Abstract
Sex and gender dimorphisms are found in a large variety of diseases, including sepsis and septic shock which are more prevalent in men than in women. Animal models show that the host response to pathogens differs in females and males. This difference is partially explained by sex polarization of the intracellular pathways responding to pathogen–cell receptor interactions. Sex hormones seem to be responsible for this polarization, although other factors, such as chromosomal effects, have yet to be investigated. In brief, females are less susceptible to sepsis and seem to recover more effectively than males. Clinical observations produce more nuanced findings, but men consistently have a higher incidence of sepsis, and some reports also claim higher mortality rates. However, variables other than hormonal differences complicate the interaction between sex and sepsis, including comorbidities as well as social and cultural differences between men and women. Conflicting data have also been reported regarding sepsis-attributable mortality rates among pregnant women, compared with non-pregnant females. We believe that unraveling sex differences in the host response to sepsis and its treatment could be the first step in personalized, phenotype-based management of patients with sepsis and septic shock.
1. Introduction
The terms gender and sex are often used interchangeably when discussing disease differences between males and females. However, gender refers to the social representation of a group while sex relates to biological characteristics. Gender-related disparities may be addressed, at least in part, by changing the socially constructed roles and behaviors of a population. A typical example is the decreasing rate of sexually transmitted diseases in populations at risk. Addressing sex-related differences requires in-depth epidemiological and biological understanding of disease processes.
Sex-based differences in disease are widely reported in the literature. For example, females have a higher prevalence of autoimmune disease [1], whereas males have a higher prevalence of cancer [2]. Sepsis, defined as life-threatening organ dysfunction resulting from a host’s dysregulated response to infection [3,4], is more prevalent among males [5,6,7]. The difference in sepsis prevalence stems from both social and biological causes. Social aspects include, for example, greater exposure to risk factors related to lifestyle (e.g., physical activity, work outdoors) and greater exposure to violence among men. Biological aspects relate to sex-related differences in hormonal and immune system characteristics. SARS-CoV-2 is one typical example, wherein both the incidence and the severity of COVID-19 disease among males are higher [8]. Women have a higher lifetime risk and worse disease expression of specific infections, such as tuberculosis and malaria [9]. Sex differences in hormonal and immune profiles are most extreme during pregnancy and the peripuerum. In the developing world, during this period one in five women contract a clinically significant infection [10]. This review highlights some of the more important experimental and clinical studies showing sex-specific differences in sepsis.
2. Animal Models
Multiple animal models have been established to investigate sepsis, including systemic administration of endotoxins or pathogens by various routes and cecal ligature and puncture. Animal models would enable assessment of the interaction between sex and sepsis while minimizing clinical confounders. However, the vast majority of pre-clinical sepsis studies have been performed only on male animals. The historic justification for the use of male animals only was the need to control for hormonal effects. However, early assumptions that female animals are prone to greater physiological variability due to hormonal cycles have not been supported by evidence [11,12,13,14]. Since it is becoming increasingly clear that female rodents are not small male rodents [15], this issue may render most basic research irrelevant for women. Research on female animal models is currently lagging at least 20–30 years following studies conducted on male animal models [16]. Therefore, with regards to sepsis and sex—what have we learned from animal models thus far?
2.1. Sex and the Immune System Response to Sepsis
Increasing evidence suggests that sex affects the host response to sepsis; sex hormones have been shown to target most immune system cells. For example, using a transcriptomic approach, Textoris et al. showed that 86% of genes involved in the response to Coxiella burnetii infection were associated with sex [17]. Such encompassing involvement suggests a very high likelihood of sex polarization in the host response to infection.
Overall, female sex seems protective with regards to the likelihood of sepsis; after an infectious challenge, bacteria are cleared faster in females than in males [17], whereas ovariectomized animals tend to have a bacterial clearance similar to that of male animals [17]. Females also seem to have a less reactive inflammatory response than males [17]. Estrogens induce efficient cell-mediated and humoral immune responses while androgens are suppressive [18]. The lower immune reactivity among females compared with males is believed to stem from evolutionary adaptations to pregnancy [19].
Multiple pathways lead to the difference in immune response between sexes. For example, dual-specificity phosphatase 3 (DUSP3) is linked to estrogen-mediated modulation of the macrophage response, thereby protecting female rats from endotoxemia-induced and polymicrobial-induced septic shock [20]. AMP-activated protein kinase (AMPK) isoform α1 (an enzyme with hepatoprotective effects during sepsis) modulates the susceptibility to multiple organ injury in a sex-dependent manner via its metabolic functions [21]. Adaptative immunity is also sex-dependent; estradiol boosts reactive oxygen species and promotes phagocytosis in murine sepsis models [22].
Finally, there is a close interaction between steroid receptors and sex. In fact, sex hormone receptors are also called sex steroid receptors, and they interact with sex hormones, including androgens, estrogen, and progesterone. The use of steroids in clinical practice (e.g., during septic shock) may interfere with sex hormone metabolism. While this topic has been partially explored [23], none of the studies conducted in animal models have taken into consideration confounding by external steroid use, a medication commonly used in septic shock. Clearly this requires further research [24].
2.2. Sex and the Cardiac Response to Sepsis
Several studies have reported fundamental data regarding the cardiac response to sepsis and sex dimorphism [25,26,27]. Estrogen modulates several acute injury-related myocardial responses. Endotoxin administration produces less pronounced myocardial dysfunction in females than in males [28]. Beta-blocker administration has been suggested to be beneficial for patients with septic shock as it may improve the cardiac relaxation, left ventricular filling, and the cardiac index [29]. However, sex differences have been shown in the beta-adrenergic response of isolated rabbit hearts [30]. In an in vivo model of landiolol (a short acting beta blocker) administered after cecal ligation and puncture in rats, cardiac function worsened significantly after CLP and landiolol reversed this effect in males. Conversely, landiolol decreased left ventricle ejection fraction in females [31]. A subsequent transcriptome study showed that landiolol reverses the expression of several genes that are downregulated during sepsis in male rats. However, sepsis-induced downregulation of gene expression was less pronounced in females than in males, and was maintained in landiolol-treated female rats [32]. A third study randomized female rats to ovariectomy and showed that after CLP, cardiac performance decreased in the ovariectomized female rats. Inflammatory and apoptotic pathways, including the JAK/STAT pathway, were less activated in ovariectomized septic females than in controls, as well as adrenergic and calcium pathways, in line with decreased cardiac performance and poor hemodynamic profiles. Conversely, administration of landiolol increased calcium entry into the cardiomyocytes of ovariectomized female rats. Landiolol restored JAK2 expression and overexpressed genes responsible for calcium influx in ovariectomized septic females, whereas it did the opposite in control females [33]. This may explain why cardiac performance improves only in ovariectomized septic females (i.e., less exposure to female hormones during sepsis).
At the cellular level, there are sex-related differences in the inflammatory response; calcium signaling and apoptosis pathways and a protective cardiac effect in female animals have been associated with increased AKT and endothelial NO synthase (eNOS) phosphorylation, which may be involved in the response to beta blockers [28,31]. Overexpression of the eNOS protein [31] and decreased level of the guanylate cyclase 1 soluble subunit alpha 2 (GUCY1A2) transcript [32], regulators of cGMP, were found only in septic males. In male rats, landiolol reversed the increase in eNOS expression, whereas no such effect was observed after landiolol administration in females [32]. Cyclic guanosine monophosphate (cGMP) is protective in males and deleterious in females [27]. Sex-related differences in regulation of β-adrenergic signaling and calcium cycling were associated with failure of diastolic relaxation and impaired systolic contraction [34], decreased stroke volume index [31], and reduced survival in septic males [35]. A decrease in myofilament calcium sensitivity following troponin I hyperphosphorylation in males may explain this difference [35].
Transcriptional research also highlights male-specific regulation of the JAK/STAT, phosphoinositide-3-kinase (PI3K), and focal adhesion pathways as well as overexpression of p53-dependent cell-cycle arrest (p21 and stromal antigen 1 (STAG1)), toll-like receptor 1 (TLR1), and the myeloid differentiation primary response 88 (MyD88) [32]. These changes, not described in females, have been associated with a poor outcome [35]. Moreover, estrogen may modulate ryanodine receptor properties [36] and β-adrenergic receptors to affect Ca2+-handling proteins and the PI3K-AKT pathway [37]. Sepsis induced cardiac dysfunction improves after administration of 17-β-œstradiol [38] and immunomodulatory effect of estrogens in animal models [39] (Figure 1).
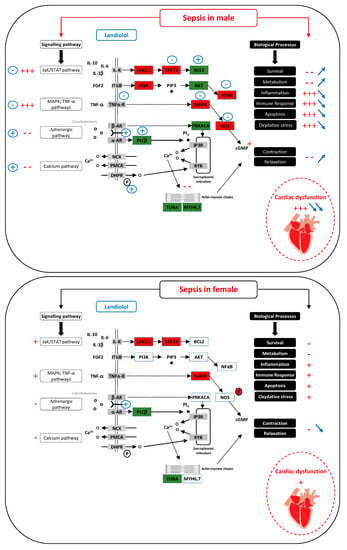
Figure 1.
In males (top panel), sepsis induces severe cardiac dysfunction by activating pathways responsible for inflammation, apoptosis, oxidative stress and by inhibiting pathways associated with excitation/contraction coupling (indications in red). Landiolol, by acting on the regulation of numerous genes, strongly improves cardiac function by reversing these biological processes (blue indications). In females (bottom panel), the effect of sepsis is markedly weaker than in males and the cardiac dysfunction is much more pronounced (indications in red). In contrast to the male, landiolol has little effect on gene regulation. It does not improve the effect of sepsis on biological processes such as inflammation or oxidative stress but, on the contrary, acts on the beta-adrenergic pathway and further decreases the contraction and relaxation capacity of the heart (blue indications). Cellular pathways according to sex. + = activation; − = inhibition;  = increase;
= increase;  = decrease; red rectangle = upregulated gene; green rectangle = downregulated gene.
= decrease; red rectangle = upregulated gene; green rectangle = downregulated gene.
 = increase;
= increase;  = decrease; red rectangle = upregulated gene; green rectangle = downregulated gene.
= decrease; red rectangle = upregulated gene; green rectangle = downregulated gene.
2.3. Sex and the Microbiome in Sepsis
The microbiome has recently become a topic of interest among several mechanisms of the host response to sepsis [40]. Microbiome diversity decreases in both male and female mice after cecal ligation and puncture. However, at day 14, the microbiome of females recovers fully while that of males still differs from controls. Notably, female recovery seems age dependent; only young females are similar to controls at day 14 [41]. The authors that showed these findings have suggested that female resilience to microbiome pathogens may explain some of the sex-related differences in sepsis outcomes.
2.4. Sex and the Central Nervous System in Sepsis
A murine model of the central nervous system response to sepsis found that sex and age influenced both the response of the brain to sepsis and its recovery after sepsis [42]. Aging was associated with delayed recovery from sepsis, and this delay was more pronounced in males. In a rat model of septic shock, transcriptional responses linked to neurogenesis and myelination were downregulated in all rats, but recovery rates differed by sex and age. Young female rats had the most rapid recovery rate. In older female rats, although most genes recovered, those linked to neurogenesis and myelination were still downregulated but no longer decreasing on day 4. In older male rats, genes linked to neurogenesis and myelination continued to decrease on day 4, while immune response genes continued to increase. A subsequent epigenetic analysis showed that on days 1 to 4 after sepsis, young female rats had better brain microRNA response profiles than young males and older females [43].
2.5. Pregnancy and Sepsis
The maternal immune system adapts during pregnancy to avoid allogenic rejection of the fetus, which is genetically distinct [44]. Most animal models of sepsis in pregnant animals have focused on fetal effects and neonatal outcomes [45]. We are unaware of animal studies specifically intended to address the effects of maternal immune system adaptations to pregnancy on maternal effects and outcomes in sepsis.
2.6. Summary of Lessons from Animal Models of Sepsis and Sex
Many papers have highlighted the role of sex dimorphism on the host response to sepsis. In brief, females are less susceptible to sepsis and seem to recover more effectively than males. Age remains an important confounder that requires further investigation; old females clearly differ from young females with regards to the host response to sepsis. While this may seem entirely attributable to changes in sex hormones, interference by other variables has not been ruled out. Among the studies conducted on interventions during sepsis (e.g., landiolol, antimicrobials, fluids), all have shown some evidence of a difference between sexes when such a difference was sought [46], but these findings are often contradictory [47,48].
Of note, animal models have several limitations with regards to the topic at hand. First, no model fully reflects the complexity and the varied presentation of sepsis. Careful planning is implemented when designing research protocols for sepsis in animal models; as physiological variability decreases, result interpretation is more precise [49]. Second, the effects of prior diseases and/or comorbidities are not really addressed in most models. Finally, most animal models of sepsis do not take into account organ support which could be an important source of confounding [50].
3. Clinical Studies
Twenty years after the European Union policy declaration intended to encourage and improve sex and gender aspects of biomedical and health-related research [51], the critical care community remains reluctant to address topics relating to differences between men and women in disease epidemiology, manifestations, and outcomes. As it is important to understand the role of sex in the epidemiology and clinical presentation of sepsis [52], the following section will address the few advances that have been made in understanding sex differences in sepsis among human subjects.
3.1. Sex Differences in Sepsis Epidemiology
Several studies have reported sex-based differences in the epidemiology of sepsis and all of these studies show a consistently higher risk of sepsis in men (Table 1). One nationwide study reported that male patients contributed 63.8% of the 187,587 septic shock episodes included in the study [53]. Even during the steady increase in the incidence of septic shock observed during 4 years, this sex discrepancy remains consistent [53].
The reasons underlying the higher rate of sepsis in men remain unclear. Several explanations have been put forward for this phenomenon, with the truth probably being a combination of all of those. Hypotheses include physiological characteristics affecting different susceptibility to infection, a greater inclination to progress from non-severe to severe infection, and gender differences in sepsis management. Additional factors may also play a role in the interaction between sepsis and sex. Smoking, alcohol consumption, and recreational activities differ between men and women. Such causes may also affect the likelihood of environmental exposure to infectious diseases (more specifically pathogens) [54,55].
3.2. Sex Differences in the Sources of Infection
Men are more commonly affected by endocarditis and bacteremia [56,57,58]. Men also tend to develop sepsis more frequently from respiratory infections (36% vs. 29% for women, p < 0.01), while women tend to develop sepsis more frequently from genitourinary infections (35% vs. 27% for men, p < 0.01) [7].
Accordingly, different pathogens are more commonly identified as causative of sepsis in men and women. For instance, bacteremia caused by Staphylococcus aureus and Pseudomonas aeruginosa is more prevalent in males, with a male-to-female ratio of 1.5 and 2.8, respectively [59,60]. Conversely, 60% of Escherichia coli bacteremias occur in females, which is consistent with the higher incidence of urinary tract infections in females [61]. Men have a higher rate of sepsis caused by Candida species; the male-to-female ratio of candidemia ranges from 1.16 to 1.65 in various reports [62,63,64].
3.3. Sex Differences in Organ Dysfunction and Treatment
A study of 18,757 patients reported that the management of sepsis was more conservative in women than in men [65,66,67]. Women were less likely to receive hemodialysis catheters (OR = 0.85; 95% CI, 0.78–0.93) [65], deep venous thrombosis prophylaxis (OR = 0.90; 95% CI, 0.84–0.97), and invasive mechanical ventilation (OR = 0.81; 95% CI, 0.76–0.86) [65].
No difference has been reported in the incidence of sepsis-associated acute kidney injury in men and women [68]. However, a meta-analysis of 21 studies including a total of 545,538 participants revealed that females were less likely to receive renal replacement therapy compared with males (adjusted OR 0.81 [0.73–0.89], I2 = 57.4%) [69].
The reported rates of sepsis-associated myocardial dysfunction range from 10% to 70% [70]. Substantial differences exist between men and women in the prevalence, manifestations and clinical presentation, and outcomes of heart disease [71,72], yet human data on this topic in relation to sepsis remains scarce. Male sex has been associated with a higher incidence of myocardial dysfunction, compared with females, in both sepsis and septic shock [73].
The administration of steroids interferes with the natural functions of endogenous sex hormones. In the ADRENAL trial (n = 3713 patients), differences were identified between men and women in the effects of hydrocortisone during sepsis: hydrocortisone increased the risk of shock recurrence in females (OR 1.48 [1.03–2.14], p = 0.03) [74].
3.4. Sex Differences in Sepsis Outcomes
The literature examining the relationship between sex and sepsis-associated mortality is inconclusive. Some studies report higher mortality rates among men with sepsis [75,76,77,78] and other studies report higher mortality rates among women with sepsis [58,65,79,80,81,82] (Table 1). However, several studies also found no statistically significant difference in the mortality rates of men and women with sepsis [6,56,83,84,85]. A systematic review and meta-analysis of 13 studies (80,520 participants) published between 2007 and 2020 also found no significant differences in all-cause hospital and ICU mortality between men and women with sepsis, but the level of evidence for this finding was very low [86]. Furthermore, meta-analysis identified higher 28-day all-cause mortality and lower 1-year all-cause mortality among women. This evidence was also classified as being of very low-certainty and low-certainty, respectively [86].
Several reasons may underlie these conflicting findings. Comorbidities may play an important role in patient outcomes [87], but few epidemiological studies adjust for these variables. As noted elsewhere, silent variables are always a potential source of bias. The larger the dataset and the smaller the number of variables adjusted for—the larger the potential effect of such bias if it does exist [88]. A typical example of a comorbidity that should be adjusted for is chronic obstructive respiratory disease, which is more prevalent among men than among women [87]. Men also display heart disease at a younger age than women [87]. In one prospective cohort which included 64,040 participants, the risk of bloodstream infection was reported to be 41% higher among men than among women and one-third of this excess risk was mediated by cardiovascular risk factors and comorbidities [89].
The rates of withholding/withdrawing of care are also rarely reported in sepsis studies. Code status limitations are ordered more often for women than for men (OR = 1.31; 95% CI, 1.18–1.47) [65]. However, while decisions to withhold or withdraw potentially life-prolonging treatment were more often made in women (28.0% vs. 22.8%, p = 0.003), these decisions may actually be based on the different disease and comorbidity profiles of men and women [90].
Additional variables that may have led to at least some of the discrepancies observed in outcomes of men and women with sepsis are the severity of sepsis, which is often not taken into consideration in retrospective and database studies [91], patient frailty [92], and the rates of organ support provided. Few, if any, studies, adjust for these important confounders, and none has taken all of these into consideration.
Finally, studies examining the relationship between sex and sepsis outcomes may have included selective populations (e.g., dependent on the characteristics of the population served or the number of available ICU beds) [93]. Different study methods (e.g., prospective versus retrospective or database studies, single versus multicenter) could also account for the variability in outcomes.

Table 1.
Details of studies reporting outcomes in sepsis and septic shock patients with respect to sex.
Table 1.
Details of studies reporting outcomes in sepsis and septic shock patients with respect to sex.
| Study | Location | Years | Population | Number of Patients | Definition of Sepsis | Main Outcome | Incidence | Type of Mortality | Crude Mortality | Adjusted OR Mortality | Conclusions | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [6] | Nationwide, U.S. | 1979–2000 | Sepsis patients | 10,319,418 sepsis patients | ICD-9-CM codes | Epidemiologic characterization of sepsis | Males > females | In-hospital mortality | 22% males | 21.8% females | Not provided | No significant difference |
| [79] | Charité Hospital, Berlin | 2006–2007 | ICU patients, subgroup of sepsis | 327 sepsis patients | SIRS + infection | ICU mortality rates for male and female patients | Males > females | ICU mortality | 13.7% males | 23.1% females | (reference male) 1.909 [1.002–3.638], p = 0.05 | Higher mortality in females |
| [80] | 24 ICUs in Italy | 2006 | Severe sepsis | 200 severe sepsis/septic shock patients | ACCP/SCCM Consensus Conference Committee definition | ICU mortality rates for male and female patients | Males > females | ICU mortality | 46.4% males | 63.5% females | (reference male) 2.33 [1.23–4.39], p = 0.010 | Higher mortality in females |
| [58] | Single centre, France | 1995–2004 | Severe nosocomial infections | 1341 severe nosocomial infections | Definition of each nosocomial infection | ICU mortality rates for male and female patients | Males > females | ICU mortality | 32% males | 37% females | (reference male) 1.50 [1.11–2.03], p = 0.008 | Higher mortality in females |
| [77] | Outcome rea database (12 French ICUs) | 1997–2005 | Severe community-acquired sepsis | 1608 severe sepsis patients | SIRS + one (or more) organ dysfunction | ICU mortality rates for male and female patients | Males > females | ICU mortality | 29% males | 26% females | (reference male) 0.75 [0.58–0.98], p = 0.03 | Lower mortality in females |
| [84] | Single centre, Germany | 1995–2000 | Patients with severe infection hospitalized in the ICU | 308 severe infections | Definition of each infection | Hospital mortality | No difference | In-hospital mortality | 31.7% males | 34% females | Not provided | No significant difference |
| [75] | Single centre, Germany | Unknown | Severe sepsis and septic shock | 11 severe sepsis, 41 septic shock | Definition of Bone et al. (1992) | Hospital mortality | Males > females | In-hospital mortality | 70% males | 26% females | Not provided | Lower mortality in females |
| [83] | State database, U.S. (California) | 2005–2010 | Severe sepsis | 1,213,219 severe sepsis | Definition of Angus et al. (2001) | Identification of patient demographic, patient health | Females > males | In-hospital mortality | 17.9% males | 16.6% females | (reference female) 1 [1-1], p < 0.001 | No significant difference |
| [81] | Single centre, U.S. | 1994–1998 | Sepsis | 1348 sepsis patients | SIRS + infection | Mortality, ICU LOS, hospital LOS, and maximal multiple organ dysfunction score | Females > males | In-hospital mortality | 21.4% males | 25.3% females | p = 0.02 (F-Test, multivariate analysis) | Higher mortality in females |
| [85] | Single centre, U.S. | 2005–2012 | Severe sepsis or septic shock | 814 severe sepsis or septic shock patients | Severe sepsis: 2 SIRS criteria + suspected infection + at least 1 organ dysfunction. Septic shock: the above + hypotension after a fluid bolus. | Completion of the SSC resuscitation bundle as previously defined by the SSC | Males > females | In-hospital mortality | 23.4% males | 25.2% females | Not provided | No significant difference |
| [74] | Nationwide, Australia | 2006–2009 | Severe sepsis and septic shock | 12,912 sepsis patients | ICD 10th Revision, Australian modification codes | Mortality, ICU LOS, hospital LOS, and readmissions | Males > females | 90-day mortality | 25.3% males | 22.5% females | Not provided | Lower mortality in females |
| [82] | Nationwide, Sweden | 2008–2015 | Severe sepsis and septic shock | 2720 severe sepsis and septic shock patients | 1992 sepsis definition | Management and outcome of sepsis patients | Males > females | 30-day mortality | 23.1% males | 25% females | (reference male) 1.28 [1.00–1.64] | Higher mortality in females |
| [65] | 98 ICUs, U.S., Canada, Brazil | 2003–2006 | Severe sepsis and septic shock | 18,757 severe sepsis or septic shock | One severe acute organ dysfunction within 3 days of a presumed infection | Hospital mortality | Males > females | In-hospital mortality | 33% males | 35% females | (reference male) 1.11 [1.04–1.19] | Higher mortality in females |
| [76] | Single centre, U.S. | 2001–2012 | Severe sepsis and septic shock | 6134 sepsis patients | Not detailed | 1 year mortality | Males > females | 1-year mortality | 55.6% males | 51.4% females | (reference female) 1.08 [1.00–1.16], p = 0.03 | Lower mortality in females |
| [56] | 2 ICUs, Netherlands | 2011–2014 | Sepsis | 1815 sepsis patients | 2001 International Sepsis Definitions | Differences in sepsis presentation and long-term outcome | Males > females | 90-day mortality | 45.5% males | 43.4% females | (reference female) 1.06 [0.83–1.35] | No significant difference |
Abbreviations: OR: odds ratio; ICU: intensive care unit; LOS: length of stay; ICD: international classification of diseases.
3.5. Pregnancy
Sepsis is ranked as the third leading cause of death among pregnant females, accounting for approximately 11% of maternal deaths and an 8% case fatality rate [94,95]. Physiological changes during pregnancy mirror those of sepsis (i.e., higher heart and respiratory rates, increased neutrophil counts, and decreased platelet counts), thereby rendering diagnosis more challenging [96]. Furthermore, since the fetus is not classified as an “organ”, in the absence of organ dysfunction, the occurrence of miscarriage, stillbirth, and neonatal complications secondary to maternal infection are not considered maternal complications of sepsis.
Conflicting data have been reported regarding sepsis-attributable mortality rates among pregnant women, compared with non-pregnant females [94,95]. Pregnant women are particularly susceptible to viral infections and the severity of the latter is increased compared with non-pregnant females. For instance, the likelihood of death from influenza is twice higher among pregnant women than among non-pregnant women of similar age. Pregnant women have also been reported to have higher mortality rates from COVID-19 infection than their age-matched counterparts [97,98].
However, a retrospective cohort analysis of 5968 pregnant and 85,240 non-pregnant females showed that sepsis-attributable case fatality rates remained higher in non-pregnant women than in pregnant women after adjustment for socio-economic status, race, age, and chronic comorbidities, which led the authors to conclude that pregnancy is associated with a protective effect which reduces the severity of sepsis presentation [99].
Given the significant hormonal changes that occur during pregnancy, it may be inferred that hormones play a significant role in the outcome of sepsis in these cases; however, this needs further elucidation.
3.6. Sex Differences Related to Culture
The recent pandemic has highlighted the glaring ethical implications of excluding pregnant and lactating women and women of childbearing age from clinical trials [100,101,102,103]. To date, most large randomized controlled trials have excluded women of childbearing age and pregnant women are still being excluded from trials designed by intensivists [104]. Continuing this practice perpetuates the lack of data and potential treatment inequities.
The medical community has already recognized the male advantage in rapid recognition of heart disease and rapid treatment [105]. The presence of similar bias has been suggested for sepsis. One study evaluated timely identification of sepsis using a clinical vignette in 120 physicians. Respondents identified sepsis with greater accuracy in women than in men [106]. Contrastingly, in the department of emergency medicine, the likelihood of progression from sepsis to septic shock has been found to be six times higher among women than among men [107], suggesting late identification and/or treatment. Indeed, registry data have shown that women treated by emergency medical service and emergency department staff were less likely to receive intravenous fluid and oxygen among men and that men receive antibiotics more rapidly than women (65 min (IQR 30-136) vs 87 min (IQR 39-172) (p = 0.0001)) [82].
Of even greater concern is the possibility that outcomes of men and women may differ according to the sex of the treating clinician. A Canadian database study examining 21 surgical procedures (n = 1,320,108 patients) found worse outcomes among women patients treated by male surgeons [108]. This finding was not supported in a study of patient outcomes after out-of-hospital cardiac arrest treated by male or female physicians [109].
Finally, physiological differences in the response to treatment may also affect outcome despite the best performance and intentions.
3.7. Perspectives
In terms of future strategies, the cornerstone of these findings should be first the principle of equity in care. As previously mentioned, studies highlighted disparities in treatment between male and female patients, as well as differences in outcomes according to the gender of physicians. Consequently, it is imperative to dedicate efforts towards the education of young medical professionals, with a focus on raising awareness about implicit biases related to gender. This awareness will contribute to reduce the impact of such biases on clinical decision making, and ultimately promote equitable treatment and outcomes for all patients [67,110,111,112].
Furthermore, it is also critical to focus on pharmacokinetics as another key area for intervention. There are known differences in drug metabolism according to gender. For example, drugs that prolong the QT interval are more likely to cause lethal ventricular arrhythmias in females than males [113]. As mentioned above, the increased efficacy of beta blockers in male animals than in their female counterparts [31,32] raises questions regarding their indiscriminate use in the management of sepsis and septic shock in human patients. Experimental evidence on this topic remains limited, as underlined by the study of Zhang et al. who encountered methodological challenges while attempting to conduct a comprehensive meta-analysis on the effect of biological sex on treatment response to fluid and antibiotic therapy in animal models of sepsis [46]. This was due to the paucity of available literature with only two relevant articles being identified through their search process [46]. Therefore, it is important to integrate considerations of sex differences in pharmacokinetics into clinical practice, as well as further research into the development of more personalized and tailored treatments based on sex-related factors.
4. Conclusions
Many important physiological issues relating to the relation between sex and sepsis remain to be studied. The lifetime risk for heart failure with reduced left ventricular ejection fraction is much higher among men than among women [114]. Women tend to have heart failure with preserved ejection fraction (i.e., right heart failure) and are also more symptomatic than their male counterparts [115]. Whether this affects sepsis outcomes remains to be seen. Women tend to have more complications after cardiovascular interventions and have more medication side effects [116,117,118]. Whether this also pertains to medications and interventions during sepsis is unknown. Women with heart disease wait longer before seeking treatment following an acute myocardial infarction [105,119]. Perhaps the opposite is true of men with infection.
At this time, the quality of the existing evidence does not enable drawing any definitive conclusions regarding the association between sex and sepsis-associated mortality. In order to claim equity exists, it does not suffice to show similar outcomes. Risk factors and process-related variables must be studied in depth. For all we know, the mortality rates attributed to sepsis in either men or women may still be overly high due to easily correctible factors that remain unaddressed.
In males (top panel), sepsis induces severe cardiac dysfunction by activating pathways responsible for inflammation, apoptosis, and oxidative stress, and by inhibiting pathways associated with excitation/contraction coupling (indications in red). Landiolol, by acting on the regulation of several genes, strongly improves cardiac function by reversing these biological processes (blue indications).
In females (bottom panel), the effect of sepsis is markedly weaker than in males and the cardiac dysfunction is much more pronounced (indications in red). In contrast to the male, landiolol has little effect on gene regulation. It does not improve the effect of sepsis on biological processes such as inflammation or oxidative stress but, on the contrary, acts on the b-adrenergic pathway and further decreases the contraction and relaxation capacity of the heart (blue indications).
Author Contributions
Conceptualization, I.L., S.E. and M.L.; methodology, I.L. and S.E.; software, N.L.; validation, S.E., I.M.-L. and M.L.; formal analysis, M.L.; investigation, I.L., S.E., N.L. and B.P.; resources, M.L.; data curation, I.L., S.E., B.P. and M.L.; writing—original draft preparation, I.L., S.E., N.L., I.M.-L., B.P. and M.L.; writing—review and editing, S.E., I.M.-L. and M.L.; supervision, M.L.; project administration, S.E. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Disclosure
M.L. received fees as consultant by AOP Pharma, LFB, Viatris and as speaker by AOP Pharma. S.E. serves as editor for Cochrane Critical Care Anesthesia and Emergency Medicine and for the journals Anesthesiology, Anesthesia Critical Care and Pain (ACCPM) and Journal of Anesthesia Analgesia and Critical Care (JACC) but has no COIs to disclose. BP received fees as consultant for Edwards Lifesciences. I.M.-L., I.L. and S.E. have no disclosure in relation with this manuscript.
References
- Ortona, E.; Pierdominici, M.; Maselli, A.; Veroni, C.; Aloisi, F.; Shoenfeld, Y. Sex-based differences in autoimmune diseases. Ann. Dell’istituto Super. Sanita 2016, 52, 205–212. [Google Scholar] [CrossRef]
- Cook, M.B.; Dawsey, S.M.; Freedman, N.D.; Inskip, P.D.; Wichner, S.M.; Quraishi, S.M.; Devesa, S.S.; McGlynn, K.A. Sex Disparities in Cancer Incidence by Period and Age. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1174–1182. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensiv. Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The Epidemiology of Sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef]
- Esper, A.M.; Moss, M.; Lewis, C.A.; Nisbet, R.; Mannino, D.M.; Martin, G.S.M. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit. Care Med. 2006, 34, 2576–2582. [Google Scholar] [CrossRef]
- Lakbar, I.; Luque-Paz, D.; Mege, J.-L.; Einav, S.; Leone, M. COVID-19 gender susceptibility and outcomes: A systematic review. PLoS ONE 2020, 15, e0241827. [Google Scholar] [CrossRef]
- Gerberding, J.L. Women and Infectious Diseases. Emerg. Infect. Dis. 2004, 10, 1965–1967. [Google Scholar] [CrossRef]
- Modi, G.; Borchi, B.; Giaché, S.; Campolmi, I.; Trotta, M.; Di Tommaso, M.; Strambi, N.; Bartoloni, A.; Zammarchi, L. Emerging Infectious Diseases in Pregnant Women in a Non-Endemic Area: Almost One Out of Four Is at Risk. Pathogens 2021, 10, 56. [Google Scholar] [CrossRef]
- Mogil, J.S.; Chanda, M.L. The case for the inclusion of female subjects in basic science studies of pain. Pain 2005, 117, 1–5. [Google Scholar] [CrossRef]
- Shansky, R.M. Are hormones a “female problem” for animal research? Science 2019, 364, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, B.J.; Onishi, K.G.; Zucker, I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2014, 40, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zajitschek, S.R.; Zajitschek, F.; Bonduriansky, R.; Brooks, R.C.; Cornwell, W.; Falster, D.S.; Lagisz, M.; Mason, J.; Senior, A.M.; Noble, D.W.; et al. Sexual dimorphism in trait variability and its eco-evolutionary and statistical implications. Elife 2020, 9, e63170. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.A.B.; Zajitschek, S.R.K.; Lagisz, M.; Mason, J.; Haselimashhadi, H.; Nakagawa, S. Sex differences in allometry for phenotypic traits in mice indicate that females are not scaled males. Nat. Commun. 2022, 13, 7502. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef]
- Textoris, J.; Ban, L.H.; Capo, C.; Raoult, D.; Leone, M.; Mege, J.-L. Sex-Related Differences in Gene Expression Following Coxiella burnetii Infection in Mice: Potential Role of Circadian Rhythm. PLoS ONE 2010, 5, e12190. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Textoris, J.; Capo, C.; Mège, J.-L. Sex Hormones and Bacterial Infections. In Sex Hormones; Dubey, R., Ed.; InTech: London, UK, 2012; ISBN 978-953-307-856-4. [Google Scholar]
- Narayan, B.; Nelson-Piercy, C. Physiological Changes of the Immune System During Pregnancy. In Principles and Practice of Maternal Critical Care; Einav, S., Weiniger, C.F., Landau, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 201–213. ISBN 978-3-030-43477-9. [Google Scholar]
- Vandereyken, M.M.; Singh, P.; Wathieu, C.P.; Jacques, S.; Zurashvilli, T.; Dejager, L.; Amand, M.; Musumeci, L.; Singh, M.; Moutschen, M.P.; et al. Dual-Specificity Phosphatase 3 Deletion Protects Female, but Not Male, Mice from Endotoxemia-Induced and Polymicrobial-Induced Septic Shock. J. Immunol. 2017, 199, 2515–2527. [Google Scholar] [CrossRef]
- Kikuchi, S.; Piraino, G.; O’Connor, M.; Wolfe, V.; Ridings, K.; Lahni, P.; Zingarelli, B. Hepatocyte-Specific Deletion of AMPKα1 Results in Worse Outcomes in Mice Subjected to Sepsis in a Sex-Specific Manner. Front. Immunol. 2020, 11, 210. [Google Scholar] [CrossRef]
- Sun, Z.; Qu, J.; Xia, X.; Pan, Y.; Liu, X.; Liang, H.; Dou, H.; Hou, Y. 17β-Estradiol promotes LC3B-associated phagocytosis in trained immunity of female mice against sepsis. Int. J. Biol. Sci. 2021, 17, 460–474. [Google Scholar] [CrossRef]
- Angele, M.K.; Pratschke, S.; Hubbard, W.J.; Chaudry, I.H. Gender differences in sepsis. Virulence 2013, 5, 12–19. [Google Scholar] [CrossRef]
- Raju, R.; Chaudry, I.H. Sex Steroids/Receptor Antagonist: Their Use as Adjuncts After Trauma-Hemorrhage for Improving Immune/Cardiovascular Responses and for Decreasing Mortality from Subsequent Sepsis. Obstet. Anesth. Dig. 2008, 107, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.R.; Singh, K.; Yu, Q.; Sen, C.K.; Wang, M. Sex as Biological Variable in Cardiac Mitochondrial Bioenergetic Responses to Acute Stress. Int. J. Mol. Sci. 2022, 23, 9312. [Google Scholar] [CrossRef]
- Walker, S.L.M.; Muthoo, C.; Sanchez, J.; Del Arroyo, A.G.; Ackland, G.L. Sex-specific differences in cardiac function, inflammation and injury during early polymicrobial sepsis. Intensiv. Care Med. Exp. 2022, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Hobai, I.A.; Aziz, K.; Buys, E.S.; Brouckaert, P.; Siwik, D.A.; Colucci, W.S. Distinct Myocardial Mechanisms Underlie Cardiac Dysfunction in Endotoxemic Male and Female Mice. Shock 2016, 46, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chiazza, F.; Collino, M.; Patel, N.S.A.; Coldewey, S.M.; Thiemermann, C. Gender Dimorphism of the Cardiac Dysfunction in Murine Sepsis: Signalling Mechanisms and Age-Dependency. PLoS ONE 2014, 9, e100631. [Google Scholar] [CrossRef]
- Morelli, A.; Sanfilippo, F. Ultrashort-Acting β-Blockers. Chest 2021, 159, 2139–2140. [Google Scholar] [CrossRef]
- Hoeker, G.S.; Hood, A.R.; Katra, R.P.; Poelzing, S.; Pogwizd, S.M. Sex Differences in β-Adrenergic Responsiveness of Action Potentials and Intracellular Calcium Handling in Isolated Rabbit Hearts. PLoS ONE 2014, 9, e111411. [Google Scholar] [CrossRef]
- Mathieu, C.; Desrois, M.; Kober, F.; Lalevée, N.; Lan, C.; Fourny, N.; Iché-Torres, M.; Tran, T.T.; Lê, L.T.; Singer, M.; et al. Sex-Mediated Response to the Beta-Blocker Landiolol in Sepsis. Crit. Care Med. 2018, 46, e684–e691. [Google Scholar] [CrossRef]
- Tran, T.T.; Mathieu, C.; Torres, M.; Loriod, B.; Lê, L.T.; Nguyen, C.; Bernard, M.; Leone, M.; Lalevée, N. Effect of landiolol on sex-related transcriptomic changes in the myocardium during sepsis. Intensiv. Care Med. Exp. 2019, 7, 50. [Google Scholar] [CrossRef]
- Xerri, A.; Gallardo, F.; Kober, F.; Mathieu, C.; Fourny, N.; Tran, T.T.; Mege, J.-L.; Singer, M.; Lalevée, N.; Bernard, M.; et al. Female hormones prevent sepsis-induced cardiac dysfunction: An experimental randomized study. Sci. Rep. 2022, 12, 4939. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.J.; Yang, Z.; Hopkins, P.M.; Steele, D.S.; Harrison, S.M. TNF-α and IL-1β increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium 2010, 47, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Rudiger, A.; Dyson, A.; Felsmann, K.; Carré, J.E.; Taylor, V.; Hughes, S.; Clatworthy, I.; Protti, A.; Pellerin, D.; Lemm, J.; et al. Early functional and transcriptomic changes in the myocardium predict outcome in a long-term rat model of sepsis. Clin. Sci. 2012, 124, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.J.; Deutschman, C.S. Evaluating Myocardial Depression in Sepsis. Shock 2004, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Machuki, J.; Zhang, H.; Harding, S.; Sun, H. Molecular pathways of oestrogen receptors and β-adrenergic receptors in cardiac cells: Recognition of their similarities, interactions and therapeutic value. Acta Physiol. 2017, 222, e12978. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shan, L.; Peng, T. Rac1 mediates sex difference in cardiac tumor necrosis factor-α expression via NADPH oxidase–ERK1/2/p38 MAPK pathway in endotoxemia. J. Mol. Cell. Cardiol. 2009, 47, 264–274. [Google Scholar] [CrossRef]
- Cristofaro, P.A.; Opal, S.M.; Palardy, J.E.; Parejo, N.A.; Jhung, J.; Keith, J.C.; Harris, H.A. WAY-202196, a selective estrogen receptor-beta agonist, protects against death in experimental septic shock. Crit. Care Med. 2006, 34, 2188–2193. [Google Scholar] [CrossRef]
- Kang, H.; Thomas, R.M. Bacteria and Sepsis: Microbiome to the Rescue? J. Clin. Med. 2021, 10, 3578. [Google Scholar] [CrossRef]
- Efron, P.A.; Darden, D.B.; Li, E.C.B.; Munley, J.; Kelly, L.; Fenner, B.; Nacionales, D.C.; Ungaro, R.F.B.; Dirain, M.L.; Rincon, J.; et al. Sex differences associate with late microbiome alterations after murine surgical sepsis. J. Trauma Inj. Infect. Crit. Care 2022, 93, 137–146. [Google Scholar] [CrossRef]
- Barter, J.; Kumar, A.; Stortz, J.A.; Hollen, M.; Nacionales, D.; Efron, P.A.; Moldawer, L.L.; Foster, T.C. Age and Sex Influence the Hippocampal Response and Recovery Following Sepsis. Mol. Neurobiol. 2019, 56, 8557–8572. [Google Scholar] [CrossRef]
- Rani, A.; Barter, J.; Kumar, A.; Stortz, J.A.; Hollen, M.; Nacionales, D.; Moldawer, L.L.; Efron, P.A.; Foster, T.C. Influence of age and sex on microRNA response and recovery in the hippocampus following sepsis. Aging 2022, 14, 728–746. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Bauman, M.D.; Van de Water, J. Translational opportunities in the prenatal immune environment: Promises and limitations of the maternal immune activation model. Neurobiol. Dis. 2020, 141, 104864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Montroy, J.; Sharma, R.; Fergusson, D.A.; Mendelson, A.A.; Macala, K.F.; Bourque, S.L.; Schlechte, J.M.; Eng, M.K.; McDonald, B.; et al. The Effects of Biological Sex on Sepsis Treatments in Animal Models: A Systematic Review and a Narrative Elaboration on Sex- and Gender-Dependent Differences in Sepsis. Crit. Care Explor. 2021, 3, e0433. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.; Griepentrog, J.E.; Zhang, X.; Angus, D.C.; Seymour, C.W.; Rosengart, M.R. Prompt Administration of Antibiotics and Fluids in the Treatment of Sepsis. Crit. Care Med. 2018, 46, e426–e434. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Panda, S.K.; Agarwal, B.; Behera, S.; Ali, S.M.; Pulse, M.E.; Solomkin, J.S.; Opal, S.M.; Bhandari, V.; Acharya, S. Novel Chitohexaose Analog Protects Young and Aged mice from CLP Induced Polymicrobial Sepsis. Sci. Rep. 2019, 9, 2904. [Google Scholar] [CrossRef] [PubMed]
- Nandi, M.; Jackson, S.K.; Macrae, D.; Shankar-Hari, M.; Tremoleda, J.L.; Lilley, E. Rethinking animal models of sepsis—Wworking towards improved clinical translation whilst integrating the 3Rs. Clin. Sci. 2020, 134, 1715–1734. [Google Scholar] [CrossRef]
- Guillon, A.; Translational Research Committee of the French Intensive Care Society (Société de Réanimation de Langue Française); Preau, S.; Aboab, J.; Azabou, E.; Jung, B.; Silva, S.; Textoris, J.; Uhel, F.; Vodovar, D.; et al. Preclinical septic shock research: Why we need an animal ICU. Ann. Intensiv. Care 2019, 9, 66. [Google Scholar] [CrossRef]
- Klinge, I. Gender perspectives in European research. Pharmacol. Res. 2008, 58, 183–189. [Google Scholar] [CrossRef]
- Lakbar, I.; Leone, M. An insight depicting estradiol pathway in sepsis. Minerva Anestesiol. 2021, 87, 505–507. [Google Scholar] [CrossRef]
- Lakbar, I.; Munoz, M.; Pauly, V.; Orleans, V.; Fabre, C.; Fond, G.; Vincent, J.-L.; Boyer, L.; Leone, M. Septic shock: Incidence, mortality and hospital readmission rates in French intensive care units from 2014 to 2018. Anaesth. Crit. Care Pain Med. 2022, 41, 101082. [Google Scholar] [CrossRef] [PubMed]
- Molanorouzi, K.; Khoo, S.; Morris, T. Motives for adult participation in physical activity: Type of activity, age, and gender. BMC Public Health 2015, 15, 66. [Google Scholar] [CrossRef]
- Białas, A.J.; Kumor-Kisielewska, A.; Górski, P. Ageing, Sex, Obesity, Smoking and COVID-19—Truths, Myths and Speculations. Adv. Respir. Med. 2020, 88, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Van Vught, L.A.; Scicluna, B.P.; Wiewel, M.A.; Hoogendijk, A.J.; Klouwenberg, P.M.C.K.; Ong, D.S.Y.; Cremer, O.L.; Horn, J.; Franitza, M.; Toliat, M.R.; et al. Association of Gender With Outcome and Host Response in Critically Ill Sepsis Patients. Crit. Care Med. 2017, 45, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Sambola, A.; Fernández-Hidalgo, N.; Almirante, B.; Roca, I.; González-Alujas, T.; Serra, B.; Pahissa, A.; García-Dorado, D.; Tornos, P. Sex Differences in Native-Valve Infective Endocarditis in a Single Tertiary-Care Hospital. Am. J. Cardiol. 2010, 106, 92–98. [Google Scholar] [CrossRef]
- Combes, A.; Luyt, C.-E.; Trouillet, J.-L.; Nieszkowska, A.; Chastre, J. Gender impact on the outcomes of critically ill patients with nosocomial infections*. Crit. Care Med. 2009, 37, 2506–2511. [Google Scholar] [CrossRef]
- Benfield, T.; Espersen, F.; Frimodt-Møller, N.; Jensen, A.; Larsen, A.; Pallesen, L.; Skov, R.; Westh, H.; Skinhøj, P. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin. Microbiol. Infect. 2007, 13, 257–263. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Wilson, J.W.; Lahr, B.D.; Eckel-Passow, J.E.; Baddour, L.M. Incidence of Pseudomonas aeruginosa Bacteremia: A Population-Based Study. Am. J. Med. 2008, 121, 702–708. [Google Scholar] [CrossRef]
- Laupland, K.; Gregson, D.; Church, D.; Ross, T.; Pitout, J. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin. Microbiol. Infect. 2008, 14, 1041–1047. [Google Scholar] [CrossRef]
- Kilic, A.U.; Alp, E.; Cevahir, F.; Ture, Z.; Yozgat, N. Epidemiology and cost implications of candidemia, a 6-year analysis from a developing country. Mycoses 2016, 60, 198–203. [Google Scholar] [CrossRef]
- Horn, D.L.; Neofytos, D.; Anaissie, E.J.; Fishman, J.A.; Steinbach, W.J.; Olyaei, A.J.; Marr, K.A.; Pfaller, M.; Chang, C.; Webster, K.M. Epidemiology and Outcomes of Candidemia in 2019 Patients: Data from the Prospective Antifungal Therapy Alliance Registry. Clin. Infect. Dis. 2009, 48, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Xess, I.; Jain, N.; Hasan, F.; Mandal, P.; Banerjee, U. Epidemiology of Candidemia in a Tertiary Care Centre of North India: 5-Year Study. Infection 2007, 35, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Pietropaoli, A.P.; Glance, L.G.; Oakes, D.; Fisher, S.G. Gender differences in mortality in patients with severe sepsis or septic shock. Gend. Med. 2010, 7, 422–437. [Google Scholar] [CrossRef]
- Fowler, R.A.; Sabur, N.; Li, P.; Juurlink, D.N.; Pinto, R.; Hladunewich, M.A.; Adhikari, N.K.; Sibbald, W.J.; Martin, C.M. Sex-and age-based differences in the delivery and outcomes of critical care. Can. Med. Assoc. J. 2007, 177, 1513–1519. [Google Scholar] [CrossRef]
- Blecha, S.; Zeman, F.; Specht, S.; Pfefferle, A.L.; Placek, S.; Karagiannidis, C.; Bein, T. Invasiveness of Treatment Is Gender Dependent in Intensive Care: Results From a Retrospective Analysis of 26,711 Cases. Obstet. Anesth. Dig. 2020, 132, 1677–1683. [Google Scholar] [CrossRef]
- Peng, J.; Tang, R.; Yu, Q.; Wang, D.; Qi, D. No sex differences in the incidence, risk factors and clinical impact of acute kidney injury in critically ill patients with sepsis. Front. Immunol. 2022, 13, 895018. [Google Scholar] [CrossRef]
- Modra, L.J.M.; Higgins, A.M.B.; Abeygunawardana, V.S.; Vithanage, R.N.; Bailey, M.J.B.; Bellomo, R.M. Sex Differences in Treatment of Adult Intensive Care Patients: A Systematic Review and Meta-Analysis. Crit. Care Med. 2022, 50, 913–923. [Google Scholar] [CrossRef]
- Carbone, F.; Liberale, L.; Preda, A.; Schindler, T.H.; Montecucco, F. Septic Cardiomyopathy: From Pathophysiology to the Clinical Setting. Cells 2022, 11, 2833. [Google Scholar] [CrossRef] [PubMed]
- Winham, S.J.; de Andrade, M.; Miller, V.M. Genetics of cardiovascular disease: Importance of sex and ethnicity. Atherosclerosis 2015, 241, 219–228. [Google Scholar] [CrossRef]
- De Bellis, A.; De Angelis, G.; Fabris, E.; Cannatà, A.; Merlo, M.; Sinagra, G. Gender-related differences in heart failure: Beyond the “one-size-fits-all” paradigm. Heart Fail. Rev. 2019, 25, 245–255. [Google Scholar] [CrossRef]
- Sato, R.; Kuriyama, A.; Takada, T.; Nasu, M.; Luthe, S.K. Prevalence and risk factors of sepsis-induced cardiomyopathy. Medicine 2016, 95, e5031. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Venkatesh, B.; Hammond, N.; Taylor, C.; Finfer, S. Sex differences in response to adjunctive corticosteroid treatment for patients with septic shock. Intensiv. Care Med. 2021, 47, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.; Kahlke, V.; Staubach, K.-H.; Zabel, P.; Stüber, F. Gender Differences in Human Sepsis. Arch. Surg. 1998, 133, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tong, L.; Yao, J.; Guo, Z.; Lui, K.Y.; Hu, X.; Cao, L.; Zhu, Y.; Huang, F.; Guan, X.; et al. Association of Sex With Clinical Outcome in Critically Ill Sepsis Patients: A Retrospective Analysis of the Large Clinical Database MIMIC-III. Shock 2019, 52, 146–151. [Google Scholar] [CrossRef]
- Adrie, C.; Azoulay, E.; Francais, A.; Clec’H, C.; Darques, L.; Schwebel, C.; Nakache, D.; Jamali, S.; Goldgran-Toledano, D.; Garrouste-Orgeas, M.; et al. Influence of Gender on the Outcome of Severe Sepsis. Chest 2007, 132, 1786–1793. [Google Scholar] [CrossRef]
- Thompson, K.J.; Finfer, S.R.; Woodward, M.; Leong, R.N.F.; Liu, B. Sex differences in sepsis hospitalisations and outcomes in older women and men: A prospective cohort study. J. Infect. 2022, 84, 770–776. [Google Scholar] [CrossRef]
- Nachtigall, I.; Tafelski, S.; Rothbart, A.; Kaufner, L.; Schmidt, M.; Tamarkin, A.; Kartachov, M.; Zebedies, D.; Trefzer, T.; Wernecke, K.-D.; et al. Gender-related outcome difference is related to course of sepsis on mixed ICUs: A prospective, observational clinical study. Crit. Care 2011, 15, R151. [Google Scholar] [CrossRef]
- Sakr, Y.; Elia, C.; Mascia, L.; Barberis, B.; Cardellino, S.; Livigni, S.; Fiore, G.; Filippini, C.; Ranieri, V. The influence of gender on the epidemiology of and outcome from severe sepsis. Crit. Care 2013, 17, R50. [Google Scholar] [CrossRef]
- Eachempati, S.R.; Hydo, L.; Barie, P.S. Gender-Based Differences in Outcome in Patients With Sepsis. Arch. Surg. 1999, 134, 1342–1347. [Google Scholar] [CrossRef]
- Sunden-Cullberg, J.; Nilsson, A.; Inghammar, M. Sex-based differences in ED management of critically ill patients with sepsis: A nationwide cohort study. Intensiv. Care Med. 2020, 46, 727–736. [Google Scholar] [CrossRef]
- Banta, J.E.; Joshi, K.P.; Beeson, L.; Nguyen, H.B. Patient and hospital characteristics associated with inpatient severe sepsis mortality in California, 2005–2010. Crit. Care Med. 2012, 40, 2960–2966. [Google Scholar] [CrossRef] [PubMed]
- Angstwurm, M.W.A.; Gaertner, R.; Schopohl, J. Outcome in elderly patients with severe infection is influenced by sex hormones but not gender. Crit. Care Med. 2005, 33, 2786–2793. [Google Scholar] [CrossRef] [PubMed]
- Madsen, T.E.; Simmons, J.; Choo, E.K.; Portelli, D.; McGregor, A.J.; Napoli, A.M. The DISPARITY Study: Do gender differences exist in Surviving Sepsis Campaign resuscitation bundle completion, completion of individual bundle elements, or sepsis mortality? J. Crit. Care 2014, 29, 473.e7–473.e11. [Google Scholar] [CrossRef]
- Antequera, A.; Lopez-Alcalde, J.; Stallings, E.; Muriel, A.; Félix, B.F.; del Campo, R.; Ponce-Alonso, M.; Fidalgo, P.; Halperin, A.V.; Madrid-Pascual, O.; et al. Sex as a prognostic factor for mortality in critically ill adults with sepsis: A systematic review and meta-analysis. BMJ Open 2021, 11, e048982. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Helms, J.; De Jong, A.; Einav, S. Yentl syndrome and the ICU. Intensiv. Care Med. 2021, 47, 594–597. [Google Scholar] [CrossRef]
- Mohus, R.M.; Gustad, L.T.; Furberg, A.-S.; Moen, M.K.; Liyanarachi, K.V.; Askim, Å.; Åsberg, S.E.; DeWan, A.T.; Rogne, T.; Simonsen, G.S.; et al. Explaining sex differences in risk of bloodstream infections using mediation analysis in the population-based HUNT study in Norway. Sci. Rep. 2022, 12, 8436. [Google Scholar] [CrossRef]
- Smets, T.; Rietjens, J.A.; Chambaere, K.; Coene, G.; Deschepper, R.; Pasman, H.R.; Deliens, L. Sex-based Differences in End-of-Life Decision Making in Flanders, Belgium. Med. Care 2012, 50, 815–820. [Google Scholar] [CrossRef]
- Paoli, C.J.; Reynolds, M.A.; Sinha, M.; Gitlin, M.; Crouser, E. Epidemiology and Costs of Sepsis in the United States—An Analysis Based on Timing of Diagnosis and Severity Level. Crit. Care Med. 2018, 46, 1889–1897. [Google Scholar] [CrossRef]
- Hessey, E.; Montgomery, C.; Zuege, D.J.; Rolfson, D.; Stelfox, H.T.; Fiest, K.M.; Bagshaw, S.M. Sex-specific prevalence and outcomes of frailty in critically ill patients. J. Intensiv. Care 2020, 8, 75. [Google Scholar] [CrossRef]
- Dodek, P.; Kozak, J.-F.; Norena, M.; Wong, H. More men than women are admitted to 9 intensive care units in British Columbia. J. Crit. Care 2009, 24, 630.e1–630.e8. [Google Scholar] [CrossRef]
- Sharma, S.; Rodrigues, P.R.; Zaher, S.; Davies, L.C.; Ghazal, P. Immune-metabolic adaptations in pregnancy: A potential stepping-stone to sepsis. Ebiomedicine 2022, 86, 104337. [Google Scholar] [CrossRef]
- Greer, O.; Shah, N.M.; Sriskandan, S.; Johnson, M.R. Sepsis: Precision-Based Medicine for Pregnancy and the Puerperium. Int. J. Mol. Sci. 2019, 20, 5388. [Google Scholar] [CrossRef]
- Gat, R.; Hadar, E.; Orbach-Zinger, S.; Shochat, T.; Kushnir, S.; Einav, S. Distribution of Extreme Vital Signs and Complete Blood Count Values of Healthy Parturients. Obstet. Anesth. Dig. 2019, 129, 1595–1606. [Google Scholar] [CrossRef]
- Vousden, N.; Ramakrishnan, R.; Bunch, K.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; et al. Management and implications of severe COVID-19 in pregnancy in the UK: Data from the UK Obstetric Surveillance System national cohort. Acta Obstet. Et Gynecol. Scand. 2022, 101, 461–470. [Google Scholar] [CrossRef]
- Mullins, E.; Hudak, M.L.; Banerjee, J.; Getzlaff, T.; Townson, J.; Barnette, K.; Playle, R.; Bourne, T.; Lees, C.; PAN-COVID In-vestigators and the National Perinatal COVID-19 Registry Study Group. Pregnancy and neonatal outcomes of COVID-19: Co-reporting of common outcomes from PAN-COVID and AAP SONPM registries. Ultrasound Obstet. Gynecol. 2021, 57, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Kidson, K.M.; Henderson, W.R.; Hutcheon, J.A. Case Fatality and Adverse Outcomes Are Reduced in Pregnant Women With Severe Sepsis or Septic Shock Compared With Age-Matched Comorbid-Matched Nonpregnant Women. Crit. Care Med. 2018, 46, 1775–1782. [Google Scholar] [CrossRef]
- Einav, S.; Ippolito, M.; Cortegiani, A. Inclusion of pregnant women in clinical trials of COVID-19 therapies: What have we learned? Br. J. Anaesth. 2020, 125, e326–e328. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.L.; Walker, S.P. Consider pregnancy in COVID-19 therapeutic drug and vaccine trials. Lancet 2020, 395, e92. [Google Scholar] [CrossRef] [PubMed]
- Manca, T.A.; Sadarangani, M.; Halperin, S.A.; Langley, J.M.; McClymont, E.; MacDonald, S.E.; Top, K.A. Vaccine regulation should require and enforce the inclusion of pregnant and breastfeeding women in prelicensure clinical trials. Hum. Vaccines Immunother. 2022, 18, 2104019. [Google Scholar] [CrossRef]
- Malhamé, I.; D’Souza, R.; Cheng, M.M.P. The Moral Imperative to Include Pregnant Women in Clinical Trials of Interventions for COVID-19. Ann. Intern. Med. 2020, 173, 836–837. [Google Scholar] [CrossRef]
- Busani, S.; Tosi, M.; Mighali, P.; Vandelli, P.; D’Amico, R.; Marietta, M.; Forfori, F.; Donati, A.; Cinnella, G.; De Monte, A.; et al. Multi-centre, three arm, randomized controlled trial on the use of methylprednisolone and unfractionated heparin in critically ill ventilated patients with pneumonia from SARS-CoV-2 infection: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 724. [Google Scholar] [CrossRef]
- Mnatzaganian, G.; Braitberg, G.; Hiller, J.E.; Kuhn, L.; Chapman, R. Sex differences in in-hospital mortality following a first acute myocardial infarction: Symptomatology, delayed presentation, and hospital setting. BMC Cardiovasc. Disord. 2016, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Pikwer, A.; Carlsson, M.; Mahmoud, D.A.; Castegren, M. The Patient’s Gender Influencing the Accuracy of Diagnosis and Proposed Sepsis Treatment in Constructed Cases. Emerg. Med. Int. 2020, 2020, 4823095. [Google Scholar] [CrossRef] [PubMed]
- Capp, R.; Horton, C.L.; Takhar, S.S.; Ginde, A.A.; Peak, D.A.; Zane, R.; Marill, K.A. Predictors of Patients Who Present to the Emergency Department With Sepsis and Progress to Septic Shock Between 4 and 48 Hours of Emergency Department Arrival*. Crit. Care Med. 2015, 43, 983–988. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Jerath, A.; Coburn, N.; Klaassen, Z.; Luckenbaugh, A.N.; Magee, D.E.; Hird, A.E.; Armstrong, K.; Ravi, B.; Esnaola, N.F.; et al. Association of Surgeon-Patient Sex Concordance With Postoperative Outcomes. JAMA Surg. 2022, 157, 146. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.; Siry-Bove, B.J.; Shelton, S.K.; McDaniel, K.; Nelson, J.L.; Perman, S.M. Does Physician Gender and Gender Composition of Clinical Teams Affect Guideline Concordance and Patient Outcomes in Out-of-Hospital Cardiac Arrest? J. Women’s Health 2022, 31, 1800–1804. [Google Scholar] [CrossRef] [PubMed]
- Gopal, D.P.; Chetty, U.; O’Donnell, P.; Gajria, C.; Blackadder-Weinstein, J. Implicit bias in healthcare: Clinical practice, research and decision making. Futur. Health J. 2021, 8, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Champagne-Langabeer, T.; Hedges, A.L. Physician gender as a source of implicit bias affecting clinical decision-making processes: A scoping review. BMC Med. Educ. 2021, 21, 171. [Google Scholar] [CrossRef]
- Chapman, E.N.; Kaatz, A.; Carnes, M. Physicians and Implicit Bias: How Doctors May Unwittingly Perpetuate Health Care Disparities. J. Gen. Intern. Med. 2013, 28, 1504–1510. [Google Scholar] [CrossRef]
- Stolarz, A.J.; Rusch, N.J. Gender Differences in Cardiovascular Drugs. Cardiovasc. Drugs Ther. 2015, 29, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Omar, W.; Ayers, C.; LaMonte, M.; Klein, L.; Allen, N.B.; Kuller, L.H.; Greenland, P.; Eaton, C.B.; Gottdiener, J.S.; et al. Sex and Race Differences in Lifetime Risk of Heart Failure With Preserved Ejection Fraction and Heart Failure With Reduced Ejection Fraction. Circulation 2018, 137, 1814–1823. [Google Scholar] [CrossRef]
- Punnoose, L.R.; Lindenfeld, J. Sex-specific differences in access and response to medical and device therapies in heart failure: State of the art. Prog. Cardiovasc. Dis. 2020, 63, 640–648. [Google Scholar] [CrossRef]
- Rodenburg, E.M.; Stricker, B.H.C.; Visser, L.E. Sex-related differences in hospital admissions attributed to adverse drug reactions in the Netherlands. Br. J. Clin. Pharmacol. 2010, 71, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, G.; Boscia, E.; Bettiol, A.; Pagani, S.; Spada, G.; Vighi, G.; Bonaiuti, R.; Venegoni, M.; Vighi, G.; Vannacci, A.; et al. Risk of Hospitalization for Adverse Drug Events in Women and Men: A Post Hoc Analysis of an Active Pharmacovigilance Study in Italian Emergency Departments. Pharmaceuticals 2021, 14, 678. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, L.C.; van der Linden, P.D.; Lagro-Janssen, A.L.M.; Bemt, P.M.L.A.V.D.; Siiskonen, S.J.; Teichert, M.; Kuiper, J.G.; Herings, R.M.C.; Stricker, B.H.; Visser, L.E. Sex differences associated with adverse drug reactions resulting in hospital admissions. Biol. Sex Differ. 2021, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Gibler, W.; Armstrong, P.; Ohman, E.; Weaver, W.; Stebbins, A.L.; Gore, J.M.; Newby, L.; Califf, R.M.; Topol, E. Persistence of delays in presentation and treatment for patients with acute myocardial infarction: The GUSTO-I and GUSTO-III experience. Ann. Emerg. Med. 2002, 39, 123–130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).