Exploitation of a Type 1 Toxin–Antitoxin System as an Inducible Counter-Selective Marker for Genome Editing in the Acetogen Eubacterium limosum
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Routine Culture of Bacteria
2.2. High-Efficiency Electroporation of E. limosum
2.3. Cloning of the Toxicity Assay Vector pMTL-JM101
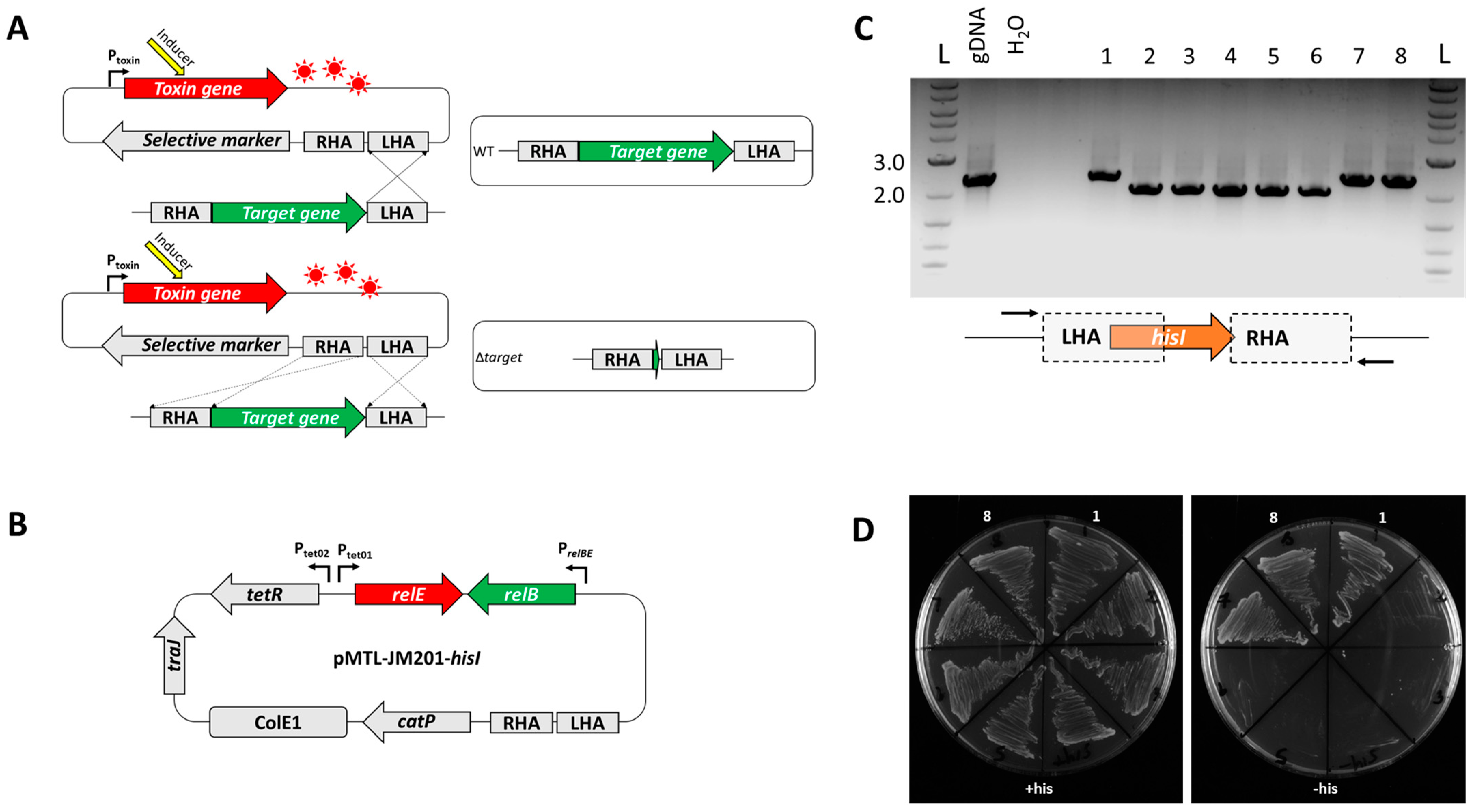
2.4. Cloning of the hisI Knockout Vector pMTL-JM201-hisI
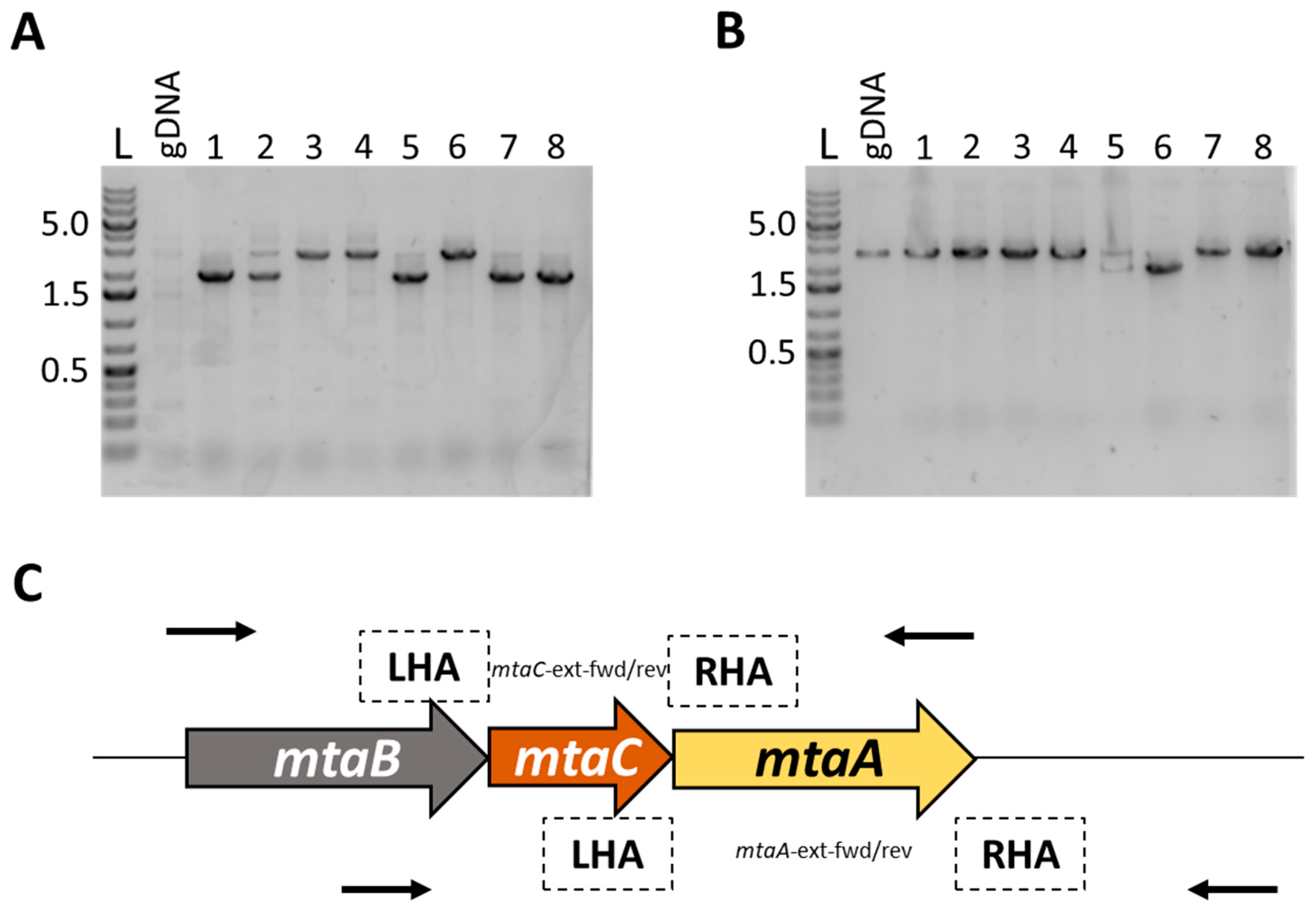
2.5. Cloning of the mtaA and mtaC Knockout Vectors pMTL-AA201-mtaA and pMTL-AA201-mtaC
2.6. Cloning of the mtcB Knockout Vector pMTL-JM201-mtcB
2.7. In Vivo RNA Staining with Thioflavin T
3. Results
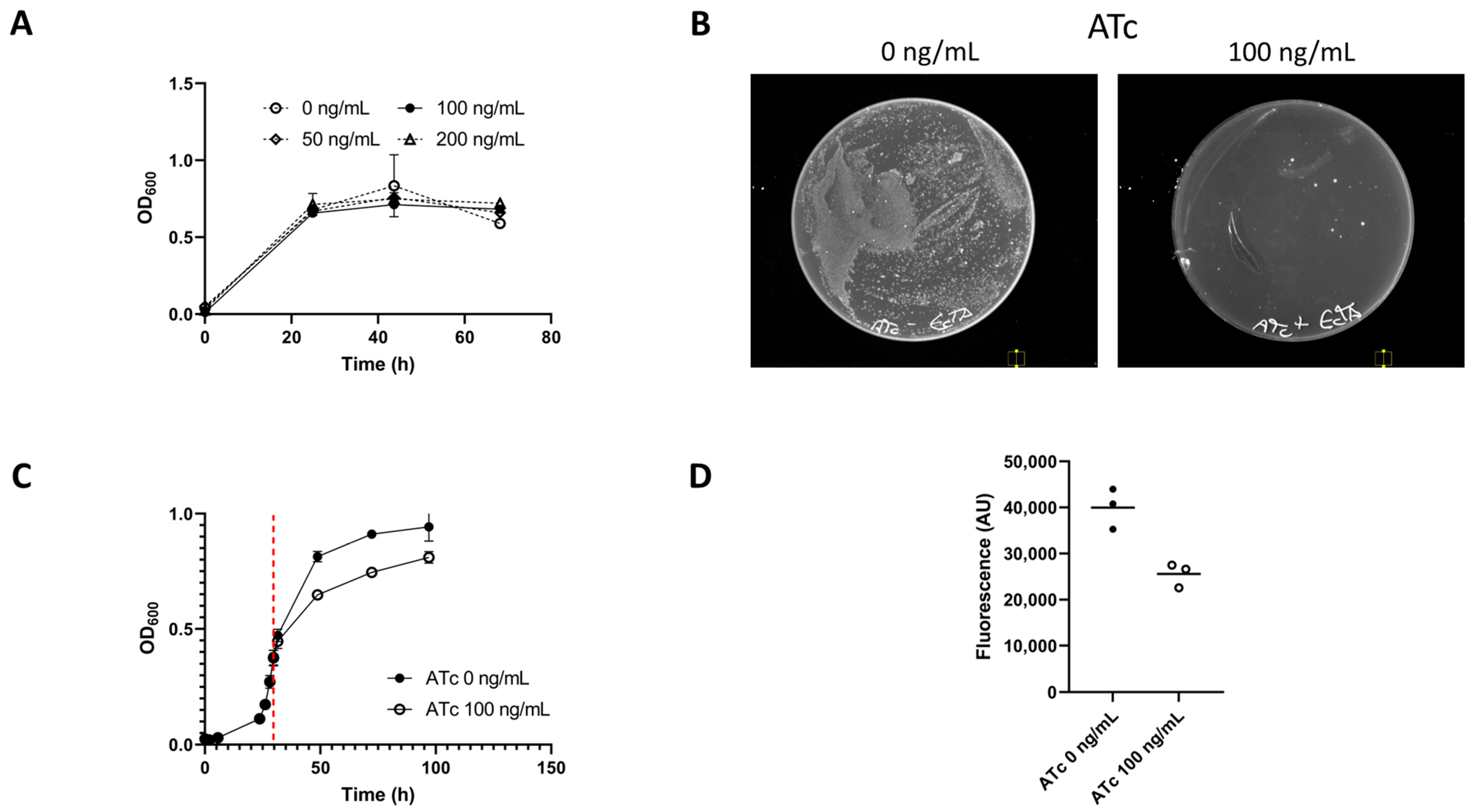
3.1. Demonstration of E. callanderi RelE toxicity in E. limosum
3.2. Exemplification of relE as a Counter-Selection Marker
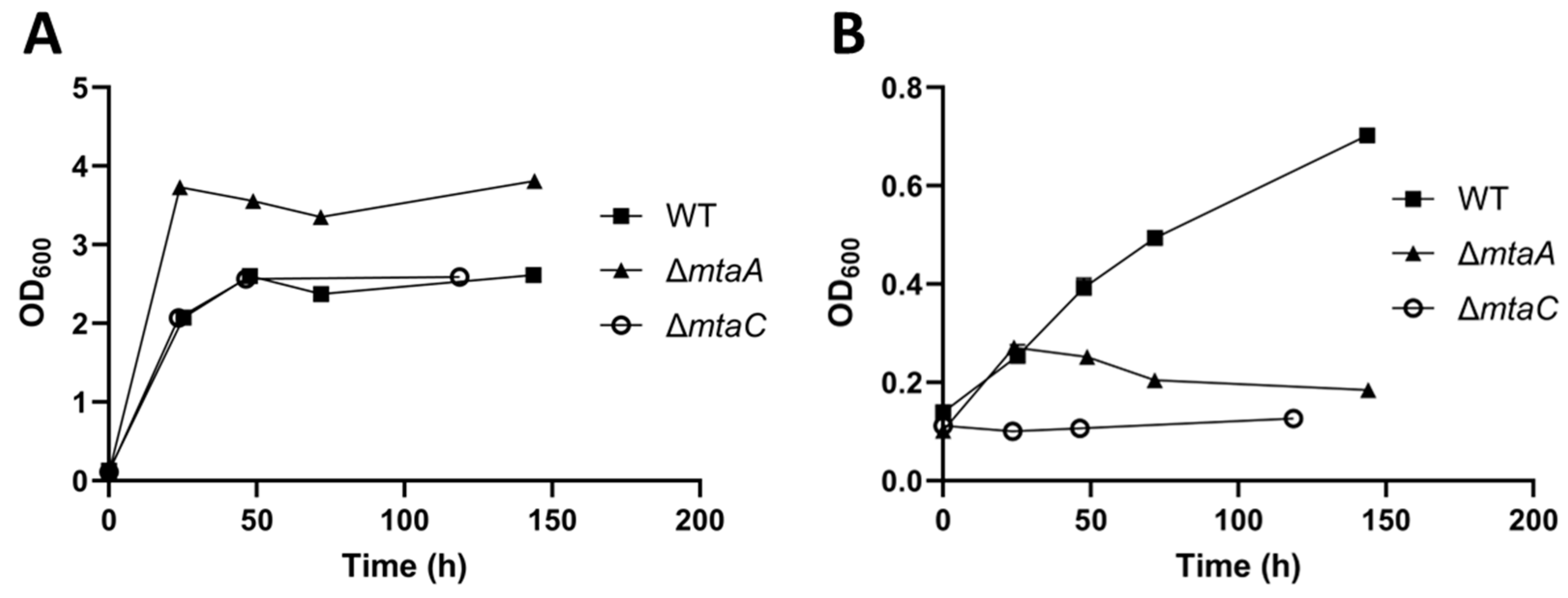
3.3. Deletion of mtaA and mtaC Genes
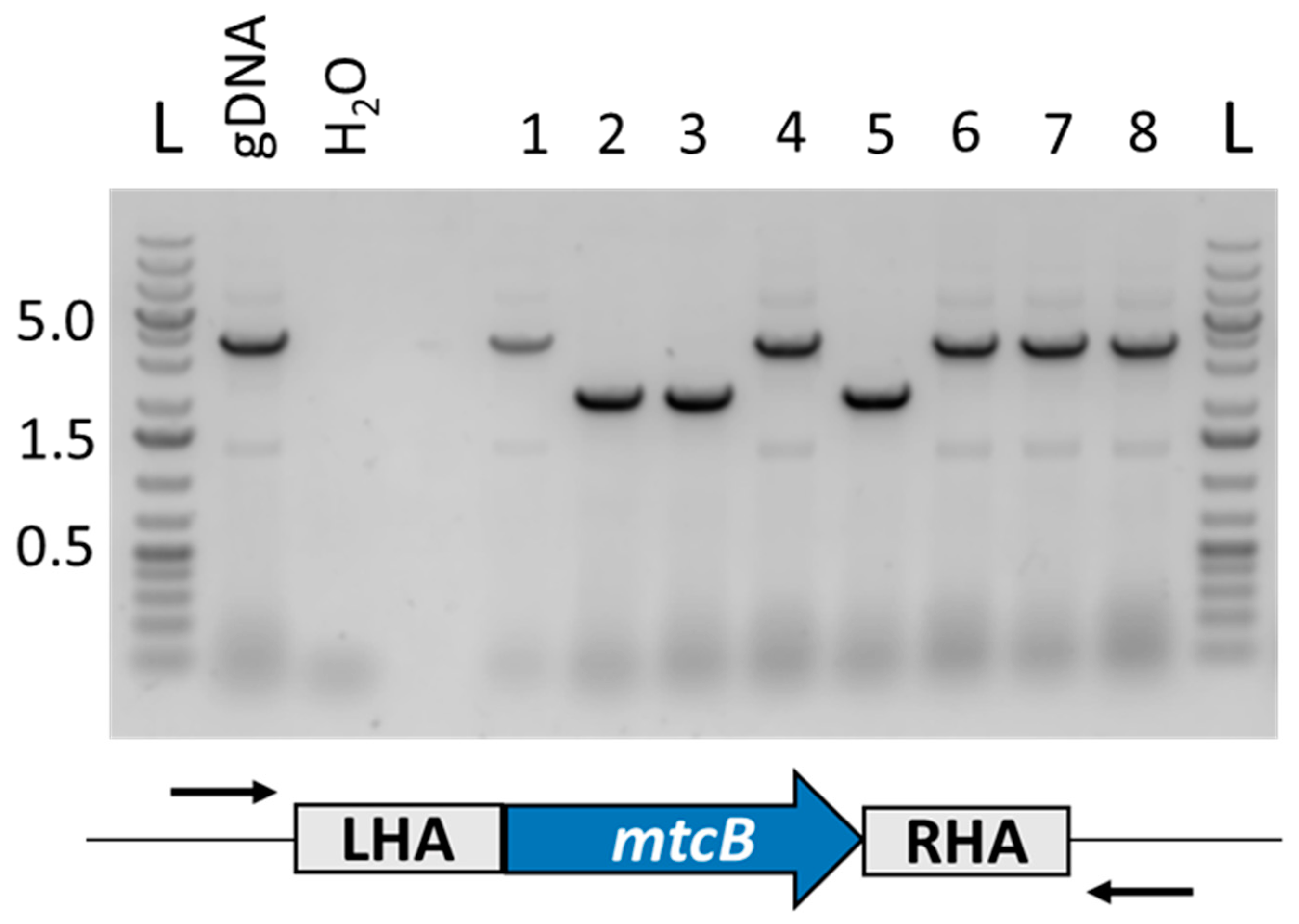
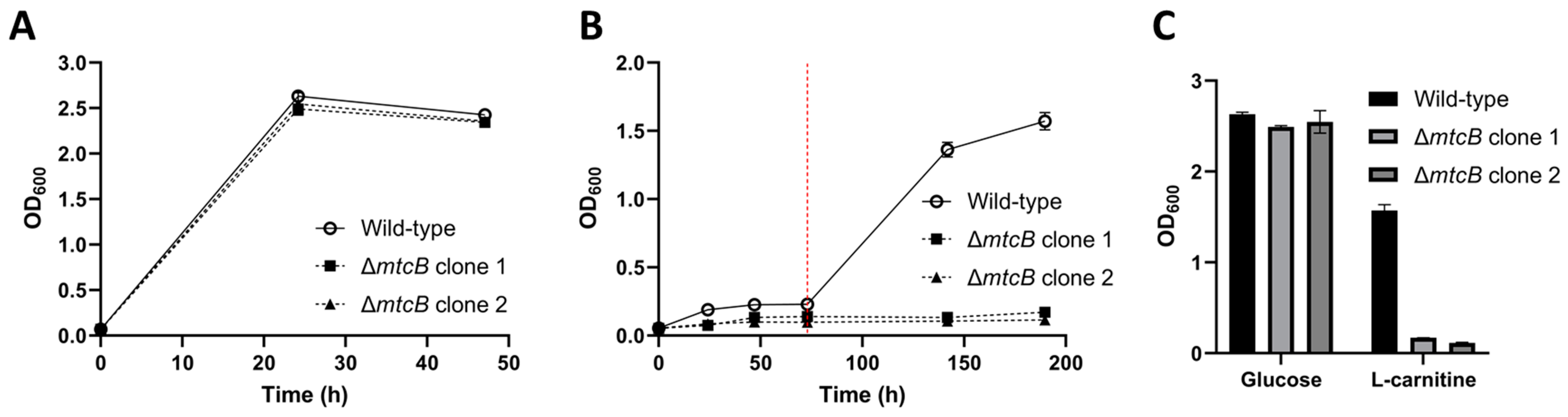
3.4. Deletion of Carnitine Demethylase Gene mtcB
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Genthner, B.R.; Davis, C.L.; Bryant, M.P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl. Environ. Microbiol. 1981, 42, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.L.; Gößner, A.S.; Daniel, S.L. Old Acetogens, New Light. Ann. N. Y. Acad. Sci. 2008, 1125, 100–128. [Google Scholar] [CrossRef] [PubMed]
- Eggerth, A.H. The Gram-positive Non-spore-bearing Anaerobic Bacilli of Human Feces. J. Bacteriol. 1935, 30, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Cotton, C.A.; Claassens, N.J.; Benito-Vaquerizo, S.; Bar-Even, A. Renewable methanol and formate as microbial feedstocks. Curr. Opin. Biotechnol. 2020, 62, 168–180. [Google Scholar] [CrossRef]
- Wood, J.C.; Marcellin, E.; Plan, M.R.; Virdis, B. High methanol-to-formate ratios induce butanol production in Eubacterium limosum. Microb. Biotechnol. 2021, 15, 1542–1549. [Google Scholar] [CrossRef]
- Flaiz, M.; Ludwig, G.; Bengelsdorf, F.R.; Dürre, P. Production of the biocommodities butanol and acetone from methanol with fluorescent FAST-tagged proteins using metabolically engineered strains of Eubacterium limosum. Biotechnol. Biofuels 2021, 14, 117. [Google Scholar] [CrossRef]
- Song, Y.; Shin, J.; Jeong, Y.; Jin, S.; Lee, J.-K.; Kim, D.R.; Kim, S.C.; Cho, S.; Cho, B.-K. Determination of the Genome and Primary Transcriptome of Syngas Fermenting Eubacterium limosum ATCC 8486. Sci. Rep. 2017, 7, 13694. [Google Scholar] [CrossRef]
- Pregnon, G.; Minton, N.P.; Soucaille, P. Genome Sequence of Eubacterium limosum B2 and Evolution for Growth on a Mineral Medium with Methanol and CO2 as Sole Carbon Sources. Microorganisms 2022, 10, 1790. [Google Scholar] [CrossRef]
- Shin, J.; Kang, S.; Song, Y.; Jin, S.; Lee, J.S.; Lee, J.-K.; Kim, D.R.; Kim, S.C.; Cho, S.; Cho, B.-K. Genome Engineering of Eubacterium limosum Using Expanded Genetic Tools and the CRISPR-Cas9 System. ACS Synth. Biol. 2019, 8, 2059–2068. [Google Scholar] [CrossRef]
- Singh, G.; Yadav, M.; Ghosh, C.; Rathore, J.S. Bacterial toxin-antitoxin modules: Classification, functions, and association with persistence. Curr. Res. Microb. Sci. 2021, 2, 100047. [Google Scholar] [CrossRef]
- Ogura, T.; Hiraga, S. Mini-F plasmid genes that couple host cell division to plasmid proliferation. Proc. Natl. Acad. Sci. USA 1983, 80, 4784–4788. [Google Scholar] [CrossRef]
- Masuda, Y.; Miyakawa, K.; Nishimura, Y.; Ohtsubo, E. chpA and chpB, Escherichia coli chromosomal homologs of the pem locus responsible for stable maintenance of plasmid R100. J. Bacteriol. 1993, 175, 6850–6856. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Yan, X.; Cui, Z.-L.; Hong, Q.; Li, S.-P. mazF, a novel counter-selectable marker for unmarked chromosomal manipulation in Bacillus subtilis. Nucleic Acids Res. 2006, 34, e71. [Google Scholar] [CrossRef]
- Al-Hinai, M.A.; Fast, A.G.; Papoutsakis, E.T. Novel System for Efficient Isolation of Clostridium Double-Crossover Allelic Exchange Mutants Enabling Markerless Chromosomal Gene Deletions and DNA Integration. Appl. Environ. Microbiol. 2012, 78, 8112–8121. [Google Scholar] [CrossRef]
- Sandoval, N.R.; Venkataramanan, K.P.; Groth, T.S.; Papoutsakis, E.T. Whole-genome sequence of an evolved Clostridium pasteurianum strain reveals Spo0A deficiency responsible for increased butanol production and superior growth. Biotechnol. Biofuels 2015, 8, 227. [Google Scholar] [CrossRef]
- Peltier, J.; Hamiot, A.; Garneau, J.R.; Boudry, P.; Maikova, A.; Hajnsdorf, E.; Fortier, L.-C.; Dupuy, B.; Soutourina, O. Type I toxin-antitoxin systems contribute to the maintenance of mobile genetic elements in Clostridioides difficile. Commun. Biol. 2020, 3, 718. [Google Scholar] [CrossRef]
- Kountz, D.J.; Behrman, E.J.; Zhang, L.; Krzycki, J.A. MtcB, a member of the MttB superfamily from the human gut acetogen Eubacterium limosum, is a cobalamin-dependent carnitine demethylase. J. Biol. Chem. 2020, 295, 11971–11981. [Google Scholar] [CrossRef]
- Pacaud, S.; Loubière, P.; Goma, G.; Lindley, N.D. Organic acid production during methylotrophic growth of Eubacterium limosum B2: Displacement towards increased butyric acid yields by supplementing with acetate. Appl. Microbiol. Biotechnol. 1986, 23, 330–335. [Google Scholar] [CrossRef]
- Heap, J.T.; Pennington, O.J.; Cartman, S.T.; Minton, N.P. A modular system for Clostridium shuttle plasmids. J. Microbiol. Methods 2009, 78, 79–85. [Google Scholar] [CrossRef]
- Roh, H.; Ko, H.-J.; Kim, D.; Choi, D.G.; Park, S.; Kim, S.; Chang, I.S.; Choi, I.-G. Complete Genome Sequence of a Carbon Monoxide-Utilizing Acetogen, Eubacterium limosum KIST612. J. Bacteriol. 2011, 193, 307–308. [Google Scholar] [CrossRef]
- Fagan, R.P.; Fairweather, N.F. Clostridium difficile Has Two Parallel and Essential Sec Secretion Systems. J. Biol. Chem. 2011, 286, 27483–27493. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S.; Arita-Morioka, K.-I.; Mizunoe, Y.; Yamanaka, K.; Ogura, T. Thioflavin T as a fluorescence probe for monitoring RNA metabolism at molecular and cellular levels. Nucleic Acids Res. 2015, 43, e92. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Christensen, S.K.; Gerdes, K. Rapid induction and reversal of a bacteriostatic condition by controlled expression of toxins and antitoxins. Mol. Microbiol. 2002, 45, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-X.; Deng, C.-Y.; Zhang, Y.-T.; Liu, Z.-M.; Wang, P.-Z.; Liu, S.-L.; Qian, W.; Yang, D.-H. Cloning, expression, and characterization of a four-component O-demethylase from human intestinal bacterium Eubacterium limosum ZL-II. Appl. Microbiol. Biotechnol. 2016, 100, 9111–9124. [Google Scholar] [CrossRef] [PubMed]
- Litty, D.; Kremp, F.; Müller, V. One substrate, many fates: Different ways of methanol utilization in the acetogen Acetobacterium woodii. Environ. Microbiol. 2022, 24, 3124–3133. [Google Scholar] [CrossRef]
- Ellenbogen, J.B.; Jiang, R.; Kountz, D.J.; Zhang, L.; Krzycki, J.A. The MttB superfamily member MtyB from the human gut symbiont Eubacterium limosum is a cobalamin-dependent γ-butyrobetaine methyltransferase. J. Biol. Chem. 2021, 297, 101327. [Google Scholar] [CrossRef]
- Minton, N.P.; Ehsaan, M.; Humphreys, C.M.; Little, G.T.; Baker, J.; Henstra, A.M.; Liew, F.; Kelly, M.L.; Sheng, L.; Schwarz, K.; et al. A roadmap for gene system development in Clostridium. Anaerobe 2016, 41, 104–112. [Google Scholar] [CrossRef]
- Fraikin, N.; Goormaghtigh, F.; Van Melderen, L. Type II Toxin-Antitoxin Systems: Evolution and Revolutions. J. Bacteriol. 2020, 202, e00763-19. [Google Scholar] [CrossRef]
- De Bast, M.S.; Mine, N.; Van Melderen, L. Chromosomal Toxin-Antitoxin Systems May Act as Antiaddiction Modules. J. Bacteriol. 2008, 190, 4603–4609. [Google Scholar] [CrossRef]
- Álvarez, R.; Ortega-Fuentes, C.; Queraltó, C.; Inostroza, O.; Díaz-Yáñez, F.; González, R.; Calderón, I.; Fuentes, J.; Paredes-Sabja, D.; Gil, F. Evaluation of functionality of type II toxin-antitoxin systems of Clostridioides difficile R20291. Microbiol. Res. 2020, 239, 126539. [Google Scholar] [CrossRef]

| Vector | Purpose | Source |
|---|---|---|
| pMTL-JM101 | Inducible toxin test vector used to demonstrate the efficacy of the E. callanderi toxin. | This study. |
| pMTL-JM201-hisI | TA knockout vector targeted against the histidine biosynthesis gene hisI. | This study. |
| pMTL-AA201-mtaA | TA knockout vector targeted against mtaA, a putative corrinoid:methyl-THF methyltransferase. | This study. |
| pMTL-AA201-mtaC | TA knockout vector targeted against mtaC, a corrinoid-binding protein associated with mtaA/B. | This study. |
| pMTL-JM201-mtcB | TA knockout vector targeted against mtcB, encoding a carnitine:corrinoid methyltransferase (WP_038351887.1). | This study. |
| pMTL83151 | E. coli-Clostridium shuttle vector. | Heap et al. [19]. |
| pMTL-tet3no | E. coli-Clostridium shuttle vector with tetracycline-inducible divergent promoter system. | SBRC Nottingham |
| Primer | 5′-3′ Oligonucleotide Sequence | Function |
|---|---|---|
| bb1_fwd | gtacccggggatcctctag | Amplification of pMTL-JM101 backbone from pMTL83151 |
| bb1_rev | cgagctcgaattcgtaatcatg | |
| tox_fwd | ggaaatacatatgaaaagctatgaggtg | Amplification of E. callanderi relE |
| tox_rev | tggtgaatgatcaattccatatatcttccag | |
| atox_fwd | atggaattgatcattcaccatatttctcctgc | Amplification of E. callanderi relB |
| atox_rev | tctagaggatccccgggtacgcaggaggcgatttgattttg | |
| tet_fwd | tgattacgaattcgagctcgttaagacccactttcacatttaag | Amplification of Tetracycline-inducible promoter system from pMTL-tet3no |
| tet_rev | agcttttcatatgtatttcctcctcttcaatatatttaag | |
| bb2_fwd | ggccggccagtgggcaag | Amplification of pMTL-JM201 vector backbone from pMTL-JM101. |
| bb2_rev | ttgtcaattgttcaaaaaaataatggcggcgcg | |
| hisI_lha_fwd | tttttttgaacaattgacaatggcggacccggtagagc | Primers for left homology arm of hisI deletion construct. |
| hisI_lha_rev | ataaggcttaattacggtagaagcaggaagtttcgc | |
| hisI_rha_fwd | ctaccgtaattaagccttattaaaaaaagaacc | Primers for right homology arm of hisI deletion construct. |
| hisI_rha_rev | aacttgcccactggccggccaatatcttttttggataaaatttgtg | |
| mtaA_lha_fwd | ttagtacacactgcgcgc | Primers for left homology arm of mtaA deletion construct |
| mtaA_lha_rev | aatgaaatcatcgggataggatgcctcctgttcgtc | |
| mtaA_rha_fwd | gacgaacaggaggcatcctatcccgatgatttcattctccaagt | Primers for right homology arm of mtaA deletion construct |
| mtaA_rha_rev | aactctccctccagctgttc | |
| mtaA_ext_fwd | aggaaatcaatgacgaagccct | External screening primers for confirming mtaA knockout. |
| mtaA_ext_rev | taagagattgatacgctccgcc | |
| mtaA_int | ataccatcaaggttatcaacgacgcagg | Internal sequencing primer for sequencing mtaA knockout locus. |
| mtaC_lha_fwd | caaggactgcgcctatgaagg | Primers for left homology arm of mtaC deletion construct |
| mtaC_lha_rev | ttcgtcaaaattttattcgctgtctgttttatatcctccgatttttgtttttattcct | |
| mtaC_rha_fwd | aaaacaaaaatcggaggatataaaacagacagcgaataaaattttgacgaa | Primers for right homology arm of mtaC deletion construct |
| mtaC_rha_rev | tcattttgccattggtgggg | |
| mtaC_ext_fwd | agactggggaatactttgcagg | External screening primers for confirming mtaC knockout. |
| mtaC_ext_rev | agttgacgtcgatataggtcgc | |
| mtaC_int | ggacatggacaaatggcatcctgaag | Internal sequencing primer for sequencing mtaC knockout locus. |
| mtcB_lha_fwd | tttttttgaacaattgacaatgctggcgcctgtaaagg | Primers for left homology arm of mtcB in-frame deletion. |
| mtcB_lha_rev | attgagcttacatttgtctctctccttaaatatctcaaaatcttttc | |
| mtcB_rha_fwd | gagacaaatgtaagctcaatggggatcg | Primers for right homology arm of mtcB in-frame deletion. |
| mtcB_rha_rev | aacttgcccactggccggcctttcggcaggatcaggaaag | |
| mtcB_ext_fwd | catccaaaaacaatgtgccgttgttgg | External sequencing primers for confirming mtcB knockout. |
| mtcB_ext_rev | gcgccgaatatatgaatgggcacc | |
| mtcB_int | ccagataagcgctgtattcaggatcatgg | Internal sequencing primer for sequencing mtcB knockout locus. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millard, J.; Agius, A.; Zhang, Y.; Soucaille, P.; Minton, N.P. Exploitation of a Type 1 Toxin–Antitoxin System as an Inducible Counter-Selective Marker for Genome Editing in the Acetogen Eubacterium limosum. Microorganisms 2023, 11, 1256. https://doi.org/10.3390/microorganisms11051256
Millard J, Agius A, Zhang Y, Soucaille P, Minton NP. Exploitation of a Type 1 Toxin–Antitoxin System as an Inducible Counter-Selective Marker for Genome Editing in the Acetogen Eubacterium limosum. Microorganisms. 2023; 11(5):1256. https://doi.org/10.3390/microorganisms11051256
Chicago/Turabian StyleMillard, James, Alexander Agius, Ying Zhang, Philippe Soucaille, and Nigel Peter Minton. 2023. "Exploitation of a Type 1 Toxin–Antitoxin System as an Inducible Counter-Selective Marker for Genome Editing in the Acetogen Eubacterium limosum" Microorganisms 11, no. 5: 1256. https://doi.org/10.3390/microorganisms11051256
APA StyleMillard, J., Agius, A., Zhang, Y., Soucaille, P., & Minton, N. P. (2023). Exploitation of a Type 1 Toxin–Antitoxin System as an Inducible Counter-Selective Marker for Genome Editing in the Acetogen Eubacterium limosum. Microorganisms, 11(5), 1256. https://doi.org/10.3390/microorganisms11051256








