Abstract
This study aimed to evaluate the effects of coconut oil and palm oil in milk replacer (MR) on the growth performance, blood lipids, rumen fermentation, rumen microbiota, and fatty acid profile of hepatic and muscle of suckling calves. Thirty-six Holstein male calves were randomly assigned to three treatments. Three milk replacers containing different fat sources were as follows: control group (CON, milk fat), coconut oil group (CCO, coconut oil powder as fat), and palm oil group (PLO, palm oil powder as fat). Calves were weighed and blood sampled at 14, 28, 42, and 56 days old, respectively, and the feed intake and fecal score were recorded daily. Fat sources in milk replacers had no effects on body weight, ADG, DMI, fecal score, or days of abnormal fecal in suckling calves among the three groups, while the PLO group tended to decrease starter intake compared with the other groups. Serum concentrations of TC, HDL-C, LDL-C, and VLDL-C in the CCO group increased compared with those of the CON group. Palm oil also decreased the serum GLU concentration of calves but had no effects on serum lipids compared with milk fat. Coconut oil or palm oil had no effects on rumen fermentation, rumen chyme enzyme activity, rumen bacterial community richness and diversity, and dominant phyla and genera when compared with milk fat. However, compared with the CON group, the CCO group increased the proportion of MCFAs and n-6 PUFAs, and decreased the proportion of UFAs and MUFAs in liver tissue, while the PLO group increased the proportion of PUFAs and decreased the proportion of n-3 PUFAs in liver tissue. In addition, compared with the CON group, the CCO group increased the proportion of MCFAs, and decreased the proportion of UFAs and n-3 PUFAs in longissimus dorsi, while the PLO group increased the proportion of PUFAs and decreased the proportion of n-3 PUFAs in longissimus dorsi. In conclusion, compared with milk fat, coconut oil or palm oil in MR had no effects on growth performance, rumen fermentation, and rumen microflora but significantly increased serum lipids concentration and changed some proportions of MCFAs and PUFAs in liver and longissimus dorsi in suckling calves. These results indicate that coconut oil or palm oil as the sole fat source for MRs has no adverse effect on calf rumen fermentation and rumen microbiota but has a detrimental effect on n-3 PUFAs deposition in the liver and longissimus dorsi muscle.
1. Introduction
Milk fat is a natural and complex fat extracted from ruminant milk rich in fatty acids. It contains more than 400 fatty acids (FAs) [1], of which saturated fatty acids (SFAs) account for 65%, followed by monounsaturated fatty acids (MUFAs, 30%) and polyunsaturated fatty acids (PUFAs, 5%) [2]. Milk fat has long been a major source of fat for milk replacers (MRs) and a major ingredient in human infant food and dairy products. However, in order to meet the human demand for milk fat and reduce the cost of MR, it is necessary to study fat substitutes with abundant sources and low prices. Numerous studies have reported the effects of various animal fats or vegetable oils, such as lard, tallow, corn oil, soybean oil, coconut oil, or palm oil, on the performance and health of suckling calves [3,4,5]. Among them, coconut oil and palm oil are cheap vegetable oils derived from coconut and palm pulp or nuts, respectively, with abundant reserves in the world, and have been extensively studied as fat sources for MRs [5,6]. The replacement of milk fat in colostrum and milk with coconut oil significantly altered fat-soluble vitamins in the serum of neonatal calves, as first found by Rajaraman et al. [4]. In addition, more energy and fat in the body were deposited in calves that were fed greater amounts of medium-chain fatty acids (MCFAs) from coconut oil under cold stress conditions [6]. Bowen et al. further demonstrated that the use of coconut oil in milk replacers benefits calf health and performance based on skeletal growth and fecal score [7]. Similarly, Panahiha et al. observed that the combination of corn forage and palm oil fat in the starter created a positive interaction on the growth performance of suckling calves [8]. In addition, some studies have shown that the addition of some short-chain or medium-chain fatty acids such as sodium butyrate and medium-chain fatty acid monoglycerides to calf MRs has some potential benefits in the rumen development [9,10]. However, more studies focused on the effects of coconut oil or palm oil used in the starter on growth performance, health, ruminal fermentation, and blood metabolites [3,11]. There are limited reports in the literature on the effects of adding coconut oil or palm oil or their major fatty acids, MCFAs or palmitic acid, to MRs on rumen fermentation, rumen chymase activity, and rumen microflora in suckling calves. This may be related to the esophagus groove reflex of pre-ruminant calves. It can deliver liquid feed directly to the abomasum, and only about 3% of the liquid feed enters the rumen for fermentation [12]. However, some studies have indicated that on average, 12% to 20% of milk or MRs may leak into or backflow into the reticulorumen in suckling calves [13,14,15], which may affect rumen fermentation and rumen microflora, provide nutrients for the development of tissues and organs, such as the rumen, and improve the growth and development of the body. Therefore, it is necessary to conduct some research that focuses on the effects of coconut oil or palm oil formulated into MRs on rumen fermentation, rumen chymase activity, and rumen microorganisms. Therefore, we hypothesized that the usage of coconut oil or palm oil as a fat source for MRs would change rumen fermentation and rumen microflora in suckling calves. One of the objectives of this study was to investigate the effects of coconut oil and palm oil as a fat source for MRs on rumen fermentation, chymase activities, and rumen microbiota in suckling calves.
In addition, palm oil is rich in palmitic acid, followed by oleic acid and linoleic acid, which contain 50% SFAs, 40% MUFAs, and 10% PUFAs [16]. The fatty acid composition of coconut oil is different from that of milk fat and palm oil, as about 90% of the fatty acids are made of SFAs [17,18], and more than about 50% of the fatty acids are made of MCFAs, such as lauric acid [2]. Previous studies have shown that different dietary fats or fatty acids can affect the composition of fatty acids in the various body tissues of calves [19,20,21]. Jenkins et al. observed that high dietary intake of linoleic acid in corn oil or PUFAs in fish oil increases PUFAs concentration, such as linoleic acid, eicosapentaenoic acid, and eicosapentaenoic acid, in calf plasma, liver, platelets, muscle, and heart, respectively [21]. Garcia et al. [22] found that supplementation with linoleic acid to newborn calf MRs significantly increases the total percentage of n-6 and n-3 PUFAs in liver tissue. However, research on the effects of adding coconut oil or palm oil or their major fatty acids to MRs on the body fatty acid composition of dairy cows is still limited. We hypothesized that the use of coconut oil or palm oil as a fat source for MRs would change the fatty acid composition in the liver and muscle of suckling calves. Therefore, another purpose of this study is to evaluate the effects of coconut or palm oil on blood lipids and fatty acid composition in the liver and muscle of suckling calves.
2. Materials and Methods
2.1. Animal Care
The experimental design and animal care and handling procedures were evaluated and approved by the Animal Ethics Committee of the Institute of Feed Research of the Chinese Academy of Agricultural Sciences (IFR-CAAS-20160715, Beijing, China).
2.2. Animals, Treatments, and Management
Thirty-six 3 ± 1 days old and healthy Holstein male calves were randomly selected for the experiment. All calves were fed colostrum and milk from 1 to 3 days after birth, and then fed fresh milk from 4 to 7 days old. Calves aged 7 days (42.8 ± 4.4 kg of body weight) were randomly assigned to one of the three treatments: (A) control group (CON), only whole milk powder as a fat source of MR (254 g/kg of crude protein (CP) and 127 g/kg of ether extract (EE); the levels of the main fatty acids including palmitic, oleic, and myristic acids were 4.15, 2.33, and 1.53 g/100 g MR, respectively); (B) coconut oil group (CCO), only coconut oil powder as a fat source of MR (259 g/kg of CP and 125 g/kg of EE; the levels of the main fatty acids including lauric and myristic acids were 4.90 and 2.00 g/100 g MR, respectively); (C) palm oil group (PLO), only palm oil powder as a fat source of MR (252 g/kg of CP and 122 g/kg of EE; the levels of the main fatty acids including palmitic and oleic acids were 6.72 and 4.19 g/100 g MR, respectively). Milk replacers were isonitrogenous and isocaloric at 250 g/kg of CP, 120 g/kg of EE, and 4.8 Mcal/kg metabolizable energy (ME) of DM. Nutrient levels and fatty acid composition of the MRs are presented in Supplementary Table S1. From 7 to 14 days old, calves were fed these MRs for an adaptation period of 1 week, then a 42-day trial was conducted. Calves were gradually weaned from 56 to 60 days old. The MRs were reconstituted to emulsions (12.5%, wet/vol) by cooling boiled water to 40 °C and then fed to calves on a DM basis at 10% of live weight twice a day (0700 and 1500 h) [23]. All calves were housed in individual hutches (1.6 × 3.6 m), fed using a bucket with a teat, and reared with ad libitum access to starter feed from 14 days old. The ingredient composition and nutrient profile of the starter are presented in Supplementary Table S2. The average ambient temperature was 9 °C (range −5~23 °C), and most of the time during the trial, it was at or below the lower critical temperature (LCT, 8~13 °C) [24,25] (Supplementary Figure S1).
2.3. Growth Performance and Feed Intake
The milk replacers and starter samples were collected weekly during the experiment to determine the content of dry matter (DM) (method 930.15), CP (method 990.03), EE (method 2003.05), ash (method 942.05), calcium (method 985.01), and phosphorus (method 985.01) according to AOAC International (2006) protocols [26]. Neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents of the starter were determined according to the method of α-amylase [27].
The body weight of calves was measured before the morning feeding at 14, 28, 42, and 56 days old, respectively. The daily intake of the MRs and starter were recorded, and the total dry matter intake (DMI) and FAs intake of MR and starter for each calf were calculated. The Feed intake: Gain ratio (F: G ratio) was calculated as the ratio of total DMI to average daily gain [28]. Fecal scores were recorded several times daily by a blind test according to a 5-point score [29]: 1 = normal, thick in consistency, 2 = normally, but less thick, 3 = abnormally thin but not watery, 4 = watery, 5 = watery with abnormal coloring.
2.4. Blood Sampling and Analysis
Blood samples were obtained using jugular venipuncture at 14, 28, 42, and 56 days old and then centrifuged at 3000× g at 4 °C for 10 min. The obtained serum was transferred into 1.5 mL centrifuges tubes and stored at −20℃ before analysis. Glucose (GLU), cholesterol (TC), and triglyceride (TG) were measured using an automatic serum biochemical analyzer (KeHua, KHB-1280, Shanghai, China). High-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), non-esterified fatty acids (NEFAs), and β-Hydroxybutyric acid (BHBA) were measured using bovine enzyme-linked immunosorbent assay kits (JianCheng Biotechnology Co., Ltd., Nanjing, China).
2.5. Ruminal Chyme Fermentation Parameters and Chyme Enzyme Activity
At 56 days old, ruminal chyme was collected through the mouth using an esophageal tube from six calves per treatment 2 h after the morning feeding as described by Paz et al. [30], and then it was divided into two tubes for microbial analysis and fermentation parameters and enzyme activities measurement, respectively. More specifically, the pH value and ammonia nitrogen (NH3-N) were measured using the methods of pH meter (206-pH2, Testo, Germany) and phenol-sodium hypochlorite colorimetric, respectively [31]. The volatile fatty acids (VFAs) were measured using a gas chromatograph (SP-3420A, Beijing Analytical Instrument Factory, Beijing, China) equipping a mega bore HP-MOLSIV column (film thickness: 30 m × 0.53 mm × 25 mm, Agilent Technologies, Palo Alto, CA, USA) [23]. The ruminal chyme enzyme activities including neutral protease, carboxymethylcellulose, α-amylase, xylanase, and lipases were measured as described previously [32].
2.6. Analysis of Microbiota
Total genome DNA from the ruminal content was extracted using the CTAB/SDS method, quantified, purified on 1% agarose gels, and finally diluted to 1 ng/μL using sterile water. Regions V4 of the bacterial 16S rRNA gene were amplified with specific primers (515F, GTGCCAGCMGCCGCGGTAA; 806R, GGACTACHVGGGTWTCTAAT). PCR products (400–450bp) were mixed in isodensity ratios. Then, the mixture of PCR products was purified with a Qiagen Gel Extraction Kit (Qiagen, Dusseldorf, Germany). Sequencing libraries were generated using TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, CA, USA) following the manufacturer’s recommendations, and index codes were added. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific, Waltham, MA, USA) and Agilent Bioanalyzer 2100 system (Agilent Technologies, Palo Alto, CA, USA). Subsequently, the library was sequenced using an Illumina HiSeq2500 platform and 250 bp paired-end reads were generated.
2.7. Sequencing Data Analysis
Paired-end reads were merged using FLASH version 1.2.7 (http://ccb.jhu.edu/software/FLASH/), and the splicing sequences were called raw tags. The high-quality clean tags were obtained according to the QIIME (version 1.8.0, http://bio.cug.edu.cn/qiime/) quality-controlled process. The chimera sequences were determined and removed by comparison between tags and the reference database (Gold database, http://drive5.com/uchime/uchime_download.html) using the UCHIME algorithm. Sequence analysis was performed using UPARSE version 2.7.1. Sequences with ≥97% similarity were assigned to the same OTUs. A representative sequence for each OTU was screened for further annotation. For each representative sequence, the GreenGene Database (http://greengenes.lbl.gov/cgi-bin/nph-index.cgi) was used based on the RDP classifier version 2.2 (http://sourceforge.net/projects/rdp-classifier/) algorithm to annotate taxonomic information. Alpha diversity was applied in analyzing the complexity of species diversity for a sample using 4 indices, including Chao1, Shannon, Simpson, and ACE, with QIIME version 1.8.0 and displayed with R version 4.0.3. A principal component analysis (PCA) was used to calculate differences in the bacterial communities’ structure using R version 4.0.3.
2.8. Fatty Acid Profile Measurements
At 60 days old, six calves were selected and slaughtered from each group. Liver and muscle samples were collected and stored at −80 °C before analysis. Lipids in the liver and muscle were extracted according to the procedure of Folch et al. [33]. Fatty acid content was measured as described by Piao et al. [34]. Briefly, fatty acid was identified using an Agilent 7890A gas chromatography system (Agilent Technologies, Palo Alto, CA, USA) equipped with an automatic injector, a CP-sil88 capillary column (100 m × 0.25 mm × 0.2 μm, CP7489, J&W Science, MA, USA) and a hydrogen flame ion detector.
2.9. Statistical Analysis
Data were analyzed using SAS 9.4 software (SAS Institute, Cary, NC, USA). The ADG, DMI, MR DMI, Starter DMI, F:G ratio, fecal score, abnormal fecal day, and serum parameters were analyzed using the MIXED procedure. The effects of treatment, day, and the interaction between treatments and day were included as fixed effects and the calf within treatments as a random effect. The fatty acids intake, liver tissue, and muscle tissue FA profile, rumen fermentation parameters, and rumen chyme activity were analyzed using a one-way ANOVA procedure. The domains among treatments were tested with the Wilcoxon rank-sum test. A p-value of <0.05 and 0.05 ≤ p < 0.10 were considered statistically significant and tendencies, respectively.
3. Results
3.1. Animal Growth Performance and Health
Except for the total DMI being affected by the interaction between treatment and day (p = 0.038; Supplementary Figure S2a), there was no interaction for any variates (p > 0.05). The intake of C6:0, C8:0, C10:0, C12:0, and C14:0 in the CCO group significantly increased (p < 0.05) when compared with those in the CON and PLO groups, while the intake of C14:1, C15:0, C16:0, C16:1, C18:0, C18:1, C18:2, C18:3, C20:0, C22:0, and C24:0 decreased significantly (p < 0.05) compared with the CON group. In addition, the intake of C16:0, C18:1, and C18:2 increased significantly in the PLO group (p < 0.05) when compared with the CON group, but the intake of C6:0, C8:0, C10:0, C12:0, and C14:0 obviously reduced (p < 0.05) compared with the CON and CCO groups (Table 1). The starter intake tended to decrease (p = 0.085) in the PLO group in comparison with the CON and CCO groups. However, the BW, ADG, DMI, and F:G ratio of suckling calves did not differ (p > 0.05) among the three groups. Moreover, the fecal scores and the average days of abnormal feces in the CCO and PLO groups had no significant difference (p > 0.05) compared with the CON group.

Table 1.
Effects of coconut oil or palm oil in MRs on growth performance and health of suckling calves.
3.2. Serum Parameters
The concentrations of BHBA and NEFA were affected by the interaction between treatment and day (p < 0.05; Supplementary Figure S2b,c), whereas these varieties were not influenced by the treatment effects (p > 0.05). The serum concentrations of TC, HDL-C, and LDL-C in the CCO group were higher (p < 0.05) than those in the CON and PLO groups (Table 2), and GLU in the CON group was higher (p < 0.05) in comparison with the PLO group. In addition, the serum level of TG in the CCO group was significantly higher (p < 0.05) when compared with the PLO group.

Table 2.
Effects of coconut oil or palm oil in MRs on serum parameters of suckling calves.
3.3. Rumen Chyme Fermentation Parameters and Chyme Digestive Enzyme Activities
There was no significant difference in the rumen chyme fermentation parameters among the three groups (Supplementary Table S3). The rumen chyme enzyme activities of α-amylase, neutral protease, carboxymethyl cellulose, xylanase, and lipase were also not significantly affected by fatty acid supplementation in MR in suckling calves (Supplementary Table S4).
3.4. Community Structure and Composition of Rumen Microbiota
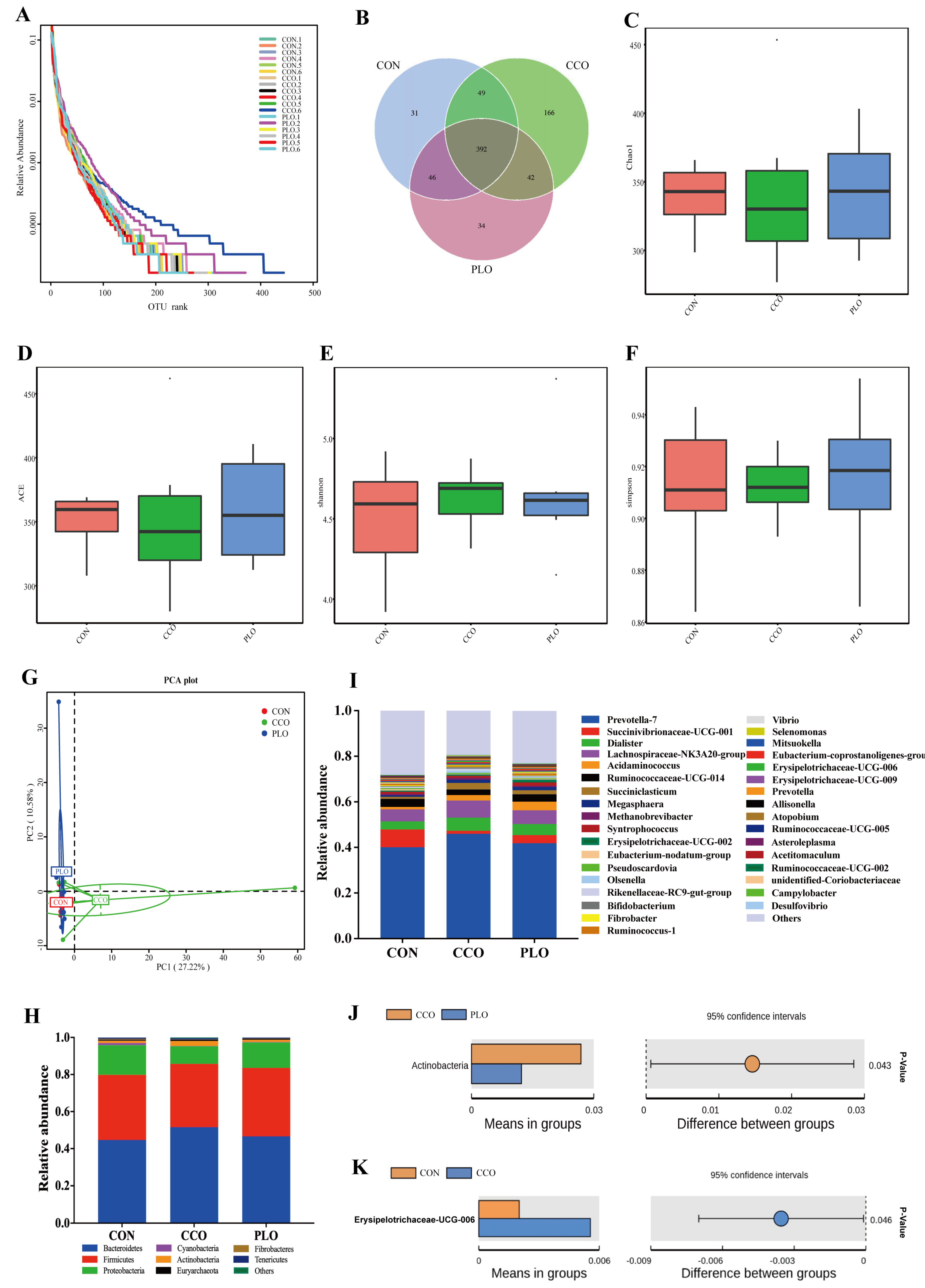
A total of 1,371,853 ± 5646 clean tags were obtained from 18 rumen samples. The stable plateau based on the rank abundance curves of the OUT level suggested that the generated sequenced data were large enough in our study (Figure 1A). A quantity of 760 OUTs was attained based on a 97% of identification rate across all samples. The Venn diagram showed that 392 OUTs were shared among all samples, accounting for 51.5% of total OUTs, particularly, 518 OUTs in the CON group, 649 OUTs in the CCO group, and 514 OUTs in the PLO group (Figure 1B). Alpha diversity indices showed that rumen bacterial community richness (Chao1, AEC; Figure 1C,D) and diversity (Simpson, Shannon; Figure 1E,F) did not show any significant difference (p > 0.05) among the three groups. Similarly, PCA plots did not display obvious distance differences in bacterial structures in the three groups (Figure 1G). At the phyla level, the top five ruminal phyla bacteria were Bacteroidetes (47.5%), Firmicutes (35.4%), Proteobacteria (13.1%), Actinobacteria (1.7%), and Cyanobacteria (0.5%) across all samples, which accounted for greater than 98% of all phyla bacteria (Figure 1H). Actinobacteria in the CCO group had greater (p < 0.05) relative abundance than that in the PLO group (Figure 1J). In addition, at the genus level, the main genera across all samples were Prevotella_7 (42.7%), followed by Succinivibrionaceae_UCG-00, Dialister, Lachnospiraceae_NK3A20_group, Acidaminococcus, Ruminococcaceae_UCG-014, Succiniclasticum, Megasphaera, Methanobrevibacter, Syntrophococcus, which accounted for more than 68% of all genera bacteria (Figure 1I). However, those dominant genera had no significant differences (p > 0.05) among the three groups. Importantly, the relative abundance of Erysipelotrichaceae_UCG-006 was higher (p < 0.05) in the CCO group than that in the CON group (Figure 1K).
Figure 1.
Rank abundance curves based on the OUT levels (A). Venn diagram of bacteria among the three groups (B). Bacterial richness and diversity among groups were estimated using the Chao1 (C), ACE (D), Shannon (E), and Simpson (F) indices. PCA analysis of rumen microtia among the three groups (G). The relative abundance of rumen microbiota at the phyla level (I). The relative abundance of rumen microbiota at the genera level (H). Comparison of microbial domains between CCO and PLO at the phyla level (J), and between CON and CCO at the genera level (K). Significantly different domains were tested with the Wilcoxon rank-sum test with an adjusted p value of <0.05.
3.5. Fatty Acid Profile of Liver and Muscle Tissues
The concentrations of total fatty acid in the liver and longissimus dorsi were not different (p > 0.05) among the three groups (Table 3). In the liver tissue, the proportions of C14:0, C20:4, C24:1, MCFAs, and n-6 PUFAs in the CCO group were higher (p < 0.05) than those in the CON group, while the proportions of C18:1, C18:3, C20:1, C20:5, C22:6, UFAs, and MUFAs in the CCO group were lower than those in the CON group (p < 0.05). In addition, the proportions of C20:4, C24:1, and n-6 PUFAs in the PLO group were significantly higher (p < 0.05) in comparison with the CON group, while the proportions of C14:0, C16:1, C18:3, C20:1, C20:5, C22:6, and n-3 PUFA were lower (p < 0.05) when compared with the CON group; In addition, when compared with the PLO group, the proportions of C14:0, C20:5, and MCFAs in the CCO group were higher (p < 0.05), and the proportions of C24:1 and MUFAs were lower (p < 0.05). In the longissimus dorsi, the proportions of C14:0 and MCFAs in the CCO group were higher (p < 0.05) than those in the CON group, while the proportions of C18:0, C18:3, C20:1, C20:5, UFAs, and n-3 PUFAs in the CON group were higher (p < 0.05). The proportions of C20:4, C24:1, MUFAs, PUFAs, and n-6 PUFAs in the PLO group were higher (p < 0.05) than those in the CON group, while the proportions of C16:1, C17:0, C18:0, C18:3, C20:1, C20:5, and n-3 PUFAs in the CON group were higher (p < 0.05). Moreover, when compared with the PLO group, the proportions of C14:0, C16:1, C20:1, MCFAs, and n-3 PUFAs in the CCO group were higher (p < 0.05), and the proportions of C20:4, C24:1, UFAs, PUFAs, and n-6 PUFAs were lower (p < 0.05).

Table 3.
Effects of coconut oil or palm oil in MRs on liver and longissimus dorsi fatty acid composition of suckling calves.
4. Discussion
Dietary fat is a crucial energy source for young calves in the functions of maintenance energy and growing energy requirements. Suckling calves, unlike mature animals, have about 3% body fat reserves, so excess energy must be provided for them to avoid negative consequences [35]. Previous studies showed that fat content, sources, and fatty acid composition in MRs could influence growth performance, health, gastrointestinal development, and immunity of suckling calves [9,25,29]. For example, Jaster et al. [36] demonstrated that additional fat addition into MRs could lead to an increase in ADG. On the contrary, some studies showed that the increase in fat content was only conducive to fat deposition, but it could cause negative effects on weight gain in suckling calves [37,38,39]. The decline in growth performance might mainly attribute to the increase in flow rates of total protein and fat out of the abomasum due to high fat (fat > 200 g/kg of DM) in MRs, and the decrease in the nutrient digestibility and starters intake [39,40]. Meanwhile, those benefits, including the improvement in the growth performance, development of gastrointestinal tract, immune response, and health in calves, resulting from adding functional fatty acids or essential fatty acids (EFAs) from flax oil [41], soybean oil [42], or porcine lard [43] into MRs, have been demonstrated by some researchers. For example, the supplementation with a blend of butyric acid, MCFAs, and linolenic acid into MRs could improve ADG and feed efficiency and reduce calf diarrhea [29]. In the present study, these parameters, such as growth performance, feed efficiency, and health of calves, did not show a significant difference between the CCO group rich in MCFAs and the PLO group rich in SFAs and MUFAs when compared with the CON group. These results differed from those studies as described above [41,42,43], which might be attributed to those differences in fat content and fatty acid composition. In this study, the intake of EFAs and some functional fatty acids such as butyric acid and α-linolenic acid in the three treatments was lower compared to the dose in other studies [41,42], which partly explains the lack of differences in growth performance and the health of suckling calves. In addition, no negative effects on starter intake were observed at the low-fat (120 g/kg DM) level, which was consistent with the results described by Hill et al. [39]. However, we observed that the PLO group tended to reduce starter intake. As far as we know, there is no relevant report about the reduction in young animals’ feed intake resulting from palm oil. Therefore, further research on the effect of feeding palm oil alone on feed intake is warranted.
The correlation between the concentrations of blood GLU, BHBA, and NEFA and the energy status of calves had been reported [44]. In the present study, the serum concentration of GLU in the PLO group decreased compared with the CCO and CON groups, which might be explained by the starter intake that tended to be lower in the PLO group than in the other groups. In the present study, the serum concentrations of TC, HDL-C, and LDL-C in the CCO group were higher than those in the PLO and CON groups. Compared with the CON group, the intake of SFAs increased in the CCO group (lactic acid 3.35 g/d vs. 30.71 g/d; myristic acid 9.32 g/d vs. 12.53 g/d) or the PLO group (palmitic acid 25.29 g/d vs. 40.86 g/d). However, diets with a high content of SFAs could increase the levels of blood cholesterol [45]. Those comparisons between SFAs diets (from coconut oil, palm oil, or lauric, myristic, and palmitic acid) and other UFAs diets showed that the levels of TC, HDL-C, and LDL-C increased in SFAs diets [16,46]. Similarly, in our study, those blood lipid concentrations were increased in the lauric and myristic acid-enriched CCO group when compared with the CON group. However, the serum concentration of cholesterol in the PLO group was similar to that in the CON group and lower than that in the CCO group, which was contrary to studies on palmitic acid/palm oil showing increasing blood cholesterol [47]. Similar to previous studies [48,49], total serum concentrations of LDL- and HDL-Cs in coconut oil rich in lauric acid increased when compared with palm oil rich in palmitic acid. These results may be related to the difference in fatty acid composition, the effects of lauric acid, palmitic acid, and oleic acid diets on serum total cholesterol concentration, and HDL-C that were sorted in order of lauric acid > palmitic acid > oleic acid [50].
Unlike mature ruminants, the rumen of calves in the first few weeks of life (until 4 weeks of life) has no significant fermentation function due to the esophageal groove reflex and low starter intake during the first 3 weeks of life [51]. For pre-ruminant calves, milk or milk replacer directly enters the abomasum through the esophageal groove reflex, and normally about 3% of milk or milk replacer leaks into the rumen for fermentation [12]. However, several studies indicated that some milk or milk replacer may link or backflow into the reticulorumen in dairy calves (0 to 25%) [13,14] and veal calves (18 to 35%) [15], which may affect the development of rumen fermentation, digestive enzyme activities, and microbiome. In addition, large amounts of milk entering the rumen could increase the risk of abnormal fermentation (acidosis) and clinical diseases (flatulence and diarrhea) in calves. In the present study, we observed that a coconut oil diet and palm oil diet did not affect the rumen fermentation, digestive enzyme activities, or alpha and beta diversity of bacteria. Similarly, previous studies showed that dietary fat sources (palm oil, soybean oil, tallow, flaxseed oil, or mixture oil) [3,52] and fatty acids composition (e.g., lauric acid, palmitic acid, linolenic acid) [11,53] did not affect ruminal pH, ammonia, or total VFAs concentrations in dairy calves. Moreover, palm oil rich in saturated fatty acids is generally used as rumen bypass fat because they do not have negative effects on rumen fermentation compared with unsaturated oils [54]. In contrast, more studies in vitro and in vivo showed that dietary fatty acids could change the rumen fermentation and rumen microorganisms [55,56]. Studies in dairy cows and calves showed that unsaturated fatty acids (e.g., linoleic acid, linolenic acid) have adverse effects on rumen microbiota and its fermentative activity compared with saturated fatty acids (palmitic acid) [57,58] probably due to more toxic of dietary PUFAs to rumen microbiota than SFAs. The PUFAs were converted into UFAs by hydrogenation of rumen microorganisms, and it would inhibit the growth of cellulolytic bacteria and some butyrate-producing bacteria, including the stearic acid producer Clostridium proteoclasticum, Butyrivibrio hungatei, and Eubacterium ruminantium [59]. In contrast, Jasem et al. reported that supplemental α-linolenic acid from flax oil increased the concentrations of total VFAs and proportion of butyrate in dairy calves [41], which could promote rumen epithelial cell proliferation in weaning calves [9]. Furthermore, numerous studies reported that coconut oil or lauric acid could change rumen protozoal numbers, microbial populations, and ruminal fermentation patterns in dairy cows and then affect nutrient digestion and methane emission [60,61,62]. However, Burdick et al. [63] reported that supplementation with MCFAs at 0.063% dietary DM in dairy cows decreases rumen bacterial richness, but it does not affect rumen fermentation. These results above might be related to fat content, fatty acid composition, fat addition methods, dietary nutrient level, rumen maturity, and cattle age. Rumen digestive enzymes (amylase, protease, xylanase, CMCase, and lipases) encoded and secreted by microbiota were associated with starch, fiber, and fatty acid metabolism [64,65,66]. Yabuuchi et al. [66] observed that supplemental coconut oil rich in lauric acid increase amylase activity in vitro rumen fermentation. Hristov et al. [67] observed that supplementation with sodium laurate could reduce CMCase and xylanase activities of ruminal contents in dairy cows compared to the control group. The effects of coconut oil or lauric acid on rumen digestive enzymes may be related to the effect on rumen microorganisms. Liu et al. [68] found that supplementation with glycerol monolaurate or the combination of glycerol monolaurate and tributyrin increased the relative abundance of Actinobacteria and Verrucomicrobia and decreased the relative abundance of Ruminococcus. However, the digestive enzyme activities of the rumen contents did not show differences between coconut oil rich in lauric acid or palm oil rich in palmitic acid and the CON group, which was consistent with the results of rumen microbial diversity and relative abundance. In this study, the Firmicutes, Bacteroidetes, and Proteobacteria dominated the rumen by accounting for ~95% at the phyla level, which was similar to the dominant rumen flora reported by Castro et al. [51] in dairy calves. Interestingly, the relative abundance of Actinobacteria in the CCO group increased at the phyla level compared with the PLO group, and its main function was responsible for the production of a variety of antibiotics, such as streptomycin, oxytetracycline, tetracycline, gentamicin, etc. Moreover, the relative abundance of unclassified members of Erysipelotrichaceae (Erysipelotrichaceae_UCG-006) at the genus level in the CCO group increased compared with the milk fat group. Erysipelotrichaceae in the rumen has been reported to have a strong negative correlation with methane emissions [69]. These results showed that coconut oil has a potential benefit on the development of rumen microflora and reducing methane emissions.
Previous studies showed that the fatty acid esterification and triacylglycerol accumulation in the hepatic cells increase in pre-ruminant calves that are fed MRs containing coconut oil rich in MCFAs compared to those of calves fed beef tallow rich in SFAs and MUFAs [70]. However, some studies observed that calves fed MRs containing coconut oil or lard fat did not show an increase the lipids infiltration in the liver compared with calves fed whole milk [6]. Similarly, the proportion of total fatty acid in the liver and longissimus dorsi of calves in the CCO group or the PLO group did not show significant changes compared with those in the CON group. This might be attributed to the fact that calves were in a cold environment for a long time (supplementary Figure S1) and the dietary protein and fat levels are relatively low in this trail, so calves need a lot of fat oxidation for energy to meet the additional energy requirement. Thus, the effect of coconut oil and palm oil on calf liver deposition under high protein and high-fat conditions need further research. In the present study, we found that coconut oil increased the intake of MCFAs and n-6 PUFAs and decreased the intake of MUFAs and n-3 PUFAs compared with milk fat. Compared with milk fat, palm oil increased the intake of SFAs, MUFAs, and n-6 PUFAs and decreased the intake of MCFAs and n-3 PUFAs. Consistent with this, coconut oil increased the proportion of MCFAs in the longissimus dorsi and the proportion of MCFAs and n-6 PUFAs in the liver and decreased the proportion of UFAs and n-3 PUFAs in the longissimus dorsi and the proportion of UFAs, MUFAs, and n-3 PUFAs in the liver. Palm oil increased the proportion of n-6 PUFAs in the liver and longissimus dorsi and decreased the proportion of C14:0 and n-3 PUFAs in the liver and the proportion of MUFAs and n-3 PUFAs in longissimus dorsi. However, although the percentage of MCFAs in the liver and longissimus dorsi increased in the coconut oil group compared with the milk fat group, the sum of the percentage of MCFAs accounted for little in the liver and longissimus dorsi. The reason might be related to the oxidative rate and metabolic pathway of varying FAs in the liver. Unlike LCFAs, MCFAs could be rapidly absorbed from the gut via a portal vein into the liver, and they are mostly used for oxidation in the liver cells [17,18]. Therefore, the decreased percentage of long-chain saturated and unsaturated FAs might be related to most of the FAs absorbed into the liver being directly oxidized and few FAs acting as substrates for de novo synthesis in the CCO group compared with the CON group. In addition, the dominant fatty acid was stearic (C18:0), followed by oleic (C18:1), linoleic (C18:2), palmitic (C16:0), behenic (C22:0), and arachidonic acid (C20:4) in the liver, and the dominant fatty acid was oleic (C18:1), followed by linoleic (C18:2), palmitic acid (C16:0), stearic (C18:0), arachidonic (C20:4), and behenic acid (C22:0) in the longissimus dorsi. This indicated that the fat source did not significantly change the proportion of major fatty acids in the liver or longissimus dorsi. As far as we know, there has been no study to report that dietary coconut oil changes the percentage of FAs in the liver or longissimus dorsi of suckling calves. Thus, a future study is warranted to elucidate the mechanisms of coconut oil or palm oil causing the difference in fatty acid composition in the liver and longissimus dorsi.
5. Conclusions
Coconut oil or palm oil replacements for milk fat in MRs had no effects on growth, health, rumen fermentation and enzyme activity, or rumen microbial abundance and diversity. Compared with milk fat, palm oil decreased blood glucose concentration, and coconut oil increased some blood lipid concentrations. Moreover, coconut oil and palm oil had no effects on the level of total fatty acids but significantly changed some proportions of MCFAs and PUFAs in the liver and longissimus dorsi in suckling calves.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11030655/s1, Supplementary Table S1: Nutrition levels and fatty acid composition of milk replacers; Table S2: The ingredient composition and nutrient profile of the starter; Table S3: Effect of medium-chain fatty acids and palmitic acid supplementation on fermentation parameters of suckling calves; Table S4: Effect of medium-chain fatty acids and palmitic acid supplementation on enzyme activity of suckling calves (per gram of true protein); Figure S1: The maximum (Max), average (Ave), and minimum (Min) ambient temperature the daily during the trial.
Author Contributions
Y.T. contributed to the design of this study and project management. F.H. conducted the animal experiments, statistical analysis, and wrote the manuscript. M.P. and Q.D. revised the manuscript. C.Y. collected biological materials. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Beijing Innovation Consortium of Livestock Research System (BAIC05) and the Agricultural Science and Technology Innovation Program (CAAS- ASTIP-2017-FRI-04).
Institutional Review Board Statement
The experiment design and animal care and handling procedures were evaluated and approved by the Animal Ethics Committee of Institute of Feed Research of the Chinese Academy of Agricultural Sciences (IFR-CAAS-20160715, Beijing, China).
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author (Y.T.) on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santos, H.O.; Howell, S.; Earnest, C.P.; Teixeira, F.J. Coconut oil intake and its effects on the cardiometabolic profile—A structured literature review. Prog. Cardiovasc Dis. 2019, 62, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Soyeurt, H.; Dehareng, F.; Gengler, N.; McParland, S.; Wall, E.; Berry, D.P.; Coffey, M.; Dardenne, P. Mid-infrared prediction of bovine milk fatty acids across multiple breeds, production systems, and countries. J. Dairy Sci. 2011, 94, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, E.; Azad-Shahraki, M.; Khorvash, M. Effect of different fat supplements on performance of dairy calves during cold season. J. Dairy Sci. 2017, 100, 5319–5328. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, V.; Nonnecke, B.J.; Horst, R.L. Effects of replacement of native fat in colostrum and milk with coconut oil on fat-soluble vitamins in serum and immune function in calves. J. Dairy Sci. 1997, 80, 2380–2390. [Google Scholar] [CrossRef]
- Huuskonen, A.; Khalili, H.; Kiljala, J.; Joki-Tokola, E.; Nousiainen, J. Effects of vegetable fats versus lard in milk replacers on feed intake, digestibility, and growth in Finnish Ayrshire bull calves. J. Dairy Sci. 2005, 88, 3575–3581. [Google Scholar] [CrossRef]
- Mills, J.K.; Ross, D.A.; Van Amburgh, M.E. The effects of feeding medium-chain triglycerides on the growth, insulin responsiveness, and body composition of Holstein calves from birth to 85 kg of body weight. J. Dairy Sci. 2010, 93, 4262–4273. [Google Scholar] [CrossRef]
- Bowen Yoho, W.S.; Swank, V.A.; Eastridge, M.L.; O’Diam, K.M.; Daniels, K.M. Jersey calf performance in response to high-protein, high-fat liquid feeds with varied fatty acid profiles: Intake and performance. J. Dairy Sci. 2013, 96, 2494–2506. [Google Scholar] [CrossRef]
- Panahiha, P.; Mirzaei-Alamouti, H.; Kazemi-Bonchenari, M.; Aschenbach, J.R. Growth performance, nutrient digestibility, and ruminal fermentation of dairy calves fed starter diets with alfalfa hay versus corn silage as forage and soybean oil versus palm fatty acids as fat source. J. Dairy Sci. 2022, 105, 9597–9609. [Google Scholar] [CrossRef]
- Ragionieri, L.; Cacchioli, A.; Ravanetti, F.; Botti, M.; Ivanovska, A.; Panu, R.; Righi, F.; Quarantelli, A.; Gazza, F. Effect of the supplementation with a blend containing short and medium chain fatty acid monoglycerides in milk replacer on rumen papillae development in weaning calves. Ann. Anat. 2016, 207, 97–108. [Google Scholar] [CrossRef]
- Gorka, P.; Kowalski, Z.M.; Pietrzak, P.; Kotunia, A.; Jagusiak, W.; Holst, J.J.; Guilloteau, P.; Zabielski, R. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J. Dairy Sci. 2011, 94, 5578–5588. [Google Scholar] [CrossRef]
- Kadkhoday, A.; Riasi, A.; Alikhani, M.; Dehghan-Banadaky, M.; Kowsar, R. Effects of fat sources and dietary C-18:2 to C-18:3 fatty acids ratio on growth performance, ruminal fermentation and some blood components of Holstein calves. Livest. Sci. 2017, 204, 71–77. [Google Scholar] [CrossRef]
- Tadeu Dos Santos, G.; Toullec, R.; Roger, R.; de la Grange, H.; Guilloteau, P. Caractéristiques digestives des veaux de boucherie s’adaptant mal en atelier d’engraissement. Reprod. Nutr. Développement 1986, 26, 1217. [Google Scholar] [CrossRef]
- Abe, M.; Iriki, T.; Kondoh, K.; Shibui, H. Effects of nipple or bucket feeding of milk-substitute on rumen by-pass and on rate of passage in calves. Br. J. Nutr. 1979, 41, 175–181. [Google Scholar] [CrossRef]
- Guilhermet, R.; Mathieu, C.M.; Toullec, R.; Beaufils, M.; Mansion, M.; Manis, Y. Transit Des Aliments Liquides Au Niveau De La GouttiÈre Œsophagienne Chez Le Veau PrÉruminant Et Ruminant. Ann. De Zootech. 1975, 24, 69–79. [Google Scholar] [CrossRef]
- Berends, H.; van den Borne, J.J.; Stockhofe-Zurwieden, N.; Gilbert, M.S.; Zandstra, T.; Pellikaan, W.F.; van Reenen, C.G.; Bokkers, E.A.; Gerrits, W.J. Effects of solid feed level and roughage-to-concentrate ratio on ruminal drinking and passage kinetics of milk replacer, concentrates, and roughage in veal calves. J. Dairy Sci. 2015, 98, 5621–5629. [Google Scholar] [CrossRef]
- Fattore, E.; Fanelli, R. Palm oil and palmitic acid: A review on cardiovascular effects and carcinogenicity. Int J Food Sci Nutr 2013, 64, 648–659. [Google Scholar] [CrossRef]
- Marten, B.; Pfeuffer, M.; Schrezenmeir, J. Medium-chain triglycerides. Int. Dairy J. 2006, 16, 1374–1382. [Google Scholar] [CrossRef]
- Guillot, E.; Vaugelade, P.; Lemarchal, P.; Rerat, A. Intestinal absorption and liver uptake of medium-chain fatty acids in non-anaesthetized pigs. Br. J. Nutr. 1993, 69, 431–442. [Google Scholar] [CrossRef]
- Erwin, E.S.; Sterner, W. Dietary Lipids and Fatty Acid Deposition in Various Calf Tissues. Am. J. Physiol. 1963, 205, 1151–1153. [Google Scholar] [CrossRef]
- Jenkins, K.J.; Kramer, J.K.G. Differential effects of dietary fatty acids on fatty acid composition of phosphotidylinositol in calf tissues. Nutr. Res. 1991, 11, 177–183. [Google Scholar] [CrossRef]
- Jenkins, K.J.; Kramer, J.K. Effects of dietary corn oil and fish oil concentrate on lipid composition of calf tissues. J. Dairy Sci. 1990, 73, 2940–2951. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Greco, L.F.; Lock, A.L.; Block, E.; Santos, J.E.P.; Thatcher, W.W.; Staples, C.R. Supplementation of essential fatty acids to Holstein calves during late uterine life and first month of life alters hepatic fatty acid profile and gene expression. J. Dairy Sci. 2016, 99, 7085–7101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, W.-B.; Bi, Y.-l.; Tu, Y.; Beckers, Y.; Du, H.-C.; Diao, Q.-Y. Early Feeding Regime of Waste Milk, Milk, and Milk Replacer for Calves Has Different Effects on Rumen Fermentation and the Bacterial Community. Animals 2019, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Young, B.A. Cold Stress as it Affects Animal Production. J. Anim. Sci. 1981, 52, 154–163. [Google Scholar] [CrossRef]
- Litherland, N.B.; Da Silva, D.N.; LaBerge, R.J.; Schefers, J.; Kertz, A. Supplemental fat for dairy calves during mild cold stress. J. Dairy Sci. 2014, 97, 2980–2989. [Google Scholar] [CrossRef]
- 26. A.I. Official Methods of Analysis, 18th ed.; AOAC Int.: Washington, DC, USA, 2006.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Braundmeier-Fleming, A.G.; Skenandore, C.S.; Gil, L.; Jacobsen, V.; Cregger, M.; Badger, T.; Karr, M.; Wu, G.; Smith, S.B.; Newell-Fugate, A.E. Dietary substitution of soybean oil with coconut oil in the absence of dietary antibiotics supports growth performance and immune function in nursery and grower pigs. J. Anim. Sci. Biotechnol. 2020, 11, 27. [Google Scholar] [CrossRef]
- Hill, T.M.; Vandehaar, M.J.; Sordillo, L.M.; Catherman, D.R.; Bateman, H.G., 2nd; Schlotterbeck, R.L. Fatty acid intake alters growth and immunity in milk-fed calves. J. Dairy Sci. 2011, 94, 3936–3948. [Google Scholar] [CrossRef]
- Paz, H.A.; Anderson, C.L.; Muller, M.J.; Kononoff, P.J.; Fernando, S.C. Rumen Bacterial Community Composition in Holstein and Jersey Cows Is Different under Same Dietary Condition and Is Not Affected by Sampling Method. Front Microbiol. 2016, 7, 1206. [Google Scholar] [CrossRef]
- Wang, S.; Ma, T.; Zhao, G.; Zhang, N.; Tu, Y.; Li, F.; Cui, K.; Bi, Y.; Ding, H.; Diao, Q. Effect of Age and Weaning on Growth Performance, Rumen Fermentation, and Serum Parameters in Lambs Fed Starter with Limited Ewe-Lamb Interaction. Animals 2019, 9, 825. [Google Scholar] [CrossRef]
- Wang, B.; Yang, C.T.; Diao, Q.Y.; Tu, Y. The influence of mulberry leaf flavonoids and Candida tropicalis on antioxidant function and gastrointestinal development of preweaning calves challenged with Escherichia coli O141:K99. J. Dairy Sci. 2018, 101, 6098–6108. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Piao, M.Y.; HU, F.M.; Kong, F.L.; Liu, Y.L.; Wang, S.; Cui, K.; Sun, T.; Diao, Q.Y.; Tu, Y. Effects of dietary amylose to amylopectin ratio on growth performance, carcass quality characteristics and meat fatty acids in Chinese Qinchuan cattle. J. Integr. Agric. 2021, 20, 14. [Google Scholar] [CrossRef]
- Davis, C.L.; Drackley, J.K. The Development, Nutrition, and Management of the Young Calf; Iowa State University Press: Ames, IA, USA, 1998; p. 339. [Google Scholar]
- Jaster, E.H.; McCoy, G.C.; Spanski, N.; Tomkins, T. Effect of Extra Energy as Fat or Milk Replacer Solids in Diets of Young Dairy Calves on Growth During Cold Weather. J. Dairy Sci. 1992, 75, 2524–2531. [Google Scholar] [CrossRef]
- Bascom, S.A.; James, R.E.; McGilliard, M.L.; Van Amburgh, M. Influence of dietary fat and protein on body composition of Jersey bull calves. J. Dairy Sci. 2007, 90, 5600–5609. [Google Scholar] [CrossRef]
- Hill, S.R.; Knowlton, K.F.; Daniels, K.M.; James, R.E.; Pearson, R.E.; Capuco, A.V.; Akers, R.M. Effects of milk replacer composition on growth, body composition, and nutrient excretion in preweaned Holstein heifers. J. Dairy Sci. 2008, 91, 3145–3155. [Google Scholar] [CrossRef]
- Hill, T.M.; Bateman, H.G., 2nd; Aldrich, J.M.; Schlotterbeck, R.L. Effects of fat concentration of a high-protein milk replacer on calf performance. J. Dairy Sci. 2009, 92, 5147–5153. [Google Scholar] [CrossRef]
- Gaudreau, J.M.; Brisson, G.J. Abomasum Emptying in Dairy Calves Fed Milk Replacers with Varying Fat and Sources of Protein. J. Dairy Sci. 1980, 63, 426–440. [Google Scholar] [CrossRef]
- Jasem, M.K.; Fattahnia, F.; Mohammadi, Y.; Shokri, A.; Khalilvandi-Behroozyar, H.; Kazemi-Bonchenari, M. Effects of n-3 fatty acid supplementation from flax oil on growth performance, ruminal fermentation, and immune response in Holstein dairy calves fed either coarsely ground or steam-flaked corn grain. Anim. Feed. Sci. Technol. 2022, 290, 115372. [Google Scholar] [CrossRef]
- Garcia, M.; Shin, J.H.; Schlaefli, A.; Greco, L.F.; Maunsell, F.P.; Thatcher, W.W.; Santos, J.E.; Staples, C.R. Increasing intake of essential fatty acids from milk replacer benefits performance, immune responses, and health of preweaned Holstein calves. J. Dairy Sci. 2015, 98, 458–477. [Google Scholar] [CrossRef]
- Garcia, M.; Greco, L.F.; Favoreto, M.G.; Marsola, R.S.; Wang, D.; Shin, J.H.; Block, E.; Thatcher, W.W.; Santos, J.E.; Staples, C.R. Effect of supplementing essential fatty acids to pregnant nonlactating Holstein cows and their preweaned calves on calf performance, immune response, and health. J. Dairy Sci. 2014, 97, 5045–5064. [Google Scholar] [CrossRef] [PubMed]
- Klopp, R.N.; Hernandez Franco, J.F.; Hogenesch, H.; Dennis, T.S.; Cowles, K.E.; Boerman, J.P. Effect of medium-chain fatty acids on growth, health, and immune response of dairy calves. J. Dairy Sci. 2022, 105, 7738–7749. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, W.M.M.; Jacobs, D.R.; Bloemberg, B.P.M.; Kromhout, D.; Menotti, A.; Aravanis, C.; Blackburn, H.; Buzina, R.; Dontas, A.S.; Fidanza, F.; et al. Serum Total Cholesterol and Long-term Coronary Heart Disease Mortality in Different Cultures: Twenty-five—Year Follow-up of the Seven Countries Study. JAMA 1995, 274, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Durand, D.; Bauchart, D.; Picherit, C.; Gruffat, D.; Graulet, B. Effects of dietary coconut oil on the density distribution and the chemical composition of plasma lipoproteins in the preruminant calf. Reprod. Nutr. Dev. 1998, 38, 205. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, D.; Yang, Y.; Zhang, L. Effect of palm oil consumption on plasma lipid concentrations related to cardiovascular disease: A systematic review and meta-analysis. Asia Pac. J. Clin. Nutr. 2019, 28, 495–506. [Google Scholar] [CrossRef]
- Ng, T.K.; Hassan, K.; Lim, J.B.; Lye, M.S.; Ishak, R. Nonhypercholesterolemic effects of a palm-oil diet in Malaysian volunteers. Am. J. Clin. Nutr. 1991, 53, 1015S–1020S. [Google Scholar] [CrossRef]
- Tholstrup, T.; Marckmann, P.; Jespersen, J.; Sandstrom, B. Fat high in stearic acid favorably affects blood lipids and factor VII coagulant activity in comparison with fats high in palmitic acid or high in myristic and lauric acids. Am. J. Clin. Nutr. 1994, 59, 371–377. [Google Scholar] [CrossRef]
- Temme, E.H.; Mensink, R.P.; Hornstra, G. Comparison of the effects of diets enriched in lauric, palmitic, or oleic acids on serum lipids and lipoproteins in healthy women and men. Am. J. Clin. Nutr. 1996, 63, 897–903. [Google Scholar] [CrossRef]
- Castro, J.J.; Gomez, A.; White, B.; Loften, J.R.; Drackley, J.K. Changes in the intestinal bacterial community, short-chain fatty acid profile, and intestinal development of preweaned Holstein calves. 2. Effects of gastrointestinal site and age. J. Dairy Sci. 2016, 99, 9703–9715. [Google Scholar] [CrossRef]
- Jolazadeh, A.R.; Mohammadabadi, T.; Dehghan-Banadaky, M.; Chaji, M.; Garcia, M. Effect of supplementation fat during the last 3 weeks of uterine life and the preweaning period on performance, ruminal fermentation, blood metabolites, passive immunity and health of the newborn calf. Br. J. Nutr. 2019, 122, 1346–1358. [Google Scholar] [CrossRef]
- Yabuuchi, Y.; Tani, M.; Matsushita, Y.; Otsuka, H.; Kobayashi, Y. Effects of lauric acid on physical, chemical and microbial characteristics in the rumen of steers on a high grain diet. Anim. Sci. J. 2007, 78, 387–394. [Google Scholar] [CrossRef]
- Jenkins, T.C. Lipid metabolism in the rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar] [CrossRef]
- Liu, S.J.; Bu, D.P.; Wang, J.Q.; Liu, L.; Liang, S.; Wei, H.Y.; Zhou, L.Y.; Li, D.; Loor, J.J. Effect of incremental levels of fish oil supplementation on specific bacterial populations in bovine ruminal fluid. J. Anim. Physiol. Anim. Nutr. 2012, 96, 9–16. [Google Scholar] [CrossRef]
- Yang, Z.T.; Liu, S.Y.; Xie, T.; Wang, Q.Q.; Wang, Z.H.; Yang, H.J.; Li, S.L.; Wang, W. Effect of Unsaturated Fatty Acid Ratio In Vitro on Rumen Fermentation, Methane Concentration, and Microbial Profile. Fermentation 2022, 8, 540. [Google Scholar] [CrossRef]
- Van Gastelen, S.; Visker, M.; Edwards, J.E.; Antunes-Fernandes, E.C.; Hettinga, K.A.; Alferink, S.J.J.; Hendriks, W.H.; Bovenhuis, H.; Smidt, H.; Dijkstra, J. Linseed oil and DGAT1 K232A polymorphism: Effects on methane emission, energy and nitrogen metabolism, lactation performance, ruminal fermentation, and rumen microbial composition of Holstein-Friesian cows. J. Dairy Sci. 2017, 100, 8939–8957. [Google Scholar] [CrossRef]
- Karimi, A.; Alijoo, Y.A.; Kazemi-Bonchenari, M.; Mirzaei, M.; Sadri, H. Effects of supplemental fat sources and forage feeding levels on growth performance, nutrient digestibility, ruminal fermentation, and nitrogen utilization in dairy calves. Animal 2021, 15, 100179. [Google Scholar] [CrossRef]
- Maia, M.R.G.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2007, 91, 303–314. [Google Scholar] [CrossRef]
- Yanza, Y.R.; Szumacher-Strabel, M.; Jayanegara, A.; Kasenta, A.M.; Gao, M.; Huang, H.; Patra, A.K.; Warzych, E.; Cieslak, A. The effects of dietary medium-chain fatty acids on ruminal methanogenesis and fermentation in vitro and in vivo: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2021, 105, 874–889. [Google Scholar] [CrossRef]
- Parra, A.K. The effect of dietary fats on methane emissions, and its other effects on digestibility, rumen fermentation and lactation performance in cattle: A meta-analysis. Livest. Sci. 2013, 155, 244–254. [Google Scholar] [CrossRef]
- Klop, G.; van Laar-van Schuppen, S.; Pellikaan, W.F.; Hendriks, W.H.; Bannink, A.; Dijkstra, J. Changes in in vitro gas and methane production from rumen fluid from dairy cows during adaptation to feed additives in vivo. Animal 2017, 11, 591–599. [Google Scholar] [CrossRef]
- Burdick, M.; Zhou, M.; Guan, L.L.; Oba, M. Effects of medium-chain fatty acid supplementation on performance and rumen fermentation of lactating Holstein dairy cows. Animal 2022, 16, 100491. [Google Scholar] [CrossRef] [PubMed]
- Arriola, K.G.; Oliveira, A.S.; Ma, Z.X.; Lean, I.J.; Giurcanu, M.C.; Adesogan, A.T. A meta-analysis on the effect of dietary application of exogenous fibrolytic enzymes on the performance of dairy cows. J. Dairy Sci. 2017, 100, 4513–4527. [Google Scholar] [CrossRef] [PubMed]
- Prive, F.; Newbold, C.J.; Kaderbhai, N.N.; Girdwood, S.G.; Golyshina, O.V.; Golyshin, P.N.; Scollan, N.D.; Huws, S.A. Isolation and characterization of novel lipases/esterases from a bovine rumen metagenome. Appl. Microbiol. Biotechnol. 2015, 99, 5475–5485. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, Y.; Matsushita, Y.; Otsuka, H.; Fukamachi, K.; Kobayashi, Y. Effects of supplemental lauric acid-rich oils in high-grain diet on in vitro rumen fermentation. Anim. Sci. J. 2006, 77, 300–307. [Google Scholar] [CrossRef]
- Hristov, A.N.; Grandeen, K.L.; Ropp, J.K.; McGuire, M.A. Effect of sodium laurate on ruminal fermentation and utilization of ruminal ammonia nitrogen for milk protein synthesis in dairy cows. J. Dairy Sci. 2004, 87, 1820–1831. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.Z.; Zhang, Y.L.; Li, X.L.; Jiang, X.R.; Ding, H.B. Effects of Dietary Supplementation with Glycerol Monolaurate (GML) or the Combination of GML and Tributyrin on Growth Performance and Rumen Microbiome of Weaned Lambs. Animals 2022, 12, 1309. [Google Scholar] [CrossRef]
- Granja-Salcedo, Y.T.; Fernandes, R.M.; de Araujo, R.C.; Kishi, L.T.; Berchielli, T.T.; de Resende, F.D.; Berndt, A.; Siqueira, G.R. Long-Term Encapsulated Nitrate Supplementation Modulates Rumen Microbial Diversity and Rumen Fermentation to Reduce Methane Emission in Grazing Steers. Front Microbiol. 2019, 10, 614. [Google Scholar] [CrossRef]
- Graulet, B.; Gruffat-Mouty, D.; Durand, D.; Bauchart, D. Effects of milk diets containing beef tallow or coconut oil on the fatty acid metabolism of liver slices from preruminant calves. Br. J. Nutr. 2007, 84, 309–318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).