Modes of Action of Biocontrol Agents and Elicitors for sustainable Protection against Bacterial Canker of Tomato
Abstract
1. Introduction
2. Overview of Clavibacter Sequenced Genomes
3. Importance of Bacterial Canker Disease
4. How Does C. michiganensis subsp. michiganensis Attack Tomato Plants?
5. Characteristics and Transmission Modes of C. michiganensis subsp. michiganensis
6. Disease Management Strategies
7. The Use of PGPRs as an Alternative Biocontrol Strategy
7.1. Mechanisms of PGPRs to Control Bacterial Canker
7.1.1. Direct Mechanisms
7.1.2. Indirect Mechanisms
- 1.
- Antibiotics and VOCs production
- 2.
- Inhibitory siderophores production
- 3.
- Lytic enzymes production
- 4.
- Lipopeptides surfactants production
| Biocontrol Agent | Bacterial Traits | References | |
|---|---|---|---|
| Pseudomonas fluorescens | Siderophores production | Pyoverdine (pseudobactin) Pyochelin | [67,84] |
| Antimicrobial compounds | HCN Phenazines (PCA) | [67,85] [86] | |
| Growth promoting factors | IAA Phosphate solubilization | [85] [67] | |
| Pseudomonas Brassicacearum LBUM300-LBUM323 | Siderophores production | [64] | |
| Antimicrobial compounds | DAPG HCN PCA | [66] [64] | |
| Growth promoting factors | Biofilm formation | [66] | |
| Pseudomonas aeruginosa FG106-BRp3 | Siderophores production | Pyoverdine Pyochelin | [87] [59] |
| Antimicrobial compounds | HCN Phenazines: (PCA-PCN-PYO) Pyoluteorin | [74] [88] | |
| Biosurfactant | Rhamnolipids | [74] | |
| Growth promoting factors | IAA Phosphate Solubilization Potassium Solubilization Ammonium production ACC deaminase activity Biofilm formation | [74] [88] | |
| Enzymes | Proteases (Elastase, Alkaline protease) Chitinase | [74] | |
| Pseudomonas chlororaphis M71/UFB2 | Siderophores production | [68] | |
| Antibiotic compounds | DAPG HCN Phenazines (PCA) | [69] [77] [68] | |
| VOCs | Methanethiol Dimethyl disulfide | [68] | |
| Hydrolytic enzymes | Protease Lipase | ||
| Growth promoting factors | Biofilm production | ||
| Production of AHLs | |||
| Pseudomonas entomophila 23 S | Siderophores production | [75] | |
| Antibiotic compounds | HCN | ||
| Growth promoting factors | IAA Phosphate Solubilization | ||
| Biocontrol Agent | Bacterial Traits | References | |
|---|---|---|---|
| Bacillus subtilis | Siderophores production | [67] | |
| Antimicrobial compounds | HCN Surfactin Butanol | [80] [67] | |
| Growth promoting factors | IAA | [89] | |
| Bacillus amyloliquefaciens | Siderophores production | [90] | |
| Growth promoting factors | IAA Phosphate solubilization Growth in nitrogen-free medium | [67] | |
| Enzymes production | Cellulase Chitinase Lipase Protease | [90] | |
| Bacillus cereus | Growth promoting factors | Gibberellic Acid (GA3) Phosphate solubilization | [72] |
| Bacillus velezensis 9D-6 Bacillus sp. 1D-12 | Antibiotic compounds | Surfactin A Surfactin B/C | [82,83] |
- 5.
- Induced plant defense responses
8. The Use of Elicitors as a New Target in Agriculture
8.1. Salicylic Acid (SA)
8.2. Beneficial Microbes
8.3. Fungal Elicitors
8.4. Synthetic Elicitors
8.4.1. Acibenzolar—S–Methyl (ASM)
8.4.2. INA and DPMP
9. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prosekov, A.Y.; Ivanova, S.A. Food security: The challenge of the present. Geoforum 2018, 91, 73–77. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations; International Fund for Agricultural Development; UNICEF; World Food Programme; World Health Organization. The State of Food Security and Nutrition in the World: Safeguarding against Economic Slowdowns and Downturns, 2019th ed.; United Nations: New York, NY, USA, 2019; ISBN 9789251315705. [Google Scholar]
- Pautasso, M.; Döring, T.F.; Garbelotto, M.; Pellis, L.; Jeger, M.J. Impacts of climate change on plant diseases—Opinions and trends. Eur. J. Plant Pathol. 2012, 133, 295–313. [Google Scholar] [CrossRef]
- Strange, R.N.; Scott, P.R. Plant Disease: A Threat to Global Food Security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Eichenlaub, R.; Gartemann, K.-H. The Clavibacter michiganensis Subspecies: Molecular Investigation of Gram-Positive Bacterial Plant Pathogens. Annu. Rev. Phytopathol. 2011, 49, 445–464. [Google Scholar] [CrossRef]
- Davis, M.J.; Gillaspie, A.G.; Vidaver, A.K.; Harris, R.W. Clavibacter: A New Genus Containing Some Phytopathogenic Coryneform Bacteria, Including Clavibacter xyli subsp. xyli sp. nov., subsp. nov. and Clavibacter xyli subsp. cynodontis subsp. nov., Pathogens That Cause Ratoon Stunting Disease of Sugarcane and Bermudagrass Stunting Disease. Int. J. Syst. Evol. Microbiol. 1984, 34, 107–117. [Google Scholar] [CrossRef]
- Gartemann, K.-H.; Kirchner, O.; Engemann, J.; Gräfen, I.; Eichenlaub, R.; Burger, A. Clavibacter Michiganensis subsp. michiganensis: First steps in the understanding of virulence of a Gram-positive phytopathogenic bacterium. J. Biotechnol. 2003, 106, 179–191. [Google Scholar] [CrossRef]
- Chalupowicz, L.; Barash, I.; Reuven, M.; Dror, O.; Sharabani, G.; Gartemann, K.-H.; Eichenlaub, R.; Sessa, G.; Manulis-Sasson, S. Manulis-Sasson, S. Differential contribution of Clavibacter michiganensis ssp. michiganensis virulence factors to systemic and local infection in tomato. Mol. Plant Pathol. 2017, 18, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Blank, L.; Cohen, Y.; Borenstein, M.; Shulhani, R.; Lofthouse, M.; Sofer, M.; Shtienberg, D. Variables Associated with Severity of Bacterial Canker and Wilt Caused by Clavibacter michiganensis subsp. michiganensis in Tomato Greenhouses. Phytopathology 2016, 106, 254–261. [Google Scholar] [CrossRef]
- Nandi, M.; Macdonald, J.; Liu, P.; Weselowski, B.; Yuan, Z.-C. Clavibacter michiganensis ssp. michiganensis: Bacterial canker of tomato, molecular interactions and disease management. Mol. Plant Pathol. 2018, 19, 2036–2050. [Google Scholar] [CrossRef]
- PM 7/42 (3) Clavibacter michiganensis subsp. michiganensis. EPPO Bull. 2016, 46, 202–225. [CrossRef]
- de León, L.; Siverio, F.; López, M.M.; Rodríguez, A. Clavibacter michiganesis subsp. michiganensis, a Seedborne Tomato Pathogen: Healthy Seeds Are Still the Goal. Plant Dis. 2011, 95, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Peritore-galve, F.C.; Tancos, M.A.; Smart, C.D. Taxonomy of the Clavibacter Genus Epidemiology in the Field and in Greenhouse Production. Plant Dis. 2021, 105, 1581–1595. [Google Scholar] [CrossRef] [PubMed]
- Sen, Y.; van der Wolf, J.; Visser, R.G.; van Heusden, S. Bacterial Canker of Tomato: Current Knowledge of Detection, Management, Resistance, and Interactions. Plant Dis. 2015, 99, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Kasselaki, A.M.; Goumas, D.; Tamm, L.; Fuchs, J.; Cooper, J.; Leifert, C. Effect of Alternative Strategies for the Disinfection of Tomato Seed Infected with Bacterial Canker (Clavibacter michiganensis subsp. michiganensis). NJAS—Wagening. J. Life Sci. 2011, 58, 145–147. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2020, 283, 124657. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9783030443689. [Google Scholar]
- Arredondo Valdés, R.; Hernández Castillo, F.D.; Anguiano Cabello, J.C.; Ochoa Fuentes, Y.M.; Gallegos, J.C.A.; Morales, G.; Diana, D.J.; Cantú, J.; Aguilar, C.N.; Coahuila, M. Review of Antibacterial Activity of Plant Extracts and Growth-Promoting Microorganism (Gpm) Against Phytopathogenic Bacterial Tomato Crop. Eur. J. Biotechnol. Genet. Eng. 2017, 4, 11–36. [Google Scholar]
- Singh, P.; Singh, R.K.; Zhou, Y.; Wang, J.; Jiang, Y.; Shen, N.; Wang, Y.; Yang, L.; Jiang, M. Unlocking the strength of plant growth promoting Pseudomonas in improving crop productivity in normal and challenging environments: A review. J. Plant Interact. 2022, 17, 220–238. [Google Scholar] [CrossRef]
- Jha, C.K.; Saraf, M. Plant Growth Promoting Rhizobacteria (PGPR): A Review. Int. J. Curr. Microbiol. Appl. Sci. 2015, 10, 882–886. [Google Scholar] [CrossRef]
- Saranraj, P. Biocontrol Potentiality of Plant Growth Promoting Bacteria (PGPR)—Pseudomonas Fluorescens and Bacillus Subtilis: A Review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The Influence of Plant Growth-Promoting Rhizobacteria in Plant Tolerance to Abiotic Stress: A Survival Strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef]
- Scopus Database. Available online: https//scopus.com (accessed on 22 November 2022).
- BV-BRC. Available online: https//bv-brc.org (accessed on 23 November 2022).
- ITOL. Available online: https://itol.embl.de (accessed on 22 November 2022).
- Habtamu, T.; Yigzaw, D.; Wassu, M. Influence of Mulching and Varieties on Growth and Yield of Tomato under Polyhouse. J. Hortic. For. 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Zamora-Macorra, E.J.; Ochoa-Martínez, D.L.; Valdovinos-Ponce, G.; Rojas-Martínez, R.; Ramírez-Rojas, S.; Sánchez-Navarro, J.Á.; Pallás, V.; Aparicio, F. Simultaneous Detection of Clavibacter michiganensis subsp. michiganensis, Pepino Mosaic Virus and Mexican Papita Viroid by Non-Radioactive Molecular Hybridization Using a Unique Polyprobe. Eur. J. Plant Pathol. 2015, 143, 779–787. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO) Data. Available online: https://faostat.fao.org/faostat/en/#data (accessed on 10 January 2023).
- Batnan, A.A.; Oodebji, S.; Mouden, N.; Chliyeh, M.; Touhami, A.O.; Douira, A.A. Study of Current Status and Future Prospects of Soil Disinfection in Souss-Massa and Gharb- Loukkos (Morocco). Recent Sci. Res. 2020, 6, 7895–7903. [Google Scholar]
- Burton-Freeman, B.; Reimers, K. Tomato Consumption and Health: Emerging Benefits. Am. J. Lifestyle Med. 2010, 5, 182–191. [Google Scholar] [CrossRef]
- Arah, I.K.; Amaglo, H.; Kumah, E.K.; Ofori, H. Preharvest and Postharvest Factors Affecting the Quality and Shelf Life of Harvested Tomatoes: A Mini Review. Int. J. Agron. 2015, 2015, 478041. [Google Scholar] [CrossRef]
- Thompson, E.T. The toxicity of a number of different bactericides to Clavibacter michiganense subsp. michiganense (Smith 1910) Jensen 1934 comb. nov. [basonym Corynebacterium michiganense pv. michiganense (AL)] and to the tomato plant, Lycopersicon esculentum. J. Appl. Bacteriol. 1986, 61, 427–436. [Google Scholar] [CrossRef]
- Gleason, M.L.; Gitaitis, R.D.; Ricker, M.D. Recent Progress in Understanding and Controlling Bacterial Canker of Tomato in Eastern North America. Plant Dis. 1993, 77, 1069–1076. [Google Scholar] [CrossRef]
- Kleitman, F.; Barash, I.; Burger, A.; Iraki, N.; Falah, Y.; Sessa, G.; Weinthal, D.; Chalupowicz, L.; Gartemann, K.H.; Eichenlaub, R.; et al. Characterization of a Clavibacter michiganensis subsp. michiganensis Population in Israel. Eur. J. Plant Pathol. 2008, 121, 463–475. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Tanina, K. Genetic Groups of Clavibacter michiganensis subsp. michiganensis Identified by DNA Fingerprinting and the Effects of Inoculation Methods on Disease Development. Eur. J. Plant Pathol. 2014, 140, 399–406. [Google Scholar] [CrossRef]
- De León, L.; Rodríguez, A.; Llop, P.; López, M.M.; Siverio, F. Comparative Study of Genetic Diversity of Clavibacter michiganensis subsp. michiganensis Isolates from the Canary Islands by RAPD-PCR, BOX-PCR and AFLP. Plant Pathol. 2009, 58, 862–871. [Google Scholar] [CrossRef]
- Jacques, M.A.; Durand, K.; Orgeur, G.; Balidas, S.; Fricot, C.; Bonneau, S.; Quillévéré, A.; Audusseau, C.; Olivier, V.; Grimault, V.; et al. Phylogenetic Analysis and Polyphasic Characterization of Clavibacter Michiganensis Strains Isolated from Tomato Seeds Reveal That Nonpathogenic Strains Are Distinct from C. michiganensis subsp. michiganensis. Appl. Environ. Microbiol. 2012, 78, 8388–8402. [Google Scholar] [CrossRef] [PubMed]
- Pereyra-Bistraín, L.I.; Ovando-Vázquez, C.; Rougon-Cardoso, A.; Alpuche-Solís, Á.G. Comparative Rna-seq Analysis Reveals Potentially Resistance-related Genes in Response to Bacterial Canker of Tomato. Genes 2021, 12, 1745. [Google Scholar] [CrossRef]
- Fatmi, M.; Schaad, N.W. Survival of Clavibacter michiganensis ssp. michiganensis in Infected Tomato Stems under Natural Field Conditions in California, Ohio and Morocco. Plant Pathol. 2002, 51, 149–154. [Google Scholar] [CrossRef]
- Amkraz, N.; Talibi, I.; Boubaker, H.; Msanda, F.; Saadi, B.; Boudyach, E. Antioxidant Activity, Phenols and Flavonoids Contents, and Antibacterial Activity of Some Moroccan Medicinal Plants against Tomato Bacterial Canker Agent. Afr. J. Biotechnol. 2014, 13, 4515–4522. [Google Scholar] [CrossRef]
- Sen, Y.; Feng, Z.; Vandenbroucke, H.; van der Wolf, J.; Visser, R.G.F.; van Heusden, A.W. Screening for New Sources of Resistance to Clavibacter michiganensis subsp. michiganensis (Cmm) in Tomato. Euphytica 2013, 190, 309–317. [Google Scholar] [CrossRef]
- Sharabani, G.; Manulis-Sasson, S.; Borenstein, M.; Shulhani, R.; Lofthouse, M.; Chalupowicz, L.; Shtienberg, D. The Significance of Guttation in the Secondary Spread of Clavibacter michiganensis subsp. michiganensis in Tomato Greenhouses. Plant Pathol. 2013, 62, 578–586. [Google Scholar] [CrossRef]
- Carlton, W.M.; Braun, E.J.; Gleason, M.L. Ingress of Clavibacter michiganensis subsp. michiganensis into Tomato Leaves through Hydathodes. Phytopathology 1998, 88, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Medina-Mora, C.M.; Hausbeck, M.K.; Fulbright, D.W. Bird’s Eye Lesions of Tomato Fruit Produced by Aerosol and Direct Application of Clavibacter michiganensis subsp. michiganensis. Plant Dis. 2001, 85, 88–91. [Google Scholar] [CrossRef]
- Gartemann, K.H.; Abt, B.; Bekel, T.; Burger, A.; Engemann, J.; Flügel, M.; Gaigalat, L.; Goesmann, A.; Gräfen, I.; Kalinowski, J.; et al. The Genome Sequence of the Tomato-Pathogenic Actinomycete Chvibacter michiganensis subsp. michiganensis NCPPB382 Reveals a Large Island Involved in Pathogenicity. J. Bacteriol. 2008, 190, 2138–2149. [Google Scholar] [CrossRef]
- Meletzus, D.; Bermpohl, A.; Dreier, J.; Eichenlaub, R. Evidence for Plasmid-Encoded Virulence Factors in the Phytopathogenic Bacterium Clavibacter michiganensis subsp. michiganensis NCPPB382. J. Bacteriol. 1993, 175, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Meletzus, D.; Eichenlaub, R. Transformation of the Phytopathogenic Bacterium Clavibacter michiganense subsp. michiganense by Electroporation and Development of a Cloning Vector. J. Bacteriol. 1991, 173, 184–190. [Google Scholar] [CrossRef]
- Chalupowicz, L.; Zellermann, E.M.; Fluegel, M.; Dror, O.; Eichenlaub, R.; Gartemann, K.H.; Savidor, A.; Sessa, G.; Iraki, N.; Barash, I.; et al. Colonization and Movement of GFP-Labeled Clavibacter michiganensis subsp. michiganensis during Tomato Infection. Phytopathology 2012, 102, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Jahr, H.; Dreier, J.; Meletzus, D.; Bahro, R.; Eichenlaub, R. The Endo-β-1,4-Glucanase CelA of Clavibacter michiganensis subsp. michiganensis Is a Pathogenicity Determinant Required for Induction of Bacterial Wilt of Tomato. Mol. Plant-Microbe Interact. 2000, 13, 703–714. [Google Scholar] [CrossRef]
- Peritore-Galve, F.C.; Miller, C.; Smart, C.D. Characterizing Colonization Patterns of Clavibacter michiganensis during Infection of Tolerant Wild Solanum Species. Phytopathology 2020, 110, 574–581. [Google Scholar] [CrossRef]
- Oloyede, A.R.; Ogbuagor, C.J.; Afolabi, C.G.; Akintokun, A.K. Biological Control of Bacterial Canker of Tomato (Lycopersicon esculentum Mill.) by Use of Non-Native Strains of Plant Growth-Promoting Rhizobacteria. Arch. Phytopathol. Plant Prot. 2021, 54, 1182–1203. [Google Scholar] [CrossRef]
- Mitra, D.; Mondal, R.; Khoshru, B.; Shadangi, S.; Das Mohapatra, P.K.; Panneerselvam, P. Rhizobacteria Mediated Seed Bio-Priming Triggers the Resistance and Plant Growth for Sustainable Crop Production. Curr. Res. Microb. Sci. 2021, 2, 100071. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.M.; Arslanoğlu, Ş.F.; Edbeib, M.F.; Kaya, Y.; Marakli, S. Antibacterial Activity of Calendula Officinalis and Echinacea Purpurea Extracts against the Causal Agent of Tomatoes’ Bacterial Canker: Clavibacter michiganensis subsp. michiganensis. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2021, 20, 496–502. [Google Scholar] [CrossRef]
- Amkraz, N.; Boudyach, E.H.; Boubaker, H.; Bouizgarne, B.; Ait Ben Aoumar, A. Screening for Fluorescent Pseudomonades, Isolated from the Rhizosphere of Tomato, for Antagonistic Activity toward Clavibacter michiganensis subsp. michiganensis. World J. Microbiol. Biotechnol. 2010, 26, 1059–1065. [Google Scholar] [CrossRef]
- Gray, E.J.; Smith, D.L. Intracellular and Extracellular PGPR: Commonalities and Distinctions in the Plant-Bacterium Signaling Processes. Soil. Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.C. Current Knowledge and Perspectives of Paenibacillus: A Review. Microb. Cell Fact. 2016, 15, 1–18. [Google Scholar] [CrossRef]
- Kang, S.M.; Asaf, S.; Khan, A.L.; Lubna; Khan, A.; Mun, B.G.; Khan, M.A.; Gul, H.; Lee, I.J. Complete Genome Sequence of Pseudomonas Psychrotolerans Cs51, a Plant Growth-Promoting Bacterium, under Heavy Metal Stress Conditions. Microorganisms 2020, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the Efficacy of Biological Control against Plant Diseases Likely to Be More Durable than That of Chemical Pesticides? Front. Plant Sci. 2015, 6, 566. [Google Scholar] [CrossRef]
- Yasmin, S.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.I.; Zubair, M.; Iqbal, M. Biocontrol of Bacterial Leaf Blight of Rice and Profiling of Secondary Metabolites Produced by Rhizospheric Pseudomonas Aeruginosa BRp3. Front. Microbiol. 2017, 8, 1895. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 871, 1473. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wei, Z.; Friman, V.-P.P.; Gu, S.H.; Wang, X.F.; Eisenhauer, N.; Yang, T.J.; Ma, J.; Shen, Q.R.; Xu, Y.C.; et al. Probiotic Diversity Enhances Rhizosphere Microbiome Function And. mBio 2016, 7, e01790-16. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Haas, D.; Défago, G. Biological Control of Soil-Borne Pathogens by Fluorescent Pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Lanteigne, C.; Gadkar, V.J.; Wallon, T.; Novinscak, A.; Filion, M. Production of DAPG and HCN by Pseudomonas Sp. LBUM300 Contributes to the Biological Control of Bacterial Canker of Tomato. Phytopathology 2012, 102, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Amkraz, N.; Boubaker, H.; Boudyach, H. Control of Bacterial Canker by Two Fluorescent Pseudomonas Isolates under Tomato Production Conditions Control of Bacterial Canker by Two Fluorescent Pseudomonas Isolates under Tomato Production Conditions. Eco. Environ. Conserv. 2019, 25, S99–S110. [Google Scholar] [CrossRef]
- Paulin, M.M.; Novinscak, A.; Lanteigne, C.; Gadkar, V.J.; Filion, M. Interaction between 2, 4-diacetylphloroglucinol-and hydrogen cyanide-producing Pseudomonas brassicacearum LBUM300 and Clavibacter michiganensis subsp. michiganensis in the tomato rhizosphere. Appl. Environ. Microbiol. 2017, 13, e00073-17. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.M.; Khalil Bagy, H.M.M.; Hashem, M.; Alamri, S.A.M.; Mostafa, Y.S. Biological Control of the Tomato Wilt Caused by Clavibacter michiganensis subsp. michiganensis Using Formulated Plant Growth-Promoting Bacteria. Egypt. J. Biol. Pest Control. 2019, 29, 54. [Google Scholar] [CrossRef]
- Raio, A.; Brilli, F.; Baraldi, R.; Neri, L.; Puopolo, G. Impact of Spontaneous Mutations on Physiological Traits and Biocontrol Activity of Pseudomonas Chlororaphis M71. Microbiol. Res. 2020, 239, 126517. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Wang, X.; Baird, S.M.; Lu, S.E. Complete Genome of Pseudomonas Chlororaphis Strain UFB2, a Soil Bacterium with Antibacterial Activity against Bacterial Canker Pathogen of Tomato. Stand Genom. Sci. 2015, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Arora, N.K. Secondary Metabolites of Fluorescent Pseudomonads in Biocontrol of Phytopathogens for Sustainable Agriculture. Appl. Soil Ecol. 2018, 125, 35–45. [Google Scholar] [CrossRef]
- Sansinenea, E. Bacillus spp.: As plant growth-promoting bacteria. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms: Discovery and Applications; Springer: Singapore, 2019; pp. 225–237. [Google Scholar]
- Escamilla-silva, E.M.; Luz, A. A Novel Isolate of Bacillus Cereus Promotes Growth in Tomato and Inhibits Clavibacter Michiganensis Infection under Greenhouse Conditions. Plants 2021, 10, 506. [Google Scholar]
- Vega-Celedón, P.; Canchignia Martínez, H.; González, M.; Seeger, M. Revisión Bibliográfica BIOSÍNTESIS DE ÁCIDO INDOL-3-ACÉTICO Y PROMOCIÓN DEL CRECIMIENTO DE PLANTAS POR BACTERIAS Review Biosynthesis of Indole-3-Acetic Acid and Plant Growth Promoting by Bacteria. Cultiv. Trop. 2016, 37, 33–39. [Google Scholar] [CrossRef]
- Ghadamgahi, F.; Tarighi, S.; Taheri, P.; Saripella, G.V.; Anzalone, A.; Kalyandurg, P.B.; Catara, V.; Ortiz, R.; Vetukuri, R.R. Plant Growth-Promoting Activity of Pseudomonas Aeruginosa FG106 and Its Ability to Act as a Biocontrol Agent against Potato, Tomato and Taro Pathogens. Biology 2022, 11, 140. [Google Scholar] [CrossRef]
- Takishita, Y.; Charron, J.B.; Smith, D.L. Biocontrol Rhizobacterium Pseudomonas sp. 23S Induces Systemic Resistance in Tomato (Solanum lycopersicum L.) against Bacterial Canker Clavibacter michiganensis subsp. michiganensis. Front. Microbiol. 2018, 9, 2119. [Google Scholar] [CrossRef]
- Elshahat, M.R.; Ahmed, A.A.; Enas, A.H.; Fekria, M.S. Plant Growth Promoting Rhizobacteria and Their Potential for Biocontrol of Phytopathogens. Afr. J. Microbiol. Res. 2016, 10, 486–504. [Google Scholar] [CrossRef]
- Raio, A.; Reveglia, P.; Puopolo, G.; Cimmino, A.; Danti, R.; Evidente, A. Involvement of Phenazine-1-Carboxylic Acid in the Interaction between Pseudomonas Chlororaphis Subsp. Aureofaciens Strain M71 and Seiridium Cardinale in Vivo. Microbiol. Res. 2017, 199, 49–56. [Google Scholar] [CrossRef]
- Schalk, I.J.; Rigouin, C.; Godet, J. An Overview of Siderophore Biosynthesis among Fluorescent Pseudomonads and New Insights into Their Complex Cellular Organization. Environ. Microbiol. 2020, 22, 1447–1466. [Google Scholar] [CrossRef]
- Siqueira, J.G.W.; Rodrigues, C.; de Vandenberghe, L.P.S.; Woiciechowski, A.L.; Soccol, C.R. Current Advances in On-Site Cellulase Production and Application on Lignocellulosic Biomass Conversion to Biofuels: A Review. Biomass Bioenergy 2020, 132, 105419. [Google Scholar] [CrossRef]
- Jung, W.J.; Mabood, F.; Souleimanov, A.; Whyte, L.G.; Niederberger, T.D.; Smith, D.L. Microbial Pathogenesis Antibacterial Activity of Antagonistic Bacterium Bacillus Subtilis DJM-51 against Phytopathogenic Clavibacter michiganense subsp. michiganense ATCC 7429 in Vitro. Microb. Pathog. 2014, 77, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.D.; Li, H.P.; Yuan, Q.S.; Song, X.S.; Yao, W.; He, W.J.; Zhang, J.B.; Liao, Y.C. Antagonistic Mechanism of Iturin a and Plipastatin a from Bacillus Amyloliquefaciens S76-3 from Wheat Spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef] [PubMed]
- Grady, E.N.; MacDonald, J.; Ho, M.T.; Weselowski, B.; McDowell, T.; Solomon, O.; Renaud, J.; Yuan, Z.C. Characterization and Complete Genome Analysis of the Surfactin-Producing, Plant-Protecting Bacterium Bacillus Velezensis 9D-6. BMC Microbiol. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Laird, M.; Piccoli, D.; Weselowski, B.; McDowell, T.; Renaud, J.; MacDonald, J.; Yuan, Z.C. Surfactin-Producing Bacillus Velezensis 1B-23 and Bacillus Sp. 1D-12 Protect Tomato against Bacterial Canker Caused by Clavibacter michiganensis subsp. michiganensis. J. Plant Pathol. 2020, 102, 451–458. [Google Scholar] [CrossRef]
- Matthijs, S.; Tehrani, K.A.; Laus, G.; Jackson, R.W.; Cooper, R.M.; Cornelis, P. Thioquinolobactin, a Pseudomonas Siderophore with Antifungal and Anti-Pythium Activity. Environ. Microbiol. 2007, 9, 425–434. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Screening of Free-Living Rhizospheric Bacteria for Their Multiple Plant Growth Promoting Activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef]
- Biessy, A.; Novinscak, A.; Blom, J.; Léger, G.; Thomashow, L.S.; Cazorla, F.M.; Josic, D.; Filion, M. Diversity of Phytobeneficial Traits Revealed by Whole-Genome Analysis of Worldwide-Isolated Phenazine-Producing Pseudomonas spp. Environ. Microbiol. 2019, 21, 437–455. [Google Scholar] [CrossRef]
- Lamont, I.L.; Martin, L.W.; Sims, T.; Scott, A.; Wallace, M. Characterization of a Gene Encoding an Acetylase Required for Pyoverdine Synthesis in Pseudomonas Aeruginosa. J. Bacteriol. 2006, 188, 3149–3152. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, R.K.; Guo, D.J.; Sharma, A.; Singh, R.N.; Li, D.P.; Malviya, M.K.; Song, X.P.; Lakshmanan, P.; Yang, L.T.; et al. Whole Genome Analysis of Sugarcane Root-Associated Endophyte Pseudomonas Aeruginosa B18—A Plant Growth-Promoting Bacterium with Antagonistic Potential Against Sporisorium Scitamineum. Front. Microbiol. 2021, 12, 628376. [Google Scholar] [CrossRef]
- Gautam, S.; Sharma, R.; Chauhan, A.; Shirkot, C.K.; Kaushal, R. Biocontrol Activities of Rhizobacteria Associated with Apple, Apricot and Kiwi Rhizosphere against Bacterial Canker Caused by Clavibacter michiganensis. Indian Phytopathol. 2020, 73, 45–56. [Google Scholar] [CrossRef]
- Gautam, S.; Chauhan, A.; Sharma, R.; Sehgal, R.; Shirkot, C.K. Microbial Pathogenesis Potential of Bacillus Amyloliquefaciens for Biocontrol of Bacterial Canker of Tomato Incited by Clavibacter michiganensis ssp. michiganensis. Microb. Pthogenesis 2019, 130, 196–203. [Google Scholar] [CrossRef]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of Plants with Microbial World: Exploring the Regulatory Networks for PGPR Mediated Defense Signaling. Microbiol. Res. 2020, 238, 126486. [Google Scholar] [CrossRef] [PubMed]
- Kreslavski, V.D.; Los, D.A.; Allakhverdiev, S.I.; Kuznetsov, V.V. Signaling Role of Reactive Oxygen Species in Plants under Stress. Russ. J. Plant Physiol. 2012, 59, 141–154. [Google Scholar] [CrossRef]
- Malinovsky, F.G.; Fangel, J.U.; Willats, W.G.T. The Role of the Cell Wall in Plant Immunity. Front. Plant Sci. 2014, 5, 178. [Google Scholar] [CrossRef]
- Grant, M.; Lamb, C. Systemic Immunity. Curr. Opin. Plant Biol. 2006, 9, 414–420. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Bakker, P.A.H.M. Induced Systemic Resistance as a Mechanism of Disease Suppression by Rhizobacteria. PGPR Biocontrol Biofertil. 2006, 39–66. [Google Scholar] [CrossRef]
- Soylu, S.; Soylu, E.M. Induction of Disease Resistance by the Plant Acti v Ator, Acibenzolar- S—Methyl (ASM), against Bacterial Canker (Cla v Ibacter michiganensis subsp. michiganensis) in Tomato Seedlings. Plant Sci. 2003, 165, 1069–1075. [Google Scholar] [CrossRef]
- Brisson, L.F.; Tenhaken, R.; Lamb, C. Function of Oxidative Cross-Linking of Cell Wall Structural Proteins in Plant Disease Resistance. Plant Cell 1994, 6, 1703–1712. [Google Scholar] [CrossRef]
- Conrath, U.; Thulke, O.; Katz, V.; Schwindling, S.; Kohler, A. Priming as a Mechanism in Induced Systemic Resistance of Plants. Eur. J. Plant Pathol. 2001, 107, 113–119. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van Der Ent, S.; Van Wees, S.C.M. Networking by Small-Molecule Hormones in Plant Immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef]
- Cawoy, H.; Mariutto, M.; Henry, G.; Fisher, C.; Vasilyeva, N.; Thonart, P.; Dommes, J.; Ongena, M. Plant Defense Stimulation by Natural Isolates of Bacillus Depends on Efficient Surfactin Production. Mol. Plant-Microbe Interact. 2014, 27, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting Ready for Battle. Mol. Plant-Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Medina, A.; Fernández, I.; Sánchez-Guzmán, M.J.; Jung, S.C.; Pascual, J.A.; Pozo, M.J. Deciphering the Hormonal Signaling Network behind the Systemic Resistance Induced by Trichoderma harzianum in Tomato. Front. Plant Sci. 2013, 4, 206. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.M.; Kaya, Y.; Ozturk, M.; Secgin, Z.; Onder, H.; Okumus, A. Pseudomonas Putida—Induced Response in Phenolic Profile of Tomato Seedlings (Solanum lycopersicum L.) Infected by Clavibacter michiganensis subsp. michiganensis. Biol. Control. 2017, 105, 6–12. [Google Scholar] [CrossRef]
- Kolomiiets, Y.; Grygoryuk, I.; Likhanov, A.; Butsenko, L.; Blume, Y. Induction of Bacterial Canker Resistance in Tomato Plants Using Plant Growth Promoting Rhizobacteria. Open Agric. J. 2020, 13, 215–222. [Google Scholar] [CrossRef]
- Baker, C.J.; Whitaker, B.D.; Roberts, D.P.; Mock, N.M.; Rice, C.P.; Deahl, K.L.; Aver’Yanov, A.A. Induction of Redox Sensitive Extracellular Phenolics during Plant-Bacterial Interactions. Physiol. Mol. Plant Pathol. 2005, 66, 90–98. [Google Scholar] [CrossRef]
- Sudhakar, N.; Nagendra-Prasad, D.; Mohan, N.; Murugesan, K. Induction of Systemic Resistance in Lycopersicon Esculentum Cv. PKM1 (Tomato) against Cucumber Mosaic Virus by Using Ozone. J. Virol. Methods 2007, 139, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Balaji, V.; Mayrose, M.; Sherf, O.; Jacob-Hirsch, J.; Eichenlaub, R.; Iraki, N.; Manulis-Sasson, S.; Rechavi, G.; Barash, I.; Sessa, G. Tomato Transcriptional Changes in Response to Clavibacter michiganensis subsp. michiganensis Reveal a Role for Ethylene in Disease Development. Plant Physiol. 2008, 146, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; Oh, E.J.; Oh, C.S. Transcriptional Changes of Plant Defense-Related Genes in Response to Clavibacter Infection in Pepper and Tomato. Plant Pathol. J. 2020, 36, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Yokotani, N.; Hasegawa, Y.; Sato, M.; Hirakawa, H.; Kouzai, Y.; Nishizawa, Y.; Yamamoto, E.; Naito, Y.; Isobe, S. Transcriptome Analysis of Clavibacter michiganensis subsp. michiganensis-Infected Tomatoes: A Role of Salicylic Acid in the Host Response. BMC Plant Biol. 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY Transcription Factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Bittel, P.; Robatzek, S. Microbe-Associated Molecular Patterns (MAMPs) Probe Plant Immunity. Curr. Opin. Plant Biol. 2007, 10, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.A.; Sundelin, T.; Nielsen, J.T.; Erbs, G. MAMP (Microbe-Associated Molecular Pattern) Triggered Immunity in Plants. Front. Plant Sci. 2013, 4, 139. [Google Scholar] [CrossRef]
- Boller, T.; He, S.Y. Innate Immunity in Plants: An Arms Race between Pattern Recognition Receptors in Plants and Effectors in Microbial Pathogens. Science 2009, 324, 742–743. [Google Scholar] [CrossRef]
- Zipfel, C. Pattern-Recognition Receptors in Plant Innate Immunity. Curr. Opin. Immunol. 2008, 20, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.A.H.M. Determinants of Pseudomonas Putida WCS358 Involved in Inducing. Mol. Plant Pathol. 2005, 6, 177–185. [Google Scholar] [CrossRef]
- Ochoa-Meza, L.C.; Quintana-Obregón, E.A.; Vargas-Arispuro, I.; Falcón-Rodríguez, A.B.; Aispuro-Hernández, E.; Virgen-Ortiz, J.J.; Martínez-Téllez, M.Á. Oligosaccharins as Elicitors of Defense Responses in Wheat. Polymers 2021, 13, 3105. [Google Scholar] [CrossRef]
- Höfte, M.; Bakker, P.A.H.M. Competiton for Iron and Induced 6 Competition Systemic Resistance by Siderophores of Plant Growth Promoting Rhizobacteria Role of Siderophores and Iron-Regulated Compounds in ISR. Bioscience 2007, 12, 121–133. [Google Scholar]
- Tran, H.; Ficke, A.; Asiimwe, T.; Höfte, M.; Raaijmakers, J.M. Role of the Cyclic Lipopeptide Massetolide a in Biological Control of Phytophthora Infestans and in Colonization of Tomato Plants by Pseudomonas Fluorescens. New Phytol. 2007, 175, 731–742. [Google Scholar] [CrossRef]
- Jang, H.; Kim, S.T.; Sang, M.K. Suppressive Effect of Bioactive Extracts of Bacillus Sp. H8-1 and Bacillus Sp. K203 on Tomato Wilt Caused by Clavibacter michiganensis subsp. michiganensis. Microorganisms 2022, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.A.; Harris, R.; Breuer, G.; Levy, M. Secretion-Based Modes of Action of Biocontrol Agents with a Focus on Pseudozyma Aphidis. Plants 2021, 10, 210. [Google Scholar] [CrossRef]
- Barda, O.; Shalev, O.; Alster, S.; Buxdorf, K.; Gafni, A.; Levy, M. Pseudozyma Aphidis Induces Salicylic-Acid-Independent Resistance to Clavibacter michiganensis in Tomato Plants. Plant Dis. 2015, 99, 621–626. [Google Scholar] [CrossRef]
- Bektas, Y.; Eulgem, T. Synthetic Plant Defense Elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef]
- Cesbron, S.; Thomson, S.V.; Paulin, J.; Morel, R.G. Acibenzolar-S-Methyl Induces the Accumulation of Defense-Related Enzymes in Apple and Protects from Fire Blight. Eur. J. Plant Pathol. 2000, 106, 529–536. [Google Scholar]
- Baysal, Ö.; Soylu, E.M.; Soylu, S. Induction of Defence-Related Enzymes and Resistance by the Plant Activator Acibenzolar-S-Methyl in Tomato Seedlings against Bacterial Canker Caused by Clavibacter michiganensis ssp. michiganensis. Plant Pathol. 2003, 52, 747–753. [Google Scholar] [CrossRef]
- Nicholson, R.L.; et Hammerschmidt, R. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Bektas, Y.; Rodriguez-Salus, M.; Schroeder, M.; Gomez, A.; Kaloshian, I.; Eulgem, T. The Synthetic Elicitor DPMP (2,4-Dichloro-6-{(E)-[(3-Methoxyphenyl)Imino]Methyl}phenol) Triggers Strong Immunity in Arabidopsis Thaliana and Tomato. Sci. Rep. 2016, 6, 29554. [Google Scholar] [CrossRef] [PubMed]
- Bektas, Y. The Synthetic Elicitors 2,6-Dichloro-Isonicotinic Acid (INA) and 2,4-Dichloro-6-{(E)-[(3-Methoxyphenyl)Imino]Methyl}phenol (DPMP) Enhances Tomato Resistance against Bacterial Canker Disease with Different Molecular Mechanisms. Physiol. Mol. Plant Pathol. 2021, 116, 101740. [Google Scholar] [CrossRef]
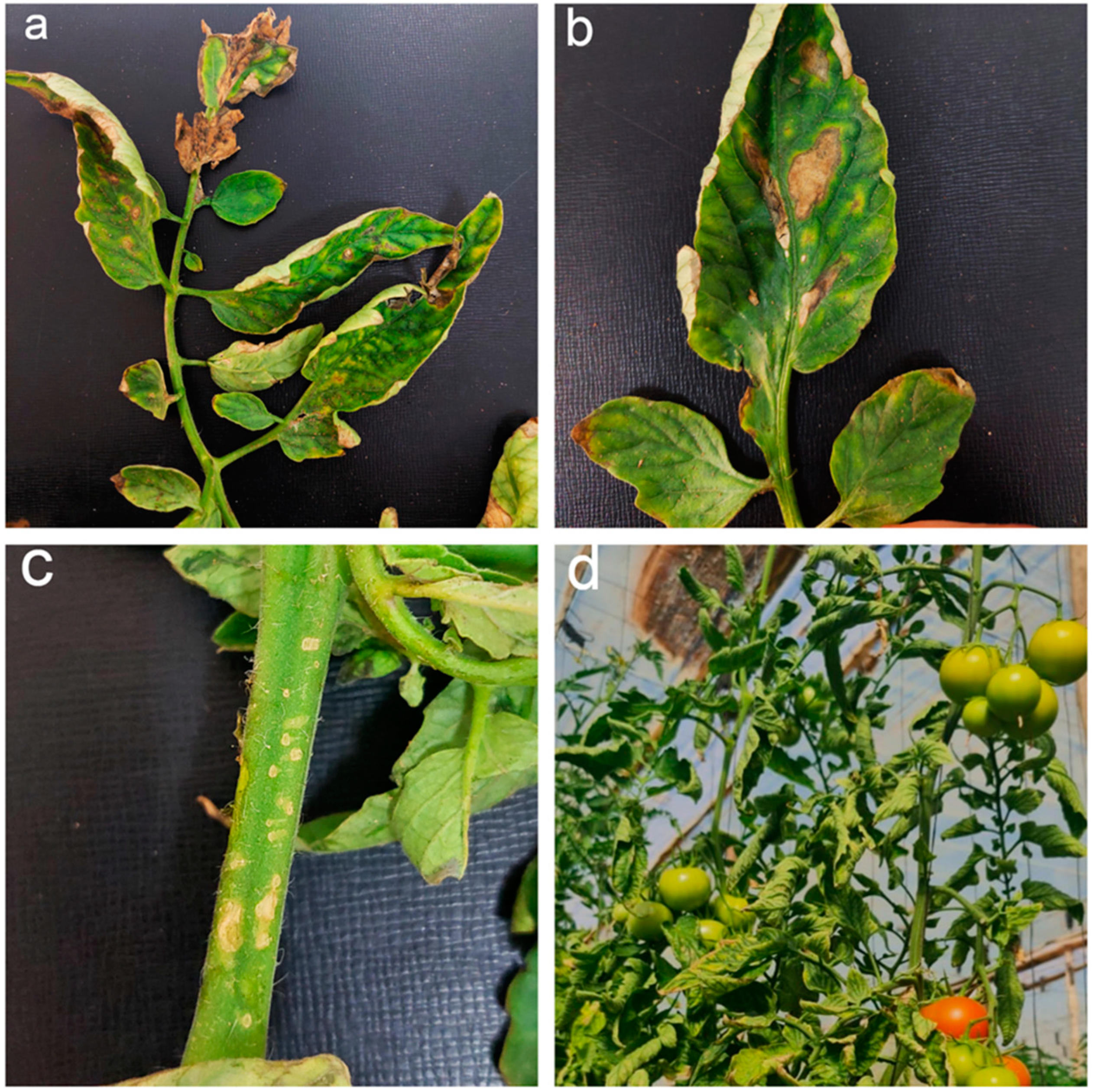
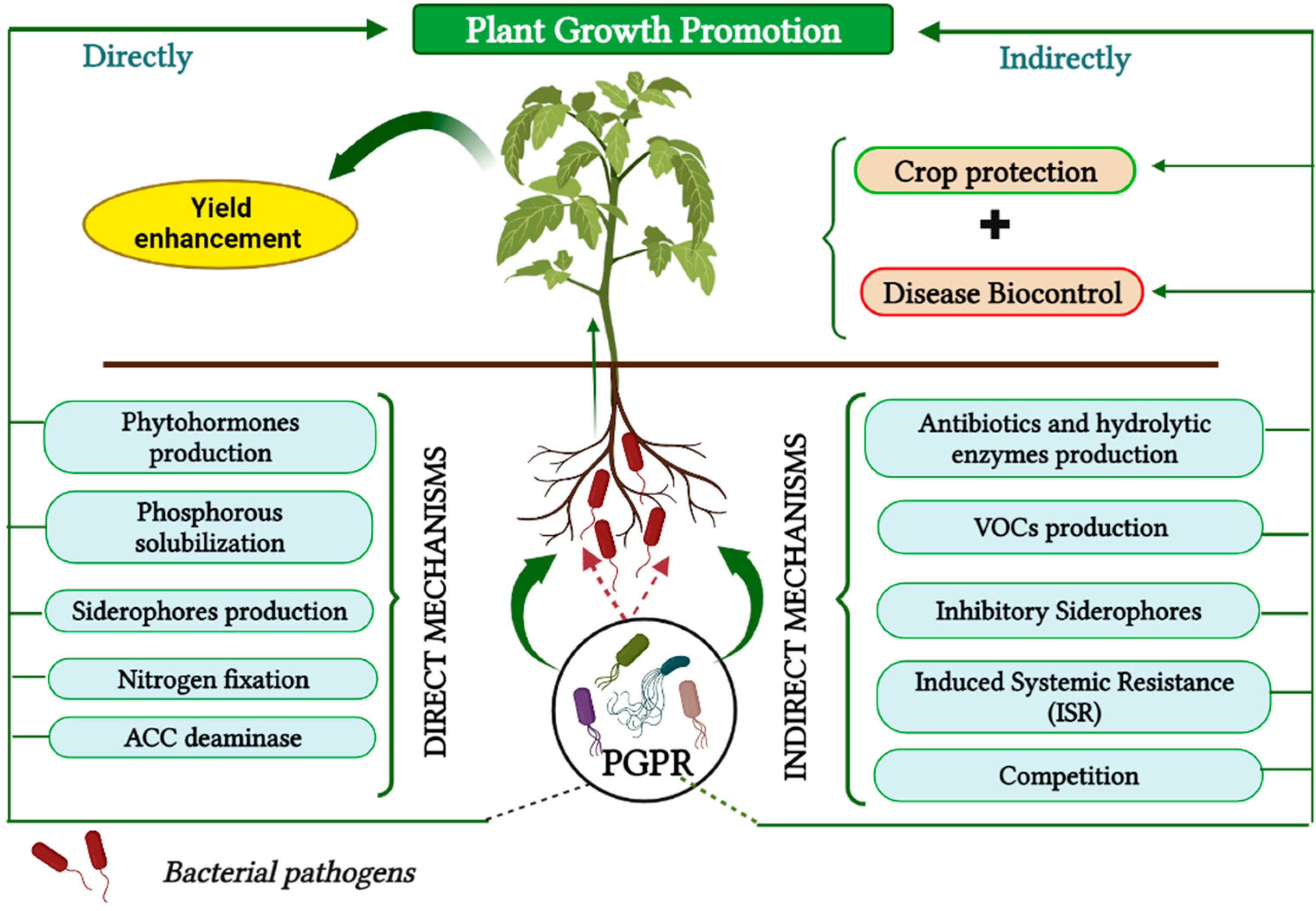
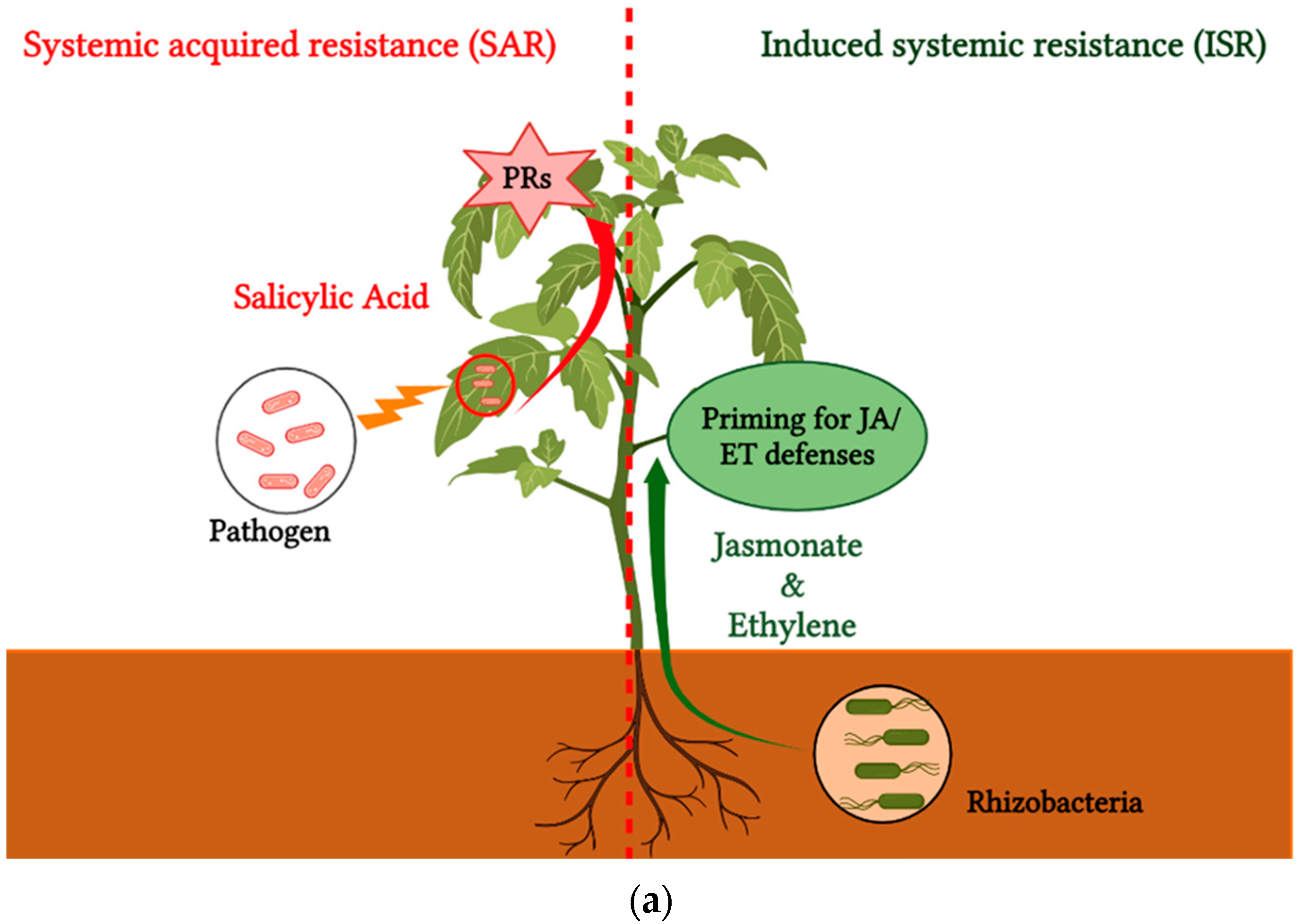
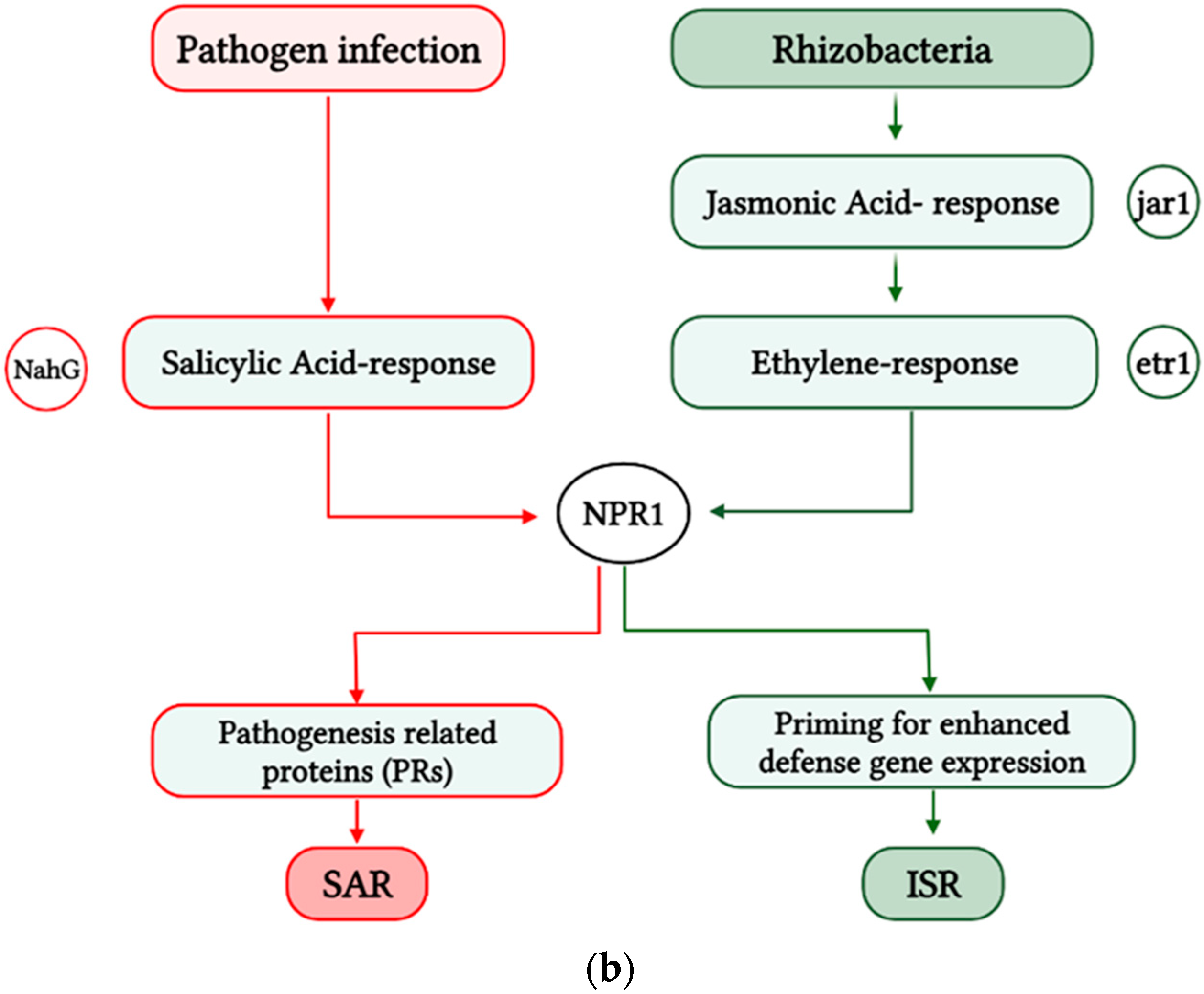

| Biocontrol Agent | Compound/Gene | References |
|---|---|---|
| Mechanism/activity | Induced systemic resistance (ISR) | |
| Pseudomonas 23 S | Through SA in its signaling pathway Up-regulation of PR-1a gene Increased the transcript level of ACO (ET) | [75] |
| Pseudomonas putida CKPp9 | Increased biosynthesis of phenolic compounds in tomato leaves (catechin, chlorogenic acid) | [104] |
| Azotobacter chroococcum | Provided an accumulation of phenols and flavonoids in tomato leaves | [105] |
| Bacillus subtilis | Increased chlorophyll (a+b) and carotenoid content in tomato leaves Increment of the Peroxidase (POX) activity | [105] |
| Bacillus cereus | Increased total chlorophyll content in tomato plants. Increment of the activity of PAL and the expression of chs (contributed to flavonoids biosynthesis) | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benchlih, S.; Esmaeel, Q.; Aberkani, K.; Tahiri, A.; Belabess, Z.; Lahlali, R.; Barka, E.A. Modes of Action of Biocontrol Agents and Elicitors for sustainable Protection against Bacterial Canker of Tomato. Microorganisms 2023, 11, 726. https://doi.org/10.3390/microorganisms11030726
Benchlih S, Esmaeel Q, Aberkani K, Tahiri A, Belabess Z, Lahlali R, Barka EA. Modes of Action of Biocontrol Agents and Elicitors for sustainable Protection against Bacterial Canker of Tomato. Microorganisms. 2023; 11(3):726. https://doi.org/10.3390/microorganisms11030726
Chicago/Turabian StyleBenchlih, Salma, Qassim Esmaeel, Kamal Aberkani, Abdessalem Tahiri, Zineb Belabess, Rachid Lahlali, and Essaid Ait Barka. 2023. "Modes of Action of Biocontrol Agents and Elicitors for sustainable Protection against Bacterial Canker of Tomato" Microorganisms 11, no. 3: 726. https://doi.org/10.3390/microorganisms11030726
APA StyleBenchlih, S., Esmaeel, Q., Aberkani, K., Tahiri, A., Belabess, Z., Lahlali, R., & Barka, E. A. (2023). Modes of Action of Biocontrol Agents and Elicitors for sustainable Protection against Bacterial Canker of Tomato. Microorganisms, 11(3), 726. https://doi.org/10.3390/microorganisms11030726









