soil. Catena 2017, 158, 82–88. [Google Scholar] [CrossRef]
- Keeley, S.C.; Johnson, A.W. A comparison of the pattern of herb and shrub growth in comparable sites in Chile and California. Am. Midl. Nat. 1997, 97, 120–132. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire in Mediterranean climate ecosystems—A comparative overview. Isr. J. Ecol. Evol. 2012, 58, 123–135. [Google Scholar]
- Bond, W.J.; Keeley, J.E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef]
- Zavala, L.; De Celis, R.; Jordán, A. How wildfires affect soil properties. A brief review. Cuad. De Investig. Geogr. 2014, 40, 311–331. [Google Scholar] [CrossRef]
- Panico, S.C.; Ceccherini, M.T.; Memoli, V.; Maisto, G.; Pietramellara, G.; Barile, R.; De Marco, A. Effects of different vegetation types on burnt soil properties and microbial communities. Int. J. Wildland Fire 2020, 29, 628–636. [Google Scholar] [CrossRef]
- Santorufo, L.; Memoli, V.; Panico, S.; Santin, G.; Baril, R.; Giarra, A.; Di Natale, G.; Trifuoggi, M.; De Marco, A.; Maisto, G. Combined Effects of Wildfire and Vegetation Cover Type on Volcanic Soil (Functions and Properties) in a Medi-terranean Region: Comparison of Two Soil Quality Indices. Int. J. Environ. Res. Public Health 2021, 18, 5926. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’—Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Yang, S.; Zheng, Q.; Yang, Y.; Yuan, M.; Ma, X.; Chiariello, N.R.; Docherty, K.M.; Field, C.B.; Gutknecht, J.L.M.; Hungate, B.A.; et al. Fire affects the taxonomic and functional composition of soil microbial communities, with cascading effects on grassland ecosystem functioning. Glob. Change Biol. 2020, 26, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-Q.; Tang, C.; Lin, J.; Yu, M.; Dai, Z.; Luo, Y.; Li, Y.; Xu, J. Recovery patterns of soil bacterial and fungal communities in Chinese boreal forests along a fire chronosequence. Sci. Total Environ. 2022, 805, 150372. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Cheng, H.; Xu, C.; Sheng, G.D. Surface characteristics of crop-residue-derived black carbon and lead(II) adsorption. Water Res. 2008, 42, 567–574. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Vallejo, V.R.; Arianoutsou, M.; Moreira, F. Fire Ecology and Post-Fire Restoration Approaches in Southern European Forest Types. In Post-Fire Management and Restoration of Southern European Forests, Managing Forest Ecosystems; Moreira, F., Arianoutsou, M., Corona, P., De las Heras, J., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2012; Volume 24. [Google Scholar] [CrossRef]
- Smith-Ramírez, C.; Castillo-Mandujano, J.; Becerra, P.; Sandoval, N.; Allende, R.; Fuentes, R. Recovery of Chilean Mediterranean vegetation after different frequencies of fires. For. Ecol. Manag. 2021, 485, 118922. [Google Scholar] [CrossRef]
- Idbella, M.; De Filippis, F.; Zotti, M.; Sequino, G.; Abd-ElGawad, A.M.; Fechtali, T.; Mazzoleni, S.; Bonanomi, G. Specific microbiome signatures under the canopy of Mediterranean shrubs. Appl. Soil Ecol. 2022, 173, 104407. [Google Scholar] [CrossRef]
- Arianoutsou-Faraggitaki, A. Post-fire successional recovery of a phryganic (East Mediterranean) ecosystem. Acta Oecol. Ecol. Plant 1984, 5, 387–394. [Google Scholar]
- Úbeda, X.; Sarricolea, P. Wildfires in Chile: A review. Glob. Planet. Chang. 2016, 146, 152–161. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pfaff, A.H.; Safford, H.D. Fire suppression impacts on postfire recovery of Sierra Nevada chaparral shrublands. Int. J. Wildland Fire 2005, 14, 255–265. [Google Scholar] [CrossRef]
- Armesto, J.J.; Bustamante-Sánchez, M.A.; Díaz, M.F.; González, M.E.; Holt, A.; Nuñez-Avila, M.; Smith-Ramírez, C. Fire Disturbance Regimes, Eco-System Recovery and Restoration Strategies in Mediterranean and Temperate Regions of Chile. In Fire Effects on Soils and Restoration Strategies, 1st ed.; Cerda, A., Robinchaud, P.R., Eds.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Francos, M.; Úbeda, X.; Pereira, P.; Alcañiz, M. Long-term impact of wildfire on soils exposed to different fire severities. A case study in Cadiretes Massif (NE Iberian Peninsula). Sci. Total Environ. 2018, 615, 664–671. [Google Scholar] [CrossRef]
- Hrelja, I.; Šestak, I.; Bogunović, I. Wildfire Impacts on Soil Physical and Chemical Properties—A Short Review of Recent Studies. Agric. Conspec. Sci. 2020, 85, 293–301. [Google Scholar]
- Borgogni, F.; Lavecchia, A.; Mastrolonardo, G.; Certini, G.; Ceccherini, M.T.; Pietramellara, G. Immediate- and Short-Term Wildfire Impact on Soil Microbial Diversity and Activity in a Mediterranean Forest Soil. Soil Sci. 2019, 184, 35–42. [Google Scholar] [CrossRef]
- Aponte, H.; Galindo-Castañeda, T.; Yáñez, C.; Hartmann, M.; Rojas, C. Microbial community-level physiological profiles and genetic prokaryotic structure of burned soils under Mediterranean sclerophyll forests in central Chile. Front. Microbiol. 2022, 13, 824813. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, A.; Díaz-Raviña, M. Fire impacts on soil microorganisms: Mass, activity, and diversity. Curr. Opin. Environ. Sci. Health 2021, 22, 100264. [Google Scholar] [CrossRef]
- Sáenz de Miera, L.E.; Pinto, R.; Gutierrez-Gonzalez, J.J.; Calvo, L.; Ansola, G. Wildfire effects on diversity and composition in soil bacterial communities. Sci. Total Environ. 2020, 726, 138636. [Google Scholar] [CrossRef]
- Whitman, T.; Whitman, E.; Woolet, J.; Flannigan, M.D.; Thompson, D.K.; Parisien, M.-A. Soil bacterial and fungal response to wildfires in the Canadian boreal forest across a burn severity gradient. Soil Biol. Biochem. 2019, 138, 107571. [Google Scholar] [CrossRef]
- Pérez-Valera, E.; Verdú, M.; Navarro-Cano, J.A.; Goberna, M. Resilience to fire of phylogenetic diversity across biological domains. Mol. Ecol. 2018, 27, 2896–2908. [Google Scholar] [CrossRef]
- Pérez-Valera, E.; Goberna, M.; Faust, K.; Raes, J.; García, C.; Verdú, M. Fire modifies the phylogenetic structure of soil bacterial cooccurrence networks. Environ. Microbiol. 2017, 19, 317–327. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, Y.; Qiu, C.; Zheng, D.; Liu, Y. Wildfire drives the transition from deterministic- to stochastic-dominated community assembly of abundant bacterial in forest soils. Catena 2022, 215, 106290. [Google Scholar] [CrossRef]
- Chase, J.M.; Myers, J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2351–2363. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Zhang, P.; Xue, K.; Liang, Y.; Van Nostrand, J.D.; Yang, Y.; He, Z.; Wu, L.; Stahl, D.A.; et al. Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc. Natl. Acad. Sci. USA 2014, 111, E836–E845. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M.; Srivastava, D.S.; Anderson, K.M.; Brown, C.D.; Jankowski, J.E.; Kleynhans, E.J.; Kraft, N.; Letaw, A.D.; Macdonald, A.A.M.; Maclean, J.E.; et al. Assessing the relative importance of neutral stochasticity in ecological communities. Oikos 2014, 123, 1420–1430. [Google Scholar] [CrossRef]
- Vellend, M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Gravel, D.; Canham, C.D.; Beaudet, M.; Messier, C. Reconciling niche and neutrality: The continuum hypothesis. Ecol. Lett. 2006, 9, 399–409. [Google Scholar] [CrossRef]
- Stamou, G.P.; Argyropoulou, M.D.; Rodriguez-Polo, I.; Boutsis, G.; Kapagianni, P.M.; Papatheodorou, E.M. A case study of nematode communities’ dynamics along successional paths in the reclaimed landfill. Diversity 2020, 12, 274. [Google Scholar] [CrossRef]
- Mayfield, M.K.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Stamou, G.P.; Monokrousos, N.; Papapostolou, A.; Papatheodorou, E.M. Recurring heavy rainfall resulting in degraded-upgraded phases in soil microbial networks that are reflected in soil functioning. Soil Ecol. Lett. 2023, 5, 220161. [Google Scholar] [CrossRef]
- Moroenyane, I.; Tripathi, B.; Dong, K.; Sherman, C.; Steinberger, Y.; Adams, J. Bulk soil bacterial community mediated by plant community in Mediterranean ecosystem, Israel. Appl. Soil Ecol. 2018, 124, 104–109. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef]
- Klein, B.; Hoel, E. The Emergence of Informative Higher Scales in Complex Networks. Hindawi Complex. 2020, 2020, 8932526. [Google Scholar] [CrossRef]
- Si, W.; Mburano, B.; Zheng, W.X.; Qiu, T. Measuring Network Robustness by Average Network Flow. IEEE Trans. Netw. Sci. Eng. 2022, 9, 1697–1712. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef]
- Katz, K.S.; Shutov, O.; Lapoint, R.; Kimelman, M.; Brister, J.R.; O’Sullivan, C. STAT: A fast, scalable, MinHash-based k-mer tool to assess Sequence Read Archive next-generation sequence submissions. Genome Biol. 2021, 22, 270. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. CoNet app: Inference of biological association networks using Cytoscape. F1000Research 2016, 5, 1519. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Brown, M.B. A method for combining non-independent, one-sided tests of significance. Biometrics 1975, 31, 987–992. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Borgatti, S.P.; Everett, M.G.; Freeman, L.C. Ucinet for Windows: Software for Social Network Analysis; Analytic Technologies: Harvard, MA, USA, 2002. [Google Scholar]
- Newman, M.E.J. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef]
- Luo, X.; Han, S.; Fu, X.; Li, X.; Wang, L.; Peng, S.; Chen, W.; Huang, Q. The microbial network in naturally fertile paddy soil possibly facilitates functional recruitment in the rice mature stage. Appl. Soil Ecol. 2019, 135, 174–181. [Google Scholar] [CrossRef]
- Humphries, M.D.; Gurney, K. Network ‘small-world-ness’: A quantitative method for determining canonical network equivalence. PLoS ONE 2008, 3, e0002051. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, M.; Wang, S.; Liu, P. A comparative study of network robustness measures. Front. Comput. Sci. 2017, 11, 568–584. [Google Scholar] [CrossRef]
- Klein, B.; Swain, A.; Byrum, T.; Scarpino, S.V.; Fagan, W.F. Exploring noise, degeneracy and determinism in biological networks with the einet package. Methods Ecol. Evol. 2022, 13, 799–804. [Google Scholar] [CrossRef]
- Tirandaz, H.; Dastgheib, S.M.M.; Amoozegar, M.A.; Shavandi, M.; de la Haba, R.R.; Ventosa, A. Pseudorhodoplanes sinuspersici gen. nov., sp. nov., isolated from oil-contaminated soil. Int. J. Syst. Evol. Microbiol. 2015, 65, 4743–4748. [Google Scholar] [CrossRef]
- Miroshnichenko, M.L.; Rainey, F.A.; Rhode, M.; Bonch-Osmolovskaya, E.A. Hippea maritima gen. nov., sp. nov., a new genus of thermophilic, sul-fur-reducing bacterium from submarine hot vents. Int. J. Syst. Bacteriol. 1999, 49, 1033–1038. [Google Scholar] [CrossRef]
- Boada, E.; Santos-Clotas, E.; Bertran, S.; Cabrera-Codony, A.; Martín, M.J.; Bañeras, L.; Gich, F. Potential use of Methylibium sp. as a biodegradation tool in organosilicon and volatile compounds removal for biogas upgrading. Chemosphere 2020, 240, 124908. [Google Scholar] [CrossRef]
- Weon, H.-Y.; Kwon, S.-W.; Son, J.-A.; Kim, S.-J.; Kim, Y.-S.; Kim, B.-Y.; Ka, J.-O. Adhaeribacter aerophilus sp. nov., Adhaeribacter aerolatus sp. nov. and Segetibacter aerophilus sp. nov., isolated from air samples. Int. J. Syst. Evol. Microbiol. 2010, 60, 2424–2429. [Google Scholar] [CrossRef]
- Sghaier, H.; Hezbri, K.; Ghodhbane-Gtari, F.; Pujic, P.; Sen, A.; Daffonchio, D.; Boudabous, A.; Tisa, L.S.; Klenk, H.-P.; Armengaud, J.; et al. Stone-dwelling actinobacteria Blastococcus saxobsidens, Modestobacter marinus and Geodermatophilus obscurus proteogenomes. ISME J. 2016, 10, 21–29. [Google Scholar] [CrossRef]
- Köberl, M.; Erlacher, A.; Ramadan, E.M.; El-Arabi, T.F.; Müller, H.; Bragina, A.; Berg, G. Comparisons of diazotrophic communities in native and agricultural desert ecosystems reveal plants as important drivers in diversity. FEMS Microbiol. Ecol. 2016, 92, 166. [Google Scholar] [CrossRef]
- Rilling, J.I.; Acuña, J.J.; Sadowsky, M.J.; Jorquera, M.A. Putative Nitrogen-Fixing Bacteria Associated with the Rhizosphere and Root Endosphere of Wheat Plants Grown in an Andisol from Southern Chile. Front. Microbiol. 2018, 9, 2710. [Google Scholar] [CrossRef]
- Von Der Weid, I.; Artursson, V.; Seldin, L.; Jansson, J.K. Antifungal and root surface colonization properties of GFP-tagged Paenibacillus brasilensis PB177. World J. Microbiol. Biotechnol. 2005, 21, 1591–1597. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Hua, Y.; Sinkkonen, A.; Romantschuk, M.; Lv, Y.; Wu, Q.; Hui, N. Meat and bone meal stimulates microbial diversity and suppresses plant pathogens in asparagus straw composting. Front. Microbiol. 2022, 13, 953783. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, S.; Wong, C.P.; Ozeki, M.; Zhang, H.; Hayashi, F.; Awakawa, T.; Asamizu, S.; Onaka, H.; Abe, I. Umezawamides, new bioactive polycyclic tetramate macrolactams isolated from a combined-culture of Umezawaea sp. and mycolic acid-containing bacterium. J. Antibiot. 2018, 71, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Drooger, S. Soil Temperatures under a Catchment Scale Experimental Fire. Master’s Thesis, Wageningen University, Wageningen, The Netherlands, 2009. [Google Scholar]
- Pantis, J.D.; Mardiris, T.A. The effects of grazing and fire on degradation processes of Mediterranean ecosystems. Isr. J. Bot. 1992, 41, 233–242. [Google Scholar]
- Bonanomi, G.; Idbella, M.; Abd-ElGawad, A.M.; Motti, R.; Ippolito, F.; Santorufo, L.; Adamo, P.; Agrelli, D.; De Marco, A.; Maisto, G.; et al. Impact of prescribed burning, mowing and abandonment on a Mediterranean grassland: A 5-year multi-kingdom comparison. Sci. Total Environ. 2022, 834, 155442. [Google Scholar] [CrossRef] [PubMed]
- Dove, N.C.; Safford, H.D.; Bohlman, G.N.; Estes, B.L.; Hart, S.C. High-severity wildfire leads to multi-decadal impacts on soil biogeochemistry in mixed-conifer forests. Ecol. Appl. 2020, 30, e02072. [Google Scholar] [CrossRef]
- Ferrenberg, S.; O’Neill, S.P.; E Knelman, J.; Todd, B.; Duggan, S.; Bradley, D.; Robinson, T.; Schmidt, S.K.; Townsend, A.R.; Williams, M.W.; et al. Changes in assembly processes in soil bacterial communities following a wildfire disturbance. ISME J. 2013, 7, 1102–1111. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, X.; Nuccio, E.E.; Yuan, M.; Zhang, N.; Xue, K.; Cohan, F.M.; Zhou, J.; Sun, B. Differentiation strategies of soil rare and abundant microbial taxa in response to changing climatic regimes. Environ. Microbiol. 2020, 22, 1327–1340. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Meunier, D.; Lambiotte, R.; Bullmore, E.T. Modular and Hierarchically Modular Organization of Brain Networks. Front. Neurosci. 2010, 4, 200. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Bahr, J.; Robinson, D.M.; Belnap, J.; Campbell, T.P.; Gill, R.A.; McMillian, B.; Clair, S.S. The Burning of Biocrusts Facilitates the Emergence of a Bare Soil Community of Poorly-Connected Chemoheterotrophic Bacteria with Depressed Ecosystem Services. Front. Ecol. Evol. 2019, 7, 467. [Google Scholar] [CrossRef]
| Rhizosphere | Bulk Soil | ||||
|---|---|---|---|---|---|
| Unburnt | Burnt | Unburnt | Burnt | ||
| Total Bacteria | |||||
| Rhizosphere | Unburnt | 1 | 0.09 | 0.09 | 0.16 |
| Burnt | 1 | 0.68 | 0.13 | ||
| Bulk soil | Unburnt | 1 | 0.13 | ||
| Burnt | 1.00 | ||||
| Actinomycetes | |||||
| Rhizosphere | Unburnt | 1 | 0 | 0 | 0.32 |
| Burnt | 1 | 1 | 0 | ||
| Bulk soil | Unburnt | 1 | 0 | ||
| Burnt | 1 | ||||
| Alphaproteobacteria | |||||
| Rhizosphere | Unburnt | 1 | 0.08 | 0 | 0.23 |
| Burnt | 1 | 0.31 | 0 | ||
| Bulk soil | Unburnt | 1 | 0 | ||
| Burnt | 1 | ||||
| Bulk Burnt (BB) | Bulk Unburnt (BU) | Rhizosphere Burnt (RB) | Rhizosphere Unburnt (RU) | |
|---|---|---|---|---|
| Nb. of nodes | 130 | 108 | 93 | 125 |
| Νb. of ties | 590 | 344 | 318 | 154 |
| Avg Νeighborhood size (ANS) | 4.538 | 3.185 | 3.419 | 2.933 |
| ANS (STDEV) | 2.37 | 1.348 | 1.247 | 1.389 |
| % Deg Centralization | 5.875 | 2.655 | 2.835 | 1.500 |
| Density | 0.035 | 0.030 | 0.037 | 0.101 |
| Density (STDEV) | 0.184 | 0.170 | 0.189 | 0.046 |
| Fragmentation | 0.708 | 0.858 | 0.839 | 0.870 |
| Modularity | 0.814 | 0.861 | 0.868 | 0.861 |
| Nb. Modules | 17 | 20 | 14 | 21 |
| Avg Distance | 5.289 | 3.892 | 3.583 | 4.644 |
| Compactness | 0.095 | 0.060 | 0.072 | 0.050 |
| Clustering Coefficient | 0.623 | 0.593 | 0.649 | 0.239 |
| Small Worldness | 10.346 | 20.097 | 16.674 | 12.985 |
| Nulls | 0.965 | 0.970 | 0.962 | 0.980 |
| Robustness-Critical faction | 0.791 | 0.637 | 0.652 | 0.554 |
| Robustness-Natural Connectivity | 5.300 | 1.954 | 2.995 | 2.000 |
| Effectiveness | 0.691 | 0.624 | 0.648 | 0.388 |
| Rhizosphere | Bulk Soil | |||
|---|---|---|---|---|
| Unburnt (RU) | Burnt (RB) | Unburnt (BU) | Burnt (BB) | |
| Nb. of influential nodes | 19 | 19 | 21 | 22 |
| % Biofertilizers | 37 | 37 | 40 | 41 |
| % Bioprotectants | 22 | 11 | 19 | 27 |
| % Decomposers | 42 | 53 | 33 | 32 |
| Rhizosphere | Bulk | |
|---|---|---|
| Unburnt |
|
|
| Burnt |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papatheodorou, E.M.; Papakostas, S.; Stamou, G.P. Fire and Rhizosphere Effects on Bacterial Co-Occurrence Patterns. Microorganisms 2023, 11, 790. https://doi.org/10.3390/microorganisms11030790
Papatheodorou EM, Papakostas S, Stamou GP. Fire and Rhizosphere Effects on Bacterial Co-Occurrence Patterns. Microorganisms. 2023; 11(3):790. https://doi.org/10.3390/microorganisms11030790
Chicago/Turabian StylePapatheodorou, Effimia M., Spiros Papakostas, and George P. Stamou. 2023. "Fire and Rhizosphere Effects on Bacterial Co-Occurrence Patterns" Microorganisms 11, no. 3: 790. https://doi.org/10.3390/microorganisms11030790
APA StylePapatheodorou, E. M., Papakostas, S., & Stamou, G. P. (2023). Fire and Rhizosphere Effects on Bacterial Co-Occurrence Patterns. Microorganisms, 11(3), 790. https://doi.org/10.3390/microorganisms11030790








