In Vitro Anti-Candida albicans Mode of Action of Enterococcus mundtii and Enterococcus faecium
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Screening of LAB for Anti-Candida Activity
2.3. Purification of the Antimicrobial Metabolites (BLIS) Produced by the Selected LAB Strains
2.4. Effect of Proteinase K on the Activity of Antimicrobial Metabolites (BLIS) Produced by the Selected LAB
2.5. Evaluation of the Hemolytic Activity of the Selected LAB
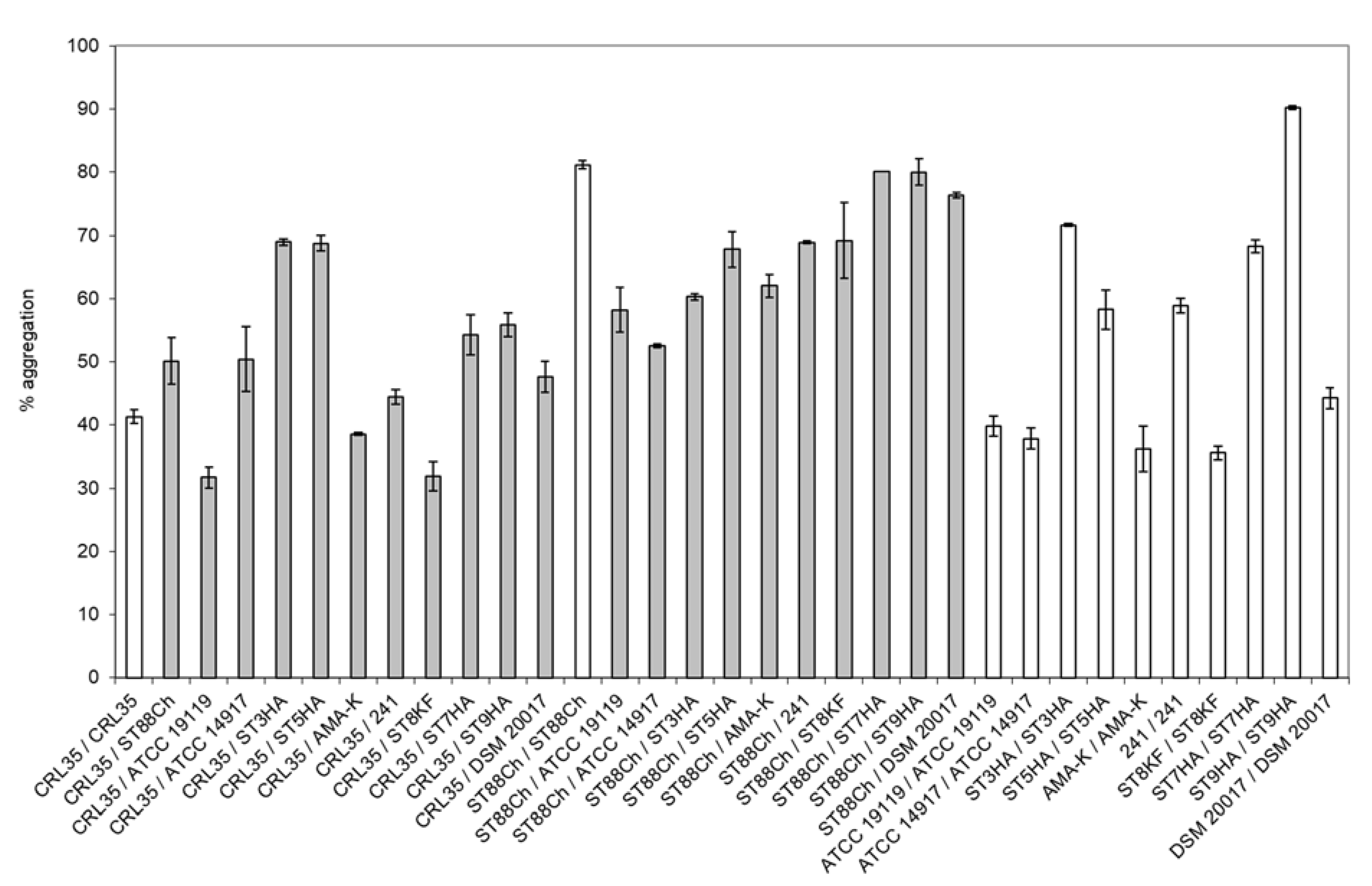
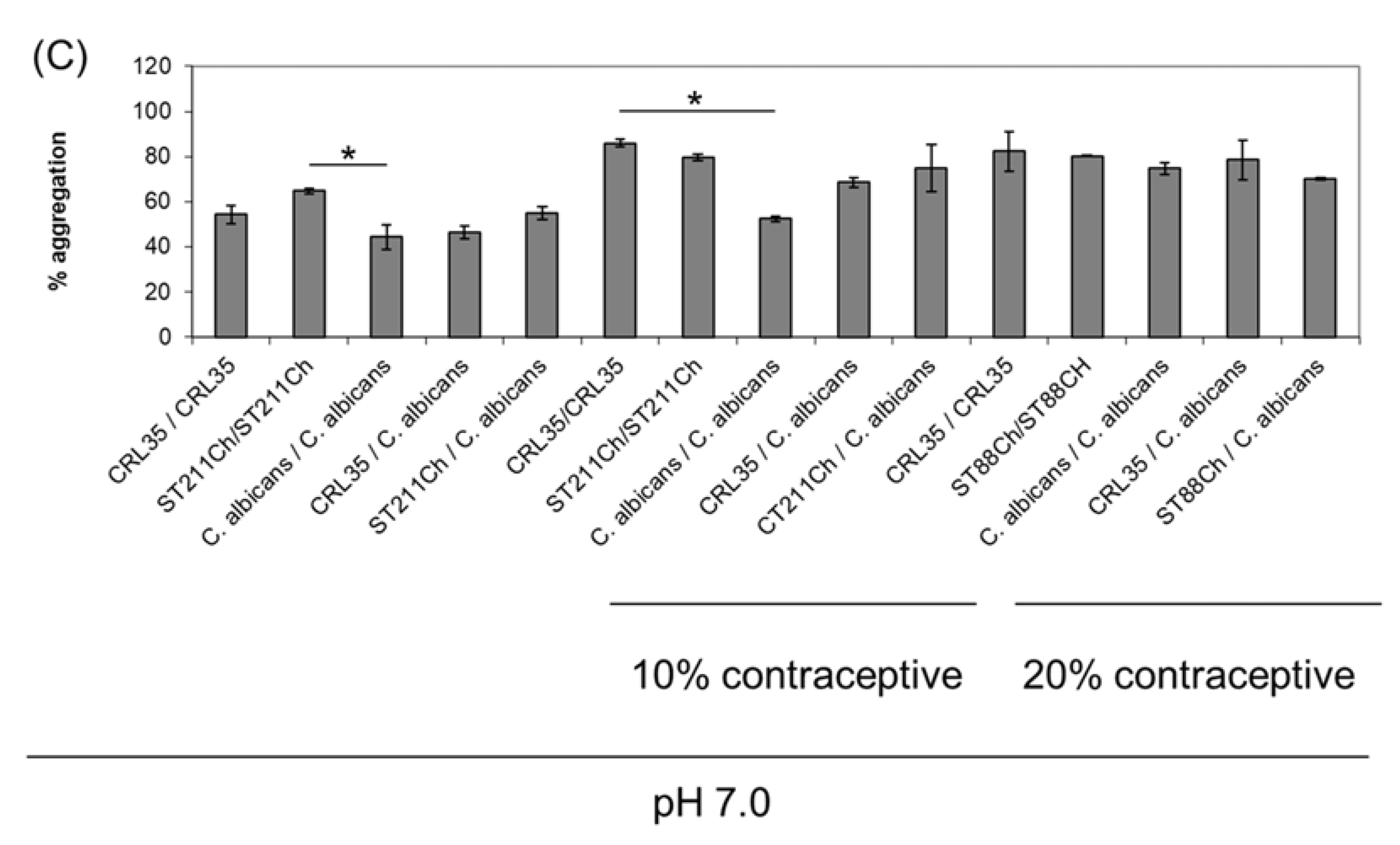
2.6. Aggregation Properties of E. mundtii CRL35 and E. faecium ST88Ch
2.7. Adsorption of BLIS to C. albicans 1281
Effect of the Contraceptive Nordette-28 on Adsorption of the Studied BLIS to C. albicans 1281
2.8. Effect of Antibiotics and Generic Drugs, including Selected Contraceptives, on the Growth of E. mundtii CRL35 and E. faecium ST88Ch
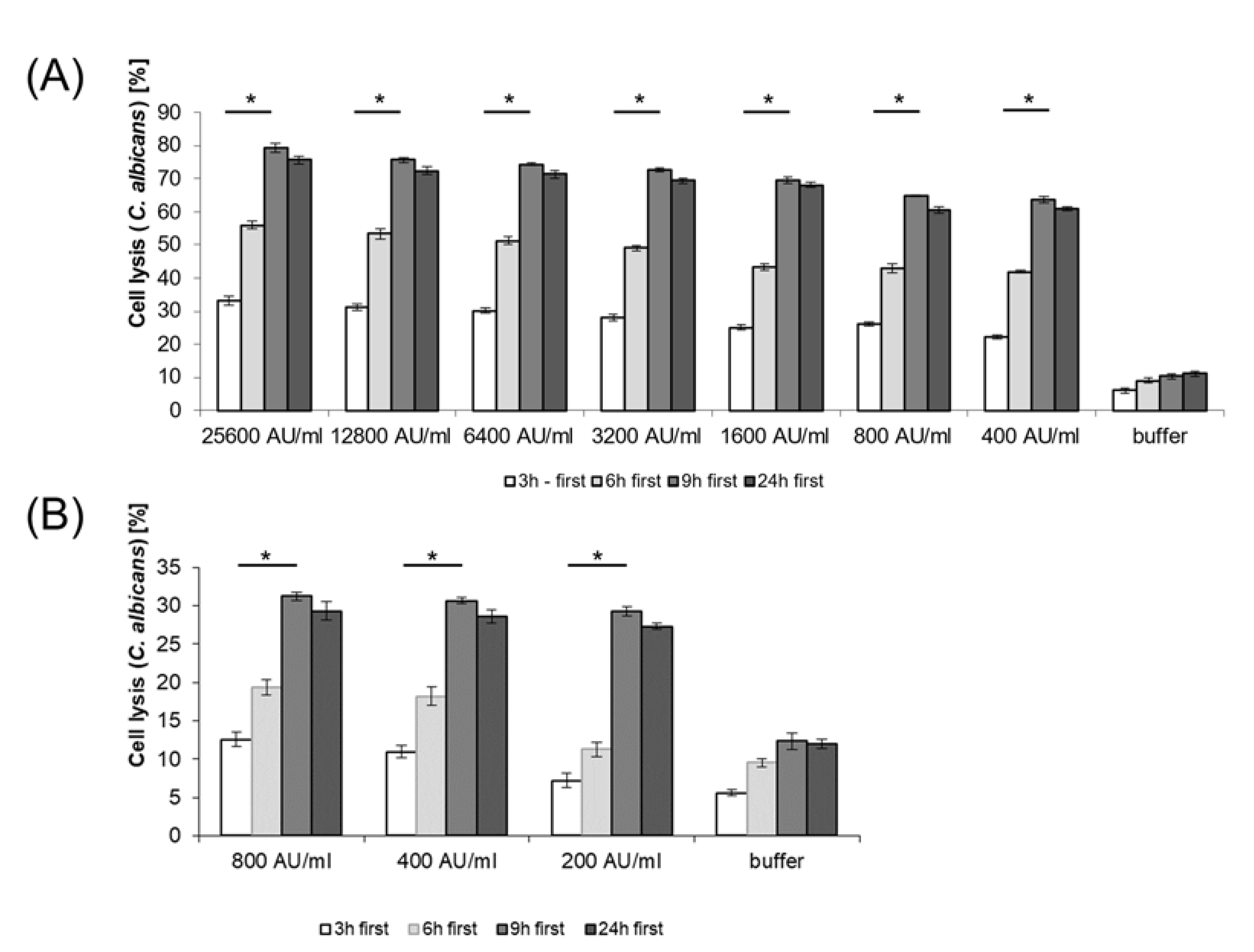
2.9. Cell Lysis of C. albicans by BLIS from E. mundtii CRL35 and E. faecium ST88Ch
2.10. Statistical Analysis
3. Results
3.1. Screening of LAB for Anti-Candida Activity
3.2. Hemolytic Activity
3.3. Aggregation Properties
3.4. Adsorption of BLIS to C. albicans
3.5. Effect of Antibiotics and Other Medicaments, including Selected Contraceptives, on the Growth of E. mundtii CRL35 and E. faecium ST88Ch
3.6. Cell Lysis of C. albicans by BLIS from E. mundtii CRL35 and E. faecium ST88Ch
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Álvarez-Artero, E.; Campo-Nuñez, A.; García-García, I.; García-Bravo, M.; Cores-Calvo, O.; Galindo-Pérez, I.; Pendones-Ulerio, J.; López-Bernus, A.; Belhassen-García, M.; Pardo-Lledías, J. Urinary tract infection caused by Enterococcus spp.: Risk factors and mortality. An observational study. Rev. Clin. Esp. 2021, 221, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulos, X.; Sakkas, H.; Ragos, V.; Tsiambas, E.; Bozidis, P.M.; Evangelou, A.; Papadopoulou, C.; Petrogiannopoulos, L.; Sofikitis, N. Impact of enterococcal urinary tract infections in immunocompromised - neoplastic patients. J. Buon. 2019, 24, 1768–1775. [Google Scholar] [PubMed]
- Li, M.; Yang, F.; Lu, Y.; Huang, W. Identification of Enterococcus faecalis in a patient with urinary-tract infection based on metagenomic next-generation sequencing: A case report. BMC Infect. Dis. 2020, 20, 467. [Google Scholar] [CrossRef] [PubMed]
- Lerma, A.F.; Palomar, M.; Insausti, J.; Olaechea, P.; Alcala, M.A.; Blanco, A. Enterococcal infections in critically ill patients admitted at ICU. Med. Clin. 2003, 13, 281–286. [Google Scholar] [CrossRef]
- Sobel, J.D. Candidal vulvovaginitis. Clin. Obstet. Gynaecol. 1993, 36, 153–165. [Google Scholar] [CrossRef]
- Reid, G.; Bruce, A.W. Urogenital infections in women: Can probiotics help? Post. Grad. Med. J. 2003, 79, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J. Vaginal candidosis: Epidemiology and etiological factors. Int. J. Gynaecol. Obstet. 2000, 71, 21–27. [Google Scholar] [CrossRef]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Dis. Mon. 2003, 49, 53–70. [Google Scholar] [CrossRef]
- Jung, W.W.; Chun, T.; Sul, D.; Hwang, K.W.; Kang, H.S.; Lee, D.J.; Han, I.K. Strategies against human papillomavirus infection and cervical cancer. J. Microbiol. 2004, 42, 255–266. [Google Scholar] [PubMed]
- Parratt, J.R.; Hay, D.P. Sexually transmitted infections. Curr. Obstet. Gynaecol. 2003, 13, 224–231. [Google Scholar] [CrossRef]
- Rhoton-Vlasak, A. Infections and infertility. Prim. Care Update. Ob./Gyns. 2000, 7, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Chaiworapongsa, T.; Kuivaniemi, H.; Tromp, G. Bacterial vaginosis, the inflammatory response and the risk of preterm birth: A role for genetic epidemiology in the prevention of preterm birth. Am. J. Obstet. Gynaecol. 2004, 190, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Greiner, L.L.; Edwards, J.L.; Shao, J.; Rabinak, C.; Entz, D.; Apicella, M.A. Biofilm formation by Neisseria gonorrhoeae. Infect. Immun. 2005, 73, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Zhou, G.; Munyon, R.; Ghannoum, M.A. Candida biofilm: A well-designed protected environment. Med. Mycol. 2005, 43, 191–208. [Google Scholar] [CrossRef]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Ladhoff, A.; Swidsinski, S.; Hale, L.P.; Lochs, H. Adherent biofilms in bacterial vaginosis. Obstet. Gynaecol. 2005, 106, 1013–1023. [Google Scholar] [CrossRef]
- Tendolkar, P.M.; Baghdayan, A.S.; Shankar, N. The N-terminal domain of enterococcal surface protein, Esp, is sufficient for Esp-mediated biofilm enhancement in Enterococcus faecalis. J. Bacteriol. 2005, 187, 6213–6222. [Google Scholar] [CrossRef]
- Singh, A.; Verma, R.; Murari, A.; Agrawal, A. Oral candidiasis: An overview. J. Oral Maxillofac. Pathol. 2014, 18 (Suppl. 1), S81–S85. [Google Scholar] [CrossRef]
- Mbizvo, E.M.; Msuya, S.E.; Stray-Pedersen, B.; Sundby, J.; Chirenje, M.Z.; Hussain, A. HIV seroprevalence and its associations with the other reproductive tract infections in asymptomatic women in Harare, Zimbabwe. Int. J. STD AIDS 2001, 12, 524–531. [Google Scholar] [CrossRef]
- van De Wijgert, J.H.; Mason, P.R.; Gwanzura, L.; Mbizvo, M.T.; Chirenje, Z.M.; Iliff, V.; Shiboski, S.; Padian, N.S. Intravaginal practices, vaginal flora disturbances, and acquisition of sexually transmitted diseases in Zimbabwean women. J. Infect. Dis. 2000, 181, 587–594. [Google Scholar] [CrossRef]
- Eschenbach, D.A.; Davick, P.R.; Williams, B.L.; Klebanoff, S.J.; Young-Smith, K.; Critchlow, C.M.; Holmes, K.K. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J. Clin. Microbiol. 1989, 27, 251–256. [Google Scholar] [CrossRef]
- Klebanoff, S.J.; Hillier, S.L.; Eschenbach, D.; Waltersdorph, A.M. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J. Infect. Dis. 1991, 164, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Petterson, M.; Mardh, P.A. Antibiosis between bacteria from the vagina of women with and without signs of bacterial vaginosis. APMIS 1991, 99, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Stapleton, A.E.; Hooton, T.M.; Roberts, P.L.; Fennell, C.L.; Stamm, W.E. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J. Infect. Dis. 1998, 178, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.L.; Kato, N.; Matsumiya, Y.; Liu, C.X.; Kato, H.; Watanabe, K. Identification of and hydrogen peroxide production by fecal and vaginal lactobacilli isolated from Japanese women and newborn infants. J. Clin. Microbiol. 1999, 37, 3062–3064. [Google Scholar] [CrossRef]
- Hoesl, C.E.; Altwein, J.E. The probiotic approach: An alternative treatment option in urology. Eur. Urol. 2005, 47, 288–296. [Google Scholar] [CrossRef]
- Fugaban, J.I.I.; Holzapfel, W.H.; Todorov, S.D. The overview of natural by-products of beneficial lactic acid bacteria as promising antimicrobial agents. Appl. Food Biotechnol. 2022, 9, 127–143. [Google Scholar] [CrossRef]
- Donders, G.; Bellen, G.; Janssens, D.; Van Bulck, B.; Hinoul, P.; Verguts, J. Influence of contraceptive choice on vaginal bacterial and fungal microflora. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 43–48. [Google Scholar] [CrossRef]
- Achilles, S.L.; Hillier, S.L. The complexity of contraceptives: Understanding their impact on genital immune cells and vaginal microbiota. AIDS 2013, 27 (Suppl. 1), S5–S15. [Google Scholar] [CrossRef]
- Pingitore, E.V.; Todorov, S.D.; Sesma, F.; Franco, B.D.G.M. Application of bacteriocinogenic Enterococcus mundtii CRL35 and Enterococcus faecium ST88Ch in the control of Listeria monocytogenes in fresh Minas cheese. Food Microbiol. 2012, 32, 38–47. [Google Scholar] [CrossRef]
- Silveira-Gomes, F.; Sarmento, D.N.; Espírito-Santo, E.P.; Souza, N.d.O.; Pinto, T.M.; Marques-da-Silva, S.H. Differentiation between Candida albicans and Candida dubliniensis using hypertonic Sabouraud broth and tobacco agar. Rev. Soc. Bras. Med. Trop. 2011, 44, 457–460. [Google Scholar] [CrossRef]
- Bhugaloo-Vial, P.; Grajek, W.; Dousset, X.; Boyaval, P. Continuous bacteriocin production with high cell density bioreactors. Enzym. Microb. Technol. 1997, 21, 450–457. [Google Scholar] [CrossRef]
- Song, D.; Zhu, M.; Gu, Q. Purification and characterization of plantaricin ZJ5, a new bacteriocin produced by Lactobacillus plantarum ZJ5. PLoS ONE 2014, 9, e105549. [Google Scholar] [CrossRef] [PubMed]
- Surovtsev, V.; Borzenkov, V.; Levchuk, V. Purification of bacteriocins by chromatographic methods. Appl. Biochem. Microbiol. 2015, 51, 881–886. [Google Scholar] [CrossRef]
- dos Santos, K.M.O.; de Matos, C.R.; Salles, H.O.; Franco, B.D.G.M.; Arellano, K.; Holzapfel, W.H.; Todorov, S.D. Exploring beneficial/virulence properties of two dairy related strains of Streptococcus infantarius subsp. infantarius. Prob. Antimicrob. Prot. 2020, 12, 1524–1541. [Google Scholar] [CrossRef] [PubMed]
- Fugaban, J.I.I.; Jung, E.S.; Todorov, S.D.; Holzapfel, W.H. Evaluation of antifungal metabolites produced by lactic acid bacteria. Prob. Antimicrob. Prot. 2022, in press. [Google Scholar] [CrossRef]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, R.-U.; Jahid, I.K. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol. 2019, 19, 253. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Measurement of aggregation properties between probiotics and pathogens: In vitro evaluation of different methods. J. Microbiol. Meth. 2007, 71, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Agaliya, P.J.; Jeevaratnam, K. Characterisation of the bacteriocins produced by two probiotic Lactobacillus isolates from idli batter. Ann. Microbiol. 2013, 63, 1525–1535. [Google Scholar] [CrossRef]
- Todorov, S.D.; Furtado, D.N.; Saad, S.M.; Tome, E.; Franco, B.D. Potential beneficial properties of bacteriocin-producing lactic acid bacteria isolated from smoked salmon. J. Appl. Microbiol. 2011, 110, 971–986. [Google Scholar] [CrossRef]
- Amaral, D.M.F.; Silva, L.F.; Casarotti, S.N.; Nascimento, L.C.S.; Penna, A.L.B. Enterococcus faecium and Enterococcus durans isolated from cheese: Survival in the presence of medications under simulated gastrointestinal conditions and adhesion properties. J. Dairy Sci. 2017, 100, 933–949. [Google Scholar] [CrossRef]
- Todorov, S.D.; Dicks, L.M. Bacteriocin production by Pediococcus pentosaceus isolated from marula (Scerocarya birrea). Int. J. Food Microbiol. 2009, 132, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Gillor, O.; Etzion, A.; Riley, M.A. The dual role of bacteriocins as anti- and probiotics. Appl. Microbiol. Biotechnol. 2008, 81, 591–606. [Google Scholar] [CrossRef]
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Heng, N.; Tagg, J. What’s in a name? Class distinction for bacteriocins. Nat. Rev. Microbiol. 2006, 4, 160. [Google Scholar] [CrossRef]
- Todorov, S.D.; Franco, B.D.G.M.; Tagg, J.R. Bacteriocins of Gram-positive bacteria having activity spectra extending beyond closely-related species. Benef. Microbs. 2019, 10, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Hefzy, E.M.; Khalil, M.A.F.; Amin, A.A.I.; Ashour, H.M.; Abdelaliem, Y.F. Bacteriocin-like inhibitory substances from probiotics as therapeutic agents for Candida vulvovaginitis. Antibiotics 2021, 10, 306. [Google Scholar] [CrossRef]
- Okkers, D.J.; Dicks, L.M.T.; Silvester, M.; Joubert, J.J.; Odendaal, H.J. Characterization of pentocin TV35b, a bacteriocin-like peptide isolated form Lactobacillus pentosus with a fungistic effect on Candida albicans. J. Appl. Microbiol. 1991, 87, 726–734. [Google Scholar] [CrossRef]
- Nieminen, M.T.; Novak-Frazer, L.; Rautemaa, W.; Rajendran, R.; Sorsa, T.; Ramage, G.; Bowyer, P.; Rautemaa, R. A novel antifungal is active against Candida albicans biofilms and inhibits mutagenic acetaldehyde production in vitro. PLoS One 2014, 9, e101859, Erratum in PLoS One 2014, 9, e97864. [Google Scholar] [CrossRef]
- Ishijima, S.A.; Hayama, K.; Burton, J.P.; Reid, G.; Okada, M.; Matsushita, Y.; Abe, S. Effect of Streptococcus salivarius K12 on the in vitro growth of Candida albicans and its protective effect inan oral candididiasis model. Appl. Environ. Microbiol. 2012, 78, 2190–2199. [Google Scholar] [CrossRef]
- Lum, K.; Tay, S.; Le, C.; Lee, V.S.; Sabri, N.H.; Velayuthan, R.D.; Hassan, H.; Sekaran, S.D. Activity of novel synthetic peptides against Candida albicans. Sci. Rep. 2015, 5, 9657. [Google Scholar] [CrossRef]
- Scillato, M.; Spitale, A.; Mongelli, G.; Privitera, G.F.; Mangano, K.; Cianci, A.; Stefani, S.; Santagati, M. Antimicrobial properties of Lactobacillus cell-free supernatants against multidrug-resistant urogenital pathogens. Microbiol. Open 2021, 10, e1173. [Google Scholar] [CrossRef] [PubMed]
- Salari, S.; Ghasemi Nejad Almani, P. Antifungal effects of Lactobacillus acidophilus and Lactobacillus plantarum against different oral Candida species isolated from HIV/ AIDS patients: An in vitro study. J. Oral Microbiol. 2020, 12, 1769386. [Google Scholar] [CrossRef] [PubMed]
- McGregor, J.A.; Chong, S.; Reid, G.; Bruce, A.W. Influence of the spermicidal compound nonoxynol-9 on the growth and adhesion of urogenital bacteria in vitro. Curr. Microbiol. 1990, 21, 219–223. [Google Scholar]
- Richardson, B.A. Nonoxynol-9 as a vaginal microbicide for prevention of sexually transmitted infections. JAMA 2002, 287, 1171–1172. [Google Scholar] [CrossRef]
- Reid, G.; Beuermann, D.; Heinemann, C.; Bruce, A.W. Probiotic Lactobacillus dose required to restore and maintain a normal vagina flora. FEMS Immunol. Med. Microbiol. 2001, 32, 37–41. [Google Scholar] [CrossRef]
- Reid, G.; Bruce, A.W.; Fraser, N.; Heinemann, C.; Owen, J.; Henning, B. Oral probiotics can resolve urogenital infections. FEMS Immunol. Med. Microbiol. 2001, 30, 49–52. [Google Scholar] [CrossRef]
- Reid, G.; Bruce, A.W.; Taylor, M. Instillation of Lactobacillus and stimulation of indigenous organisms to prevent recurrence of urinary tract infections. Microecol. Ther. 1995, 23, 32–45. [Google Scholar]
- Bayo, M.; Berlanga, M.; Agut, M. Vaginal microbiota in healthy pregnant women and prenatal screening of group B streptococci. Int. Microbiol. 2002, 5, 87–90. [Google Scholar] [CrossRef]
- Lifshitz, D.A.; Winkler, H.Z.; Gross, M.; Sulkes, J.; Baniel, J.; Livne, P.M. Predictive value of urinary cultures in assessment of microbial colonization of ureteral stents. J. Endourol. 1999, 13, 735–738. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Putra, R.D.; Lyrawati, D. Interactions between bacteriophages and eukaryotic cells. Scientifica 2020, 2020, 3589316. [Google Scholar] [CrossRef]
- Fiore, E.; Van Tyne, D.; Gilmore, M.S. Pathogenicity of enterococci. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.; Zishiri, O.T.; Adeleke, M.A. Prevalence of virulence genes in Enterococcus species isolated from companion animals and livestock. Onderstepoort J. Vet. Res. 2018, 85, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Foulquié Moreno, M.R.; Sarantinopoulos, P.; Tsakalidou, E.; De Vuyst, L. The role and application of enterococci in food and health. Int. J. Food Microbiol. 2006, 106, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Forssten, S.; Hibberd, A.A.; Lyra, A.; Stahl, B. Probiotic approach to prevent antibiotic resistance. Ann. Med. 2016, 48, 246–255. [Google Scholar] [CrossRef]
- Suvorov, A. What is wrong with enterococcal probiotics? Prob. Antimicrob Prot. 2020, 12, 1–4. [Google Scholar] [CrossRef]
- Osoba, M.Y.; Rao, A.K.; Agrawal, S.K.; Lalwani, A.K. Balance and gait in the elderly: A contemporary review. Laryngoscope Investig. Otolaryngol. 2019, 4, 143–153. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The female vaginal microbiome in health and bacterial vaginosis. Front. Cell Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Nisaa, A.A.; Oon, C.-E.; Sreenivasan, S.; Balakrishnan, V.; Tan, J.J.; Teh, C.S.-J.; Sany, S.; Todorov, S.D.; Liu, G.; Park, Y.-H.; et al. Breast milk from healthy women has higher anti-Candida properties than women with vaginal infections during pregnancy. Food Sci. Biotechnol. 2022, in press. [Google Scholar] [CrossRef]
- Paula, A.T.; Jeronymo-Ceneviva, A.B.; Silva, L.F.; Todorov, S.D.; Franco, B.D.G.M.; Penna, A.L.B. Leuconostoc mesenteroides SJRP55: A potential probiotic strain isolated from Brazilian water buffalo mozzarela cheese. Ann. Microbiol. 2015, 65, 899–910. [Google Scholar] [CrossRef]
- Todorov, S.D.; Furtado, D.N.; Saad, S.M.I.; Franco, B.D.G.M. Bacteriocin production and resistance to drugs are advantageous features for Lactobacillus acidophilus La-14, a potential probiotic strain. New Microbiol. 2011, 34, 357–370. [Google Scholar] [PubMed]

| Antibioitcs (μg per Disk) | CRL35 | ST88Ch |
|---|---|---|
| Nitrofurantoin (300) | 30 | 22 |
| Ciprofloxacin (5) | 24 | 20 |
| Fusidic acid (10) | 25 | 20 |
| Furazolidone (10) | 21 | 12 |
| Rifampicin (30) | 30 | 25 |
| Tetracycline (30) | 38 | 38 |
| Ofloxacin (5) | 16 | 16 |
| Cephazolin (30) | 13 | 15 |
| Ceftriaxone (30) | 0 | 18 |
| Erithromicin (15) | 23 | 0 |
| Chloramphenicol (30) | 33 | 30 |
| Vancomycin (30) | 24 | 0 |
| Sulphamethoxazole/trimethorpin (23.75 and 1.25) | 17 | 0 |
| Trimethoprim (5) | 16 | 12 |
| Nalidixic acid (30), Neomycin (10), Tobramycin (10), Cefuroxime (30), Clindamycin (2), Cefotaxime (30), Oxacillin (1), Compound sulphonamides (300), Cefepime (30), Amikacin (30), Ceftazidim (30), Streptomycin (10), Metronidazole (50) | 0 | 0 |
| Commercial Name | Original | Final Concentration | Composition (Active Substance) | Medication Form | Producer | Applied Quantity of Drugs (10 μL) and Diameter of the Inhibition Zone (mm) | Medication Group | |
|---|---|---|---|---|---|---|---|---|
| CRL35 | ST88Ch | |||||||
| Dimenhydrinat | 50 mg | 10 mg/mL | Dimenhydrinat 50 mg | Tablets | Pharmacia AD, Bulgaria | 0 | 0 | Antihistamine |
| Dehydratin Neo | 25 mg | 5 mg/mL | Hydrochlorothiazidin 25 mg | Tablets | Pharmacia AD, Bulgaria | 0 | 0 | Moderate diuretic |
| Famotidine | 20 mg | 4 mg/mL | Famotidine 20 mg | Film tablets | Medika AD, Sandansky, Bulgaria | 0 | 0 | Antiacid |
| Thioridazin | 10 mg | 2 mg/mL | Thioridazine hydrochloride 10 mg; starch, colorant E110 | Film tablets | Balkanpharma AD, Dupnitza, Bulgaria | 5 | 2 | Neuroleptic |
| Diclamina | 70 mg | 14 mg/mL | Cinarizina 20 mg; Heptaminol Acetilinato 50 mg | Tablets | Laboratorios Dr. Esteve S.A. Barcelona, Spain | 0 | 0 | |
| Acetylcystein 600 Stada® Tabs | 600 mg | 120 mg/mL | Acetylcystein 600 mg | Tablets | STADA Arzneimittel AG, Bad Vilder, Germany | 0 | 0 | Antitusiva, anti-inflammatory |
| Duoventrinetten N | 500 mg | 100 mg/mL | Antazidum 500 mg | Tablets | Pharma Schworer, Wiesenbach, Germany | 0 | 0 | |
| Bisalax | 5 mg | 1 mg/mL | Bisacodylum 5 mg | Film tablets | Pharmacia AD, Bulgaria | 0 | 0 | Treatment of constipation |
| Diuretidin | 37.5 mg | 7.5 mg/mL | Triamterenum 25 mg; hydrochlorothiazidum 12.5 mg | Tablets | Pharmacia AD, Bulgaria | 12 | 7 | Moderate diuretic |
| Oleum Jecoris | 37.5 mg | 7.5 mg/mL | Retinol palmitas (Vit A) 3750 IU Ergocalciferolum (Vit D2) 375 IU Oleum Jecoris Aselli to 37.5 mg | Capsules | TroyaPharma AD, Bulgaria | 0 | 0 | Hepatic and pancreatic |
| Atarax | 25 mg | 5 mg/mL | Hidroxizina diclorhidrato 25 mg | Tablets | UCB Pharma S.A. | 0 | 0 | Nevrolepta, anti-inflammatory |
| Ambro | 100 mg | 20 mg/mL | Ambroxol 100 mg | Tablets | Hexal AG | 0 | 0 | Anti-inflammatory |
| Voltaren | 50 mg | 10 mg/ml | Sodium diclofenac 50 mg | Suppository | Novartis | 4 | 8 | Antirheumatic Anti-inflammatory |
| Proalgin | 500 mg | 100 mg/mL | Metamizole sodium 500 mg | Tablets | Balkanpharma AD, Dupnitza, Bulgaria | 0 | 0 | Analgesic, anti-inflammatory |
| Novphyllin | 100 mg | 10 mg/mL | Aminophylline 100 mg | Tablets | Balkanpharma AD, Dupnitza, Bulgaria | 0 | 0 | Anti-asthmatic |
| Cerucal | 10.54 mg | 2.5 mg/mL | Metoclopramide hydrochloride 10.54 mg | Tablets | Arzneimittelwerk Dresden GmbH, Radebeul, Germany | 0 | 0 | Anti-inflammatory |
| Espumisan (Simethicon) | 40 mg | 8 mg/mL | Simethicon 40 mg; methyl-4-hydroxybenzoat 0.28 mg | Tablets | Berlin-Chemie (Menarini Group), Berlin, Germany | 0 | 0 | Analgesic |
| Hepcarsil | 70 mg | 14 mg/mL | Silymarin 70 mg | Capsules | Medica, Sandansky, Bulgaria | 0 | 0 | Hepatic and pancreatic |
| (A) | CRL35 | ST88Ch |
| pH 3.0 at 30 °C | 33% | 50% |
| pH 3.0 at 37 °C | 33% | 50% |
| pH 3.0 at 40 °C | 33% | 25% |
| pH 5.0 at 30 °C | 33% | 75% |
| pH 5.0 at 37 °C | 33% | 25% |
| pH 5.0 at 40 °C | 33% | 25% |
| pH 7.0 at 30 °C | 67% | 100% |
| pH 7.0 at 37 °C | 83% | 75% |
| pH 7.0 at 40 °C | 67% | 75% |
| (B) | CRL35 | ST88Ch |
| 37 °C | ||
| pH 3.0 | 33% | 50% |
| pH 3.0 and 10% contraceptive | 33% | 50% |
| pH 3.0 and 20% contraceptive | 50% | 50% |
| pH 5.0 | 33% | 25% |
| pH 5.0 and 10% contraceptive | 50% | 75% |
| pH 5.0 and 20% contraceptive | 50% | 50% |
| pH 7.0 | 83% | 75% |
| pH 7.0 and 10% contraceptive | 67% | 75% |
| pH 7.0 and 20% contraceptive | 67% | 75% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorov, S.D.; Weeks, R.; Popov, I.; Franco, B.D.G.d.M.; Chikindas, M.L. In Vitro Anti-Candida albicans Mode of Action of Enterococcus mundtii and Enterococcus faecium. Microorganisms 2023, 11, 602. https://doi.org/10.3390/microorganisms11030602
Todorov SD, Weeks R, Popov I, Franco BDGdM, Chikindas ML. In Vitro Anti-Candida albicans Mode of Action of Enterococcus mundtii and Enterococcus faecium. Microorganisms. 2023; 11(3):602. https://doi.org/10.3390/microorganisms11030602
Chicago/Turabian StyleTodorov, Svetoslav Dimitrov, Richard Weeks, Igor Popov, Bernadette Dora Gombossy de Melo Franco, and Michael Leonidas Chikindas. 2023. "In Vitro Anti-Candida albicans Mode of Action of Enterococcus mundtii and Enterococcus faecium" Microorganisms 11, no. 3: 602. https://doi.org/10.3390/microorganisms11030602
APA StyleTodorov, S. D., Weeks, R., Popov, I., Franco, B. D. G. d. M., & Chikindas, M. L. (2023). In Vitro Anti-Candida albicans Mode of Action of Enterococcus mundtii and Enterococcus faecium. Microorganisms, 11(3), 602. https://doi.org/10.3390/microorganisms11030602







