Abstract
The aim of the study was to determine prevalence of Anaplasmataceae-infected ticks in the Black Sea Coast and the Pleven regions of Bulgaria. A total of 350 ticks from different tick species were collected. Two hundred fifty-five ticks were removed from dogs in the Black Sea Coast region, and 95 Ixodes ricinus ticks were collected by flagging vegetation with a white flannel cloth in two areas in the region of Pleven. After the DNA isolation of the ticks, a genus-specific polymerase chain reaction (PCR) was performed to identify Anaplasmataceae. Second PCRs were performed with species-specific primers to identify Ehrlichia canis (E. canis) and Anaplasma phagocytophilum (A. phagocytophilum). The results showed that 26.9% of the Ixodes ricinus ticks were infected with Anaplasmataceae in the Black Sea Coast region and 36.8% in the Pleven region. The infection with E. canis was detected in 35.7% and A. phagocytophilum in 25.0% of positive ticks from the Black Sea Coast region. In the Pleven region, 22.9% of ticks were positive for E. canis, while 42.9% were positive for A. phagocytophilum. The molecular identification of E. canis in ticks collected from Bulgaria was performed for the first time. In conclusion, the present study revealed a higher prevalence of ticks infected with Anaplasmataceae, particularly A. phagocytophilum, in the Pleven region than in the Black Sea Coast region.
1. Introduction
Ticks represent the second vector group after mosquitoes, consisting of more than 900 tick species worldwide that transmit a broad range of pathogens to domestic animals and humans [1]. The expansion of their geographical area and the increasing incidence of tick-borne diseases over the last 20 years have significantly influenced domestic animal and human health. The most important tick species responsible for zoonotic disease transmission and spreading belong to the Ixodidae family [2].
Their successful survival depends on optimal temperature and humidity in their environment and the availability of appropriate hosts [3,4]. During blood feeding, ticks can transmit viruses, bacteria, and protozoan parasites to their hosts, mainly birds and mammals, including humans. Ticks and tick-transmitted pathogens have co-evolved with various wild animal hosts. As a result, many of these wild animals have become reservoir hosts and successfully live in a state of equilibrium with tick-borne pathogens [5]. That situation facilitates the spreading of tick-borne diseases in pets and humans. Moreover, tick species are recently present in more areas than ever, including urban green regions (city parks and gardens) and peri-urban forest areas, facilitating contact between humans and pets and infected questing ticks [6,7]. Among ixodid ticks, Ixodes ricinus is the most known species in terms of spreading and significant transmission in Europe and Bulgaria [6,8]. Moreover, transstadial transmission of pathogens in the Ixodes ricinus developmental stages is important for their epidemiological significance.
Among the bacterial pathogens transmitted by ixodid ticks, Borrelia burgdorferi s.l., Anaplasma, and Ehrlichia spp. significantly influence human health. The family Anaplasmtaceae comprises obligate intracellular Gram-negative bacteria and includes agents of Ehrlichia and Anaplasma species. Anaplasma phagocytophilum causes canine and human granulocytic anaplasmosis. Ehrlichia canis cause canine monocytic ehrlichiosis, and some cases of human infection were described [9]. Moreover, during the last decade, incidence of tick-borne diseases in Europe has increased [6]. For the last two decades, Bulgarian studies on tick-borne pathogens, particularly Anaplasmataceae, have been scarce. Little is known about the prevalence of Ehrlichia and Anaplasma spp. in ticks from different regions of Bulgaria [10,11].
In the present study, two distanced regions of Bulgaria were explored: the Black Sea Coast of Eastern Bulgaria and the Pleven region of North Bulgaria. Both regions provide the optimal environment for tick development in temperature, humidity, and potential hosts. In addition, many people frequently visit these areas. All of these epidemiological characteristics of the above-described areas make it reasonable to explore tick density and tick-borne pathogen occurrence within these regions. To date, no study has been carried out on the presence of Anaplasmataceae pathogens in ticks in these places in Bulgaria. Therefore, this study aims to detect the presence of Anaplasmataceae bacterial DNA in ticks and to establish the prevalence of Anaplasma phagocytophilum (A. phagocytophilum) and Ehrlichia canis (E. canis) in areas of Byala, St. Vlas, and the Pleven region of Bulgaria.
2. Materials and Methods
2.1. Sample Collection
The ticks (n = 350) were collected from two Bulgarian sites: Byala and Sveti Vlas on the Black Sea Coast and the Pleven region (Kaylaka park and Kartozhabene) between 2021 and 2022 (Figure 1). Geographic mapping was conducted on Quantum Geographic Information Systems (QGIS version 3.18.0, QGIS Development Team, GNU General Public License, Essen, Germany) with the World Geodetic System 1984 (WGS 84) being a standard of coordinate referencing.
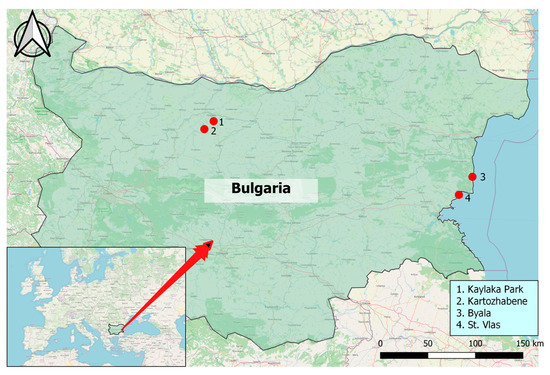
Figure 1.
Location of areas for tick collection. Pleven region in Northern Bulgaria including (1) Kaylaka park and (2) village Kartozhabene, as well as the Black Sea Coast region of Eastern Bulgaria, including the towns of (3) Byala and (4) St. Vlas.
The towns of Byala and St. Vlas are located on the Black Sea Coast of Eastern Bulgaria. This region covers the mountainous part of the Black Sea Coast, where the Balkan Mountains (Stara Planina) reach the Black Sea. It is a forested area favourable for Ixodes species. These towns are seaside resorts that are visited by many people, especially during the summer season. A total of 255 ticks were removed from stray dogs in an area of Byala town (n = 27) and domestic dogs from private veterinary clinics in St. Vlas town (n = 15). Collected ticks were maintained alive in individual 1.5 mL Eppendorf tubes and transported to the Department “Molecular Biology, Immunology and Medical Genetics” laboratory at the Medical Faculty, Trakia University, for species identification and testing for pathogens. In the laboratory, all ticks underwent taxonomic identification, developmental staging, and sexing, according to Georgieva and Gencheva [12] and Estrada-Peña et al. [13]; the identification was performed using a stereomicroscope (Olympus SZ4045, Olympus American Inc., Melville, NY, USA). Using the morphology of the mouthparts (length of the palps to the basis capituli and the shape of the basis capituli); the presence of festoons and specific patterning (ornate) on the dorsal shield; the presence of eyes; the shape of the anal groove; and the shape of the coxae, we identified 104 ticks belonging to the genus Ixodes and 151 ticks from the genus Rhipicephalus. The collected Ixodes ticks included 46 (44.2%) fed, 32 (30.8%) semi-fed and 26 (25%) unfed ticks or nymphs.
The second studied region is close to Pleven in Northern Bulgaria and includes Kaylaka Park and the village of Kartozhabene. Kaylaka Park is located near the town of Pleven and is a frequently visited place by many people. The village of Kartozhabene is around 10 km from the town of Pleven. Both spots in the Pleven region are in the karst canyons of rivers, with abundant and diverse flora and fauna, and are favourite places for outdoor activities for many visitors. A total of 95 Ixodes ticks were collected from the vegetation by flagging in urbanized and wild areas in the region of Pleven. A detailed description of sampling from the Pleven region was previously described [14].
2.2. DNA Extraction and Polymerase Chain Reaction (PCR) Identification of Tick-Borne Bacteria
Total DNA was isolated from all collected Ixodes ticks (n = 199), and from 44 randomly selected ticks from the genus Rhipicephalus, using the animal tissue genomic DNA purification mini prep kit (Gennaxxon bioscience, Ulm, Germany) or the NucleoSpin Tissue, Mini kit for DNA from cells and tissue (Macherey-Nagel KG; Düren, Germany), following the manufacturer’s instructions. The DNA extraction kits have comparable performance. The extracted DNA was stored at −70 °C until PCR analysis could be conducted.
Firstly, the gradient PCR was performed in an AERIS PCR system (Esco, Singapore) to optimize the annealing temperatures in any PCR. Then, each DNA sample was tested first for the presence of the 16S rRNA consensus sequence specific for all bacterial species of the family Anaplasmataceae, with a conventional PCR, using the primers presented in Table 1 and previously described by Inokuma et al. [15]. For the PCR run, we used 3 µL template DNA and the reaction mixture (20 µL) contained 2 µL 10XPCR buffer, 1.5 mM MgCl2, 0.2 mM each of dNTPs, 0.25 μM each of primers, and 1U Taq DNA polymerase. The amplification was performed as follows: 96 °C for 3 min, 35 cycles of 95 °C for 30 s, 55 °C for 30 s and 40 s at 72 °C, and a final extension at 72 °C for 7 min.

Table 1.
Primers and PCR conditions used for molecular identification of pathogens from family Anaplasmataceae and species identification of E. canis and A. phagocytophilum.
Subsequently, positive samples were tested with a second PCR using a species-specific primer set for Ehrlichia canis (E. canis) or Anaplasma phagocytophilum (A. phagocytophilum) and the annealing temperature presented in Table 1 [16,17]. The 5mM MgCl2 was used in a species-specific PCR for E. canis. The rest condition of the reaction mix was the same as the first PCR. The used PCR components were manufactured by Thermo Fisher Scientific, Vilnius, Lithuania, and the primers by Metabion GMBH, Planegg, Germany.
All PCR products were analysed on a 1.5% agarose gel electrophoresis stained with ethidium bromide. A DNA Ladder (by 100 bp) was applied for evaluation of the obtained product size. The results of the PCR amplification were viewed under UV light and were archived using EasyWin32 software (Herolab; Wiesloch, Germany). DNA extraction, fragment amplification, and agarose gel electrophoresis were performed in separate rooms.
2.3. Statistical Analysis
The frequency of data is reported as counts and as the percentage of the total number of ticks or the percentage of the Anaplasmataceae-positive Ixodes ricinus ticks in the regions that were studied. Differences in tick infection prevalence between sexes and localities were analysed using a nonparametric Chi-square test and 95% confidential interval (95% CI). The differences were considered significant with p values less than 0.05.
3. Results
The ticks collected from dogs in regions of Byala and St. Vlas were mainly from the genus Rhipicephalus (n = 151) and Ixodes (n = 104). The collected Ixodes ticks included 54 (51.9%) female ticks, 33 (31.7%) male ticks, and 17 (16.4%) nymphs. The ticks collected from the Pleven region (n = 95) were from the genus Ixodes, including 46 (48.4%) females, 44 (46.3%) males, and 5 (5.3%) nymphs. All Ixodes ticks (n = 199) were classified as Ixodes ricinus and were subjected to further analysis to detect the prevalence of bacteria of the Anaplasmataceae family.
We did not detect any infected Rhipicephalus sanguineus ticks with bacteria in the Anaplasmataceae family among the randomly selected 44 ticks. As a result, these tick species were excluded from further analysis.
The overall prevalence of bacteria in the Anaplasmataceae family in Ixodes ricinus ticks collected from the Black Sea Coast and Pleven regions are presented in Figure 2. Although there was a slightly higher rate of infected Ixodes ticks in the Pleven region (36.8%, 35 of 95) in comparison to the Black Sea Coast region (26.9%, 28 of 104), the statistical significance was not reached (χ2 = 2.258; df = 1; p = 0.133). The overall prevalence of ticks infected with bacteria in the Anaplasmataceae family is 31.7% (63 of 199).
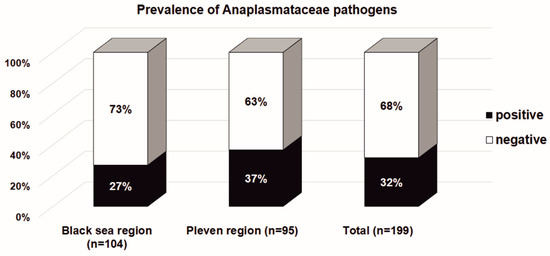
Figure 2.
The Ixodes ricinus infectivity with bacteria in the Anaplasmataceae family in the Black Sea Coast and Pleven regions. The results are presented as a percentage of the total number of tested Ixodes ricinus ticks.
As is shown in Table 2, the highest rate of infection with the Anaplasmataceae pathogens was detected amongst females, followed by male ticks in both of the studied regions. None of the tested nymphs was infected with species of Anaplasmataceae. In the Black Sea Coast region, 19 of 54 female ticks (35.2% of females) and 9 of 33 male ticks (27.3% of males) were infected (χ2 = 0.588; df = 1; p = 0.443). In the Pleven region, there was a 14-fold increase of infected female ticks compared to male ticks (OR = 14.6; 95% CI: 4.814 ÷ 44.434; p < 0.0001). Here, 30 of the 46 female ticks (65.2% of females) were infected with the Anaplasmataceae species, which was significantly higher than the infection rate seen in male ticks from the same area (5 of 44 males, 11.4% of males with χ2 = 27.444; df = 1; p < 0.001).

Table 2.
The infective rate of Ixodes ricinus ticks with bacteria in the Anaplasmataceae family, according to their stage and sex in studied regions.
Since the ticks collected from the Black Sea Coast region were removed from dogs during their feeding, we compared the prevalence of infected ticks with bacteria in the Anaplasmataceae family across the subgroups, taking into account their feeding status. Among the female infected ticks, 13 of 19 (68.4%) were fed, 4 (21.1%) were semi-fed, and 2 (10.5%) were unfed ticks. Similarly, among the male infected ticks 4 of 9 (44.4%) were fed, 2 (22.2%) were semi-fed, and 3 (33.3%) were unfed ticks (χ2 = 2.36; df = 2; p = 0.307). Altogether, the highest infective rate was detected among fed ticks (60.7%; 17 of 28), followed by semi-fed (21.4%; 6 of 28) and unfed (17.9%; 5 of 28) ticks.
Ticks that tested positive in the test for the detection of pathogens from the Anaplasmataceae family were further analysed with a species-specific PCR assay for E. canis and A. phagocytophilum. The observed results are presented in Table 3.

Table 3.
The prevalence of E. canis and A. phagocytophilum among the Anaplasmataceae-positive Ixodes ricinus ticks in studied regions.
In the Black Sea Coast region, among the Anaplasmataceae-positive ticks, 35.7% (10 of 28) were infected with E. canis and 25.0% (7 of 28) with A. phagocytophilum. Co-infection was observed in 10.7% (3 of 28) and 39.3% (11 of 28) were negative for both of the pathogens studied.
In the Pleven region, E. canis was identified in 8 ticks (22.9% of Anaplasmataceae-positive ticks and 8.6% for all tested ticks), and 15 ticks (42.9% of Anaplasmataceae-positive ticks and 15.8% of for all tested ticks) were positive for A. phagocytophilum. Co-infection was observed in 17.1% (6 of 35), and 34.3% (12 of 35) were negative for both of the pathogens studied.
The infection rate of E. canis between the two studied regions was similar when considering only Ananplasmataceae-positive ticks (35.7%; 10 of 28 vs. 22.9%; 8 of 35 with χ2 = 1.260; df = 1; p = 0.262), as well as when considering all the tested ticks (9.6%; 10 of 104 vs. 8.4%; 8 of 95; χ2 = 0.086; df = 1; p = 0.769).
A difference in the infection rate of A. phagocytophilum between the two studied regions was observed. The infection rate amongst Anaplasmataceae-positive ticks was seen to be higher in the Pleven region than in the Black Sea region (42.9%; 15 of 35 vs. 25%; 7 of 28; χ2 = 2.183; df = 1; p = 0.140). The difference reached a statistical significance when considering all of the tested ticks within this study (15.8%; 15 of 95 vs. 6.7%; 7 of 104; χ2 = 4.143; df = 1; p = 0.042). In the Black Sea region, the infection rate of E. canis was more common than infection with A. phagocytophilum, contrary to the Pleven region (Figure 3).
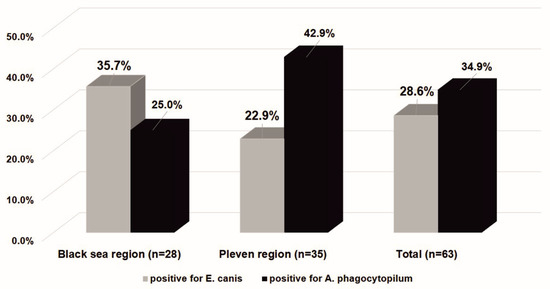
Figure 3.
The Ixodes ricinus infectivity with bacteria E. canis and A. phagocytophilum in the Black Sea Coast and Pleven regions. The results are presented as a percentage of the number of ticks positive for bacteria in the Anaplasmataceae family.
The co-infection rate was similar in both of the areas studied. Co-infection with E. canis and A. phagocytophilum was detected in 3 of 28 (10.7%) ticks from the Black Sea Coast region and 6 of 35 (17.1%) ticks from the Pleven region, with a χ2 = 0.525; df = 1; p = 0.469. A total of 14.3% of ticks were infected with both of the pathological species from the Anaplasmataceae family.
4. Discussion
Although accurate prediction by epidemiological data is not possible, they can reveal which areas are more prone to tick-borne diseases and could potentially be used for minimizing the risk of transmission of these diseases. Molecular tools such as PCR identification can provide a better understanding of the epidemiology of target pathogens.
The main investigated tick species was Ixodes ricinus, the most common and widely distributed tick in Europe and Bulgaria [18,19,20]. Furthermore, the prevalence of infection with A. phagocytophilum and B. burgdorferi in rodents within Bulgaria clarifies their role as competent reservoirs for these pathogens in nature [21].
In the present study, PCR techniques were used to detect Ehrlichia canis and Anaplasma phagocytophilum, which represent an emerging threat to public health due to their zoonotic nature. We investigated the rate of bacterial infection of Ixodes ticks, which were collected from two distant regions in Bulgaria, since both areas are frequently visited by locals and tourists. Byala and St. Vlas are seaside resorts in Central-Eastern Bulgaria, located on the Black Sea Coast, and lie in a semi-mountainous region in the eastern region of Stara Planina. Kaylaka Park is located on the periphery of the regional town of Pleven and is a local place for walks and leisure activities, with a large number of daily visitors. The village of Kartozhabene is located twelve kilometres southwest of Pleven. Our results showed the difference in infected ticks between the two studied regions. The frequencies of the ticks infected with bacteria belonging to the family Anaplasmataceae are higher in the Pleven region (36.8%) than in the region of the Black Sea Coast (26.9%), though they do not display statistical significance. Also, we detected DNA from Anaplasmataceae pathogens in Ixodes, but not in Rhipicephalus, ticks which confirms the results from other authors that Ixodes are the predominant vector for these bacteria in Europe and, therefore, in Bulgaria [6,10].
Two genera from the family Anaplasmataceae—Ehrlichia and Anaplasma—comprise tick-borne intra-cellular, non-motile, Gram-negative bacteria of medical and veterinary concern. They parasitize in human and canine neutrophils (Anaplasma spp.) or monocytes and macrophages (Ehrlichia spp.). E. canis is a causative agent of canine monocytic ehrlichiosis (CME) and is transmitted mainly by two genera of ticks, Ixodes and Rhipicephalus [3,22]. It was previously thought that this bacterium only infected dogs, until the publication of Perez et al. [9]. Other recent reports confirm that the etiological agent of CME E. canis can also be a human pathogen [23,24]. The molecular detection of E. canis in Ixodes ticks in the two investigated regions in Bulgaria was similar and varied between 8.4% (Pleven) and 9.6% (Black Sea Coast) from all of the tested ticks. To our knowledge, no other study has examined the presence of E. canis DNA in ticks from Bulgaria. The published data refers to the study of antibodies against E. canis in dog serum, with total seropositivity of dogs from southern Bulgaria at approximately 30%, with a variation between 15% and 50% for the different regions studied [25,26]. The distribution of E. canis in European countries varies greatly, which has been seen in the published work [27].
Among six species of the genus Anaplasma, only A. phagocytophilum causes a febrile disease in humans and other mammals [28]. The human infection with A. phagocytophilum has been referred to as human granulocytic anaplasmosis (HGA). A. phagocytophilum is mainly transmitted by I. ricinus, and our results confirm that none of the species belonging to R. sanguineus was positive for this pathogen. Even in ticks removed from a single animal, Anaplasma infection was not detected in co-feeding Ixodes and Ripicephalus ticks. The molecular detection of A. phagocytophilum was performed by detecting the ankA gene, which encodes a protein characterized by repeated ankyrin motifs and is involved in the molecular mechanism of host adaptation selection [29].
The prevalence of infected ticks with A. phagocytophilum in the Pleven region was higher than that found in the Black Sea Coast region, reaching the statistical significance when taking into account all of the tested ticks (χ2 = 4.143; p = 0.042). The co-infection was also higher for the Pleven region in comparison to the Black Sea Coast region (17.14% vs. 10.7%). These results are supported by the data on the spread of A. phagocytophilum in rodents (average 8.8% for rodents trapped in various regions of Bulgaria), which are the main natural reservoir [21]. Another recent study for the molecular detection of A. phagocytophilum in Ixodes ticks, collected from Strandja Nature Park in south-eastern Bulgaria [11], reported a significantly higher prevalence of that pathogen in Ixodes spp. (38.9%). In our study, a total of 34.9% (22 of 63) of Ixodes ticks were infected with A. phagocytophilum, which is similar to the rate of 33.9% reported previously among adult ticks collected near Sofia, Bulgaria [10].
All of this data collectively demonstrates the abundant distribution of A. phagocytophilum in ticks and animals in Bulgaria, but, surprisingly, Anaplasmosis is only occasionally detected in patients. The lack of correlation between the prevalence of A. phagocytophilum and the number of diagnosed patients can be explained by the unspecific clinical manifestation of Anaplasmosis, incomplete diagnosis, or a high rate of asymptomatic infections. We can suspect that Anaplasmosis is much more common in Bulgaria than the official data presented for that disease. Most European cases of Anaplasmosis are presented as a mild infection, and the suspicion of being under-diagnosed or under-reported remains. Data for A. phagocytophilum-seropositive humans in Europe is on average ~8.3%, reaching up to 31% [30]. Some serological studies reported the presence of both HGA and Lyme borreliosis antibodies, suggesting co-infection transmitted from a tick’s bite, since the vector is the same [30]. Accurate molecular tests should be recommended for patients before the treatment to avoid antibiotic overuse, and for blood donors because a blood transfusion can also transmit this pathogen.
Finally, it should be mentioned when using the method of molecular testing, many of the ticks positive for 16S rRNA of Anaplasmataceae are negative for both E. canis and A. phagocytophilum. This can be due to the presence of other pathogens in that family, and some of them can have a zoonotic potential and can be a future threat to human health. All of this requires further investigation to determine the epidemiological significance of tick-borne diseases.
5. Conclusions
In conclusion, our study presents epidemiological data for the rate of infection with pathogens belonging to the family of Anaplasmataceae in Ixodes ricinus ticks collected from two regions of Bulgaria: the Pleven and Black Sea Coast regions. The detection was performed by molecular identification of the pathogens’ DNA sequences. Generally, the Pleven region presents a higher frequency of infected ticks with studied pathogens. Although the fed or semi-fed ticks may be newly infected, since it is not known whether the pathogens’ DNA was in a tick or from the animal’s blood, all ticks collected from the region of Pleven were collected from the vegetation by flagging and were unfed. Therefore, we may accept the Pleven region as a site with a higher prevalence of ticks infected naturally with Anaplasmataceae than the Black Sea Coast region. In addition, the distribution of Ehrlichia canis in ticks from the two regions did not reach a significant difference, while the prevalence of Anaplasma phagocytophilum in the Pleven region was significantly higher than that found in the Black Sea coast region.
Author Contributions
Conceptualization, L.M. and A.B.; methodology, I.S.; software, I.S.; validation, A.B. and L.M.; formal analysis, I.S.; investigation, I.S., A.B. and L.M.; resources, I.S. and A.B.; data curation, I.S. and A.B.; writing—original draft preparation, I.S.; writing—review and editing, L.M.; visualization, A.B.; supervision, L.M.; project administration, L.M.; funding acquisition, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Trakia University, Medical Faculty, Stara Zagora, Bulgaria [Grant number: NIP 1/2022].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129 (Suppl. 1), S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Peña, A.; Horak, I.G. The Hard Ticks of the World (Acari: Ixodida: Ixodidae); Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Stańczak, J.; Gabre, R.M.; Kruminis-Łozowska, W.; Racewicz, M.; Kubica-Biernat, B. Ixodes ricinus as a vector of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti in urban and suburban forests. Ann. Agric. Environ. Med. 2004, 11, 109–114. [Google Scholar] [PubMed]
- Uspensky, I. Conditions of tick (Acari: Ixodoidea) population persistence in the urban environment. In Proceedings of the Eighth International Conference on Urban Pests, Zurich, Switzerland, 20–23 July 2014; Müller, G., Pospischil, R., Robinson, W.H., Eds.; OOK-Press Kft.: Veszprém, Hungary, 2014; pp. 203–210. [Google Scholar]
- Tsao, J.I.; Hamer, S.A.; Han, S.; Sidge, J.L.; Hickling, G.J. The contribution of wildlife hosts to the rise of ticks and Tick-Borne Diseases in North America. J. Med. Entomol. 2021, 58, 1565–1587. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Spitalsk´a, E.; et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: New hazards and relevance for public health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef]
- Bellato, A.; Pintore, M.D.; Catelan, D.; Pautasso, A.; Torina, A.; Rizzo, F.; Mandola, M.L.; Mannelli, A.; Casalone, C.; Tomassone, L. Risk of tick-borne zoonoses in urban green areas: A case study from Turin, northwestern Italy. Urban For. Urban Green. 2021, 64, 127297. [Google Scholar] [CrossRef]
- Ivanova Aleksandrova, N.; Christova, I.; Dimitrov, D.; Marinov, M.P.; Panayotova, E.; Trifonova, I.; Taseva, E.; Gladnishka, T.; Kamenov, G.; Ilieva, M.; et al. Records of Ixodid ticks on wild birds in Bulgaria. Probl. Infect. Parasit. Dis. 2021, 27, 35–39. [Google Scholar] [CrossRef]
- Perez, M.; Boodor, M.; Zhang, C.; Xiong, Q.; Rikihisa, Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. N. Y. Acad. Sci. 2006, 1078, 110–117. [Google Scholar] [CrossRef]
- Christova, I.; Schouls, L.; van de Pol, I.; Park, J.; Panayotov, S.; Lefterova, V.; Kantardjiev, T.; Dumler, J.S. High prevalence of granulocytic ehrlichiae and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Bulgaria. J. Clin. Microbiol. 2001, 39, 4172–4174. [Google Scholar] [CrossRef]
- Nader, J.; Król, N.; Pfeffer, M.; Ohlendorf, V.; Marklewitz, M.; Drosten, C.; Junglen, S.; Obiegala, A. The diversity of tick-borne bacteria and parasites in ticks collected from the Strandja Nature Park in south-eastern Bulgaria. Parasit. Vectors 2018, 11, 165. [Google Scholar] [CrossRef]
- Georgieva, G.; Gecheva, G. Fauna Bulgaria; Beron, P., Ed.; Marin Drinov Academic Publishing House: Sofia, Bulgaria, 2013; p. 226. [Google Scholar]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa: A Guide to Species Identification; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Blazhev, A.; Stanilov, I.; Miteva, L.D.; Atanasova, M.; Blazheva, S.; Stanilova, S. Prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from Kaylaka Park in Pleven, Bulgaria. Microorganisms 2022, 10, 772. [Google Scholar] [CrossRef]
- Inokuma, H.; Parola, P.; Raoult, D.; Brouqui, P. Molecular survey of Ehrlichia infection in ticks from animals in Yamaguchi Prefecture, Japan. Vet. Parasitol. 2001, 99, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Lim, S.Y.; Watanabe, M.; Sharma, R.S.; Cheng, N.A.; Watanabe, M. Molecular detection of Ehrlichia canis in dogs in Malaysia. PLoS Negl. Trop. Dis. 2013, 7, e1982. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.A.; Kalmár, Z.; Magdaş, C.; Magdaş, V.; Toriay, H.; Dumitrache, M.O.; Ionică, A.M.; D’Amico, G.; Sándor, A.D.; Mărcuţan, D.I.; et al. Anaplasma phagocytophilum in questing Ixodes ricinus ticks from Romania. Ticks Tick Borne Dis. 2015, 6, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Voyiatzaki, C.; Papailia, S.I.; Venetikou, M.S.; Pouris, J.; Tsoumani, M.E.; Papageorgiou, E.G. Climate Changes Exacerbate the Spread of Ixodes ricinus and the Occurrence of Lyme Borreliosis and Tick-Borne Encephalitis in Europe—How Climate Models Are Used as a Risk Assessment Approach for Tick-Borne Diseases. Int. J. Environ. Res. Public Health 2022, 19, 6516. [Google Scholar] [CrossRef]
- Kirkova, Z.; Iliev, P.; Vesser, M.; Knaus, M. Survey on ectoparasites of dogs (Canis familiaris) in Bulgaria. In Proceedings of the 12th International Symposium of Ectoparasites of Pets (ISEP), Munich, German, 7–9 April 2013; p. 68. [Google Scholar]
- European Centre for Disease Prevention and Control; European Food Safety Authority. Tick Maps [Internet]. Stockholm: ECDC. 2022. Available online: https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/tick-maps (accessed on 22 November 2022).
- Christova, I.; Dimitrov, H.; Trifonova, I.; Gladnishka, T.; Mitkovska, V.; Stojanova, A.; Taseva, E.; Ivanova, V. Detection of Human Tick-borne Pathogens in Rodents from Bulgaria. Acta Zool. Bulg. 2012, 64 (Suppl. 4), 111–114. [Google Scholar]
- Groves, M.G.; Dennis, G.L.; Amyx, H.L.; Huxsoll, D.L. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am. J. Vet. Res. 1975, 36, 937–940. [Google Scholar]
- Silva, A.B.; Pina, S.; Gabriel de la Torre, M.; Mayoral, A.; Mayoral, M.A.; Pérez-Campos, L.; López, J.; Pérez-Campos, E. Infeccién humanaasintomótica por contacto con perros. Un caso de ehrlichiosis humana. [Asymptomatic human infection from contact with dogs. A case of human ehrlichiosis]. Gaceta Méd. México 2014, 150, 171–174. [Google Scholar]
- Bouza-Mora, L.; Dolz, G.; Solórzano-Morales, A.; Romero-Zuñiga, J.J.; Salazar-Sánchez, L.; Labruna, M.B.; Aguiar, D.M. Novel genotype of Ehrlichia canis detected in samples of human blood bank donors in Costa Rica. Ticks Tick Borne Dis. 2017, 8, 36–40. [Google Scholar] [CrossRef]
- Tsachev, I.; Kontos, V.; Zarkov, I.; Krastev, S. Survey of antibodies reactive with Ehrlichia canis among dogs in South Bulgaria. Revue Méd. Vét. 2006, 157, 481–485. [Google Scholar]
- Pantchev, N.; Schnyder, M.; Vrhovec, M.G.; Schaper, R.; Tsachev, I. Current Surveys of the Seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in Dogs in Bulgaria. Parasitol. Res. 2015, 114 (Suppl. 1), S117–S130. [Google Scholar] [CrossRef]
- Sainz, Á.; Roura, X.; Miró, G.; Estrada-Peña, A.; Kohn, B.; Harrus, S.; Solano-Gallego, L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit. Vectors 2015, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some speciesof Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, A. PCR for detection of tick-borne Anaplasma phagocytophilum pathogens: A review. Vet. Med. 2011, 56, 529–536. [Google Scholar] [CrossRef]
- Matei, I.A.; Estrada-Peña, A.; Cutler, S.J.; Vayssier-Taussat, M.; Varela-Castro, L.; Potkonjak, A.; Zeller, H.; Mihalca, A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasit. Vectors 2019, 12, 599. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).