Isolation of Bioactive Metabolites from Soil Derived Fungus-Aspergillus fumigatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Fungal Isolation
2.2. Identification of Fungus
2.3. Fermentation and Extraction
2.4. Isolation and Identification of the Metabolites
2.5. Antimicrobial Activities
2.5.1. Preliminary Assay of Crude Extract
2.5.2. Antimicrobial Susceptibility Testing for Isolated Compounds
2.5.3. Minimum Inhibitory Concentration (MIC) of Isolated Compounds
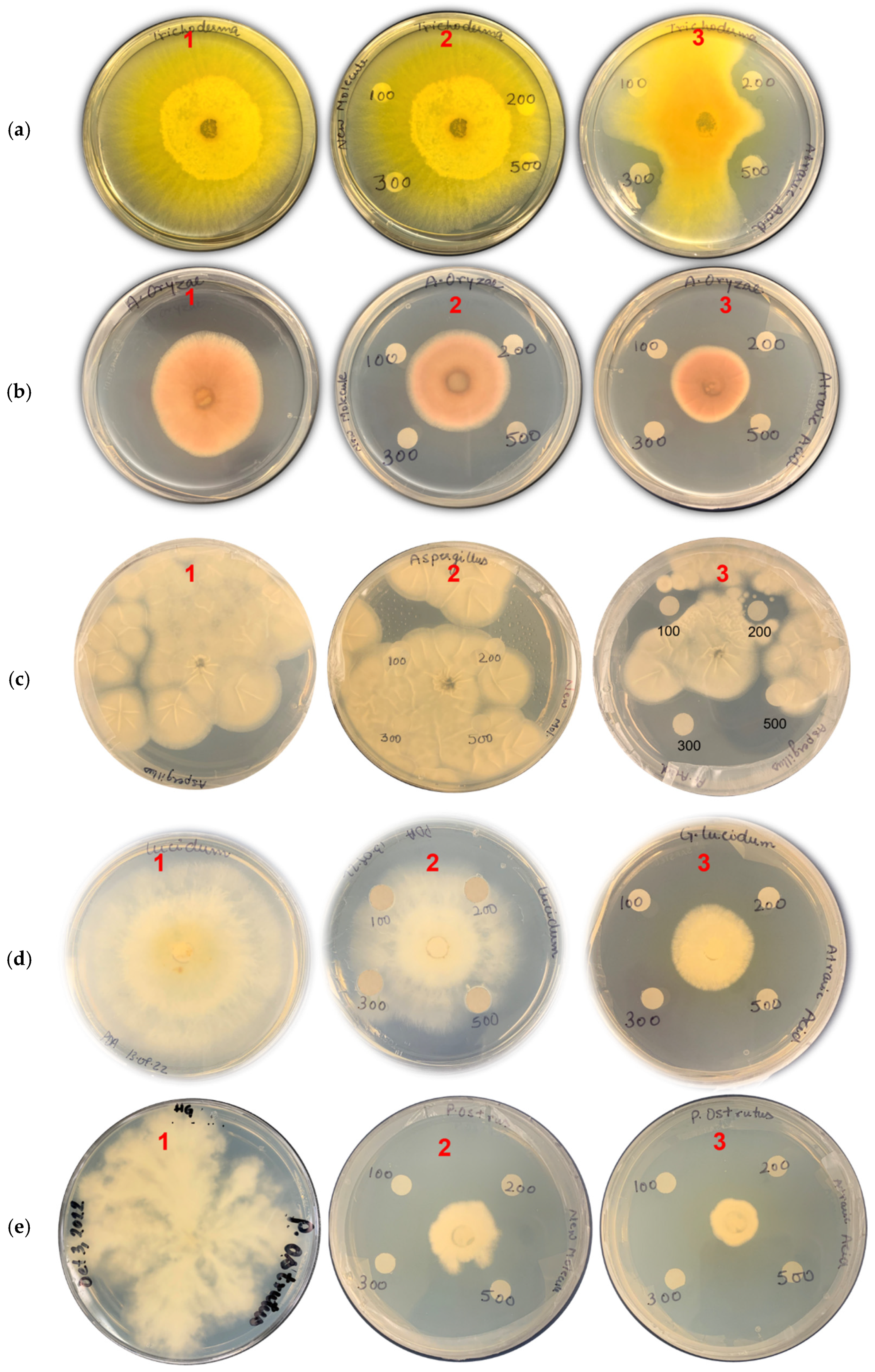
2.5.4. Antifungal Assay of Isolated Compounds
3. Results
3.1. Fungal Isolation and Preliminary Selection
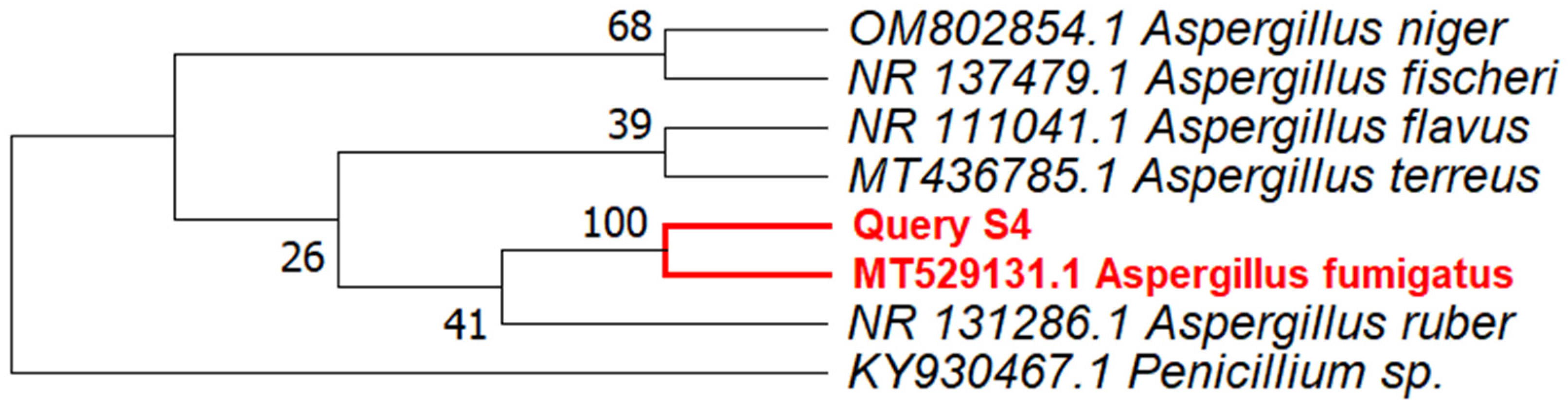
3.2. Identification of Fungus
3.3. Fermentation, Extraction, and Isolation of Compounds
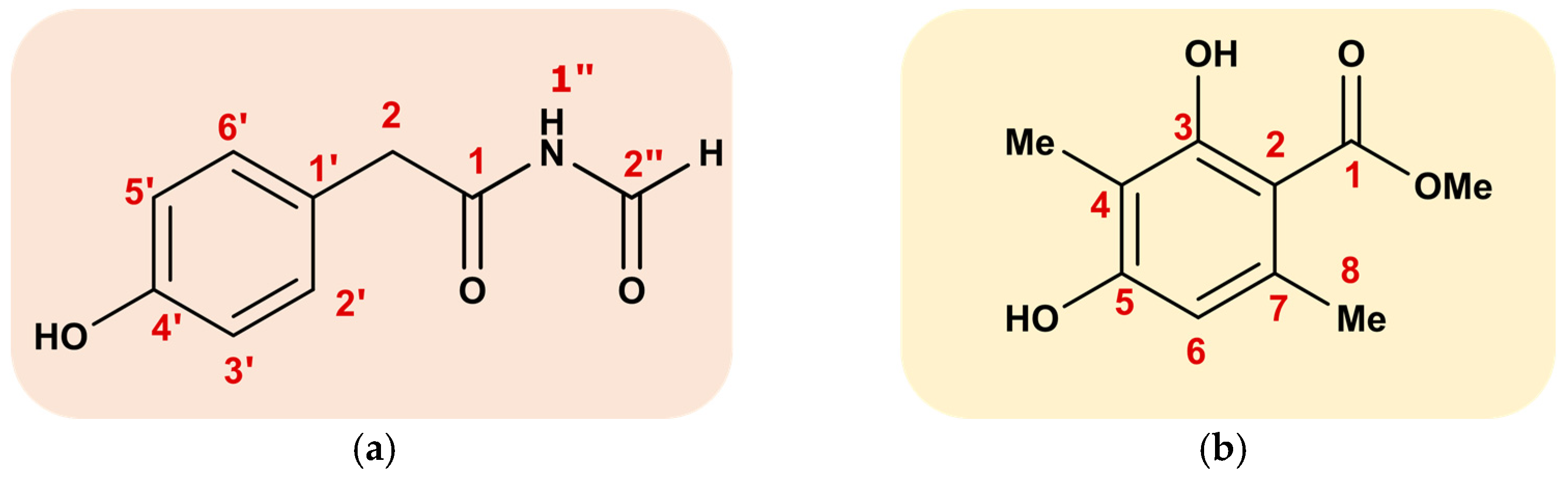
3.4. Structure Identification
3.5. Antimicrobial Activities
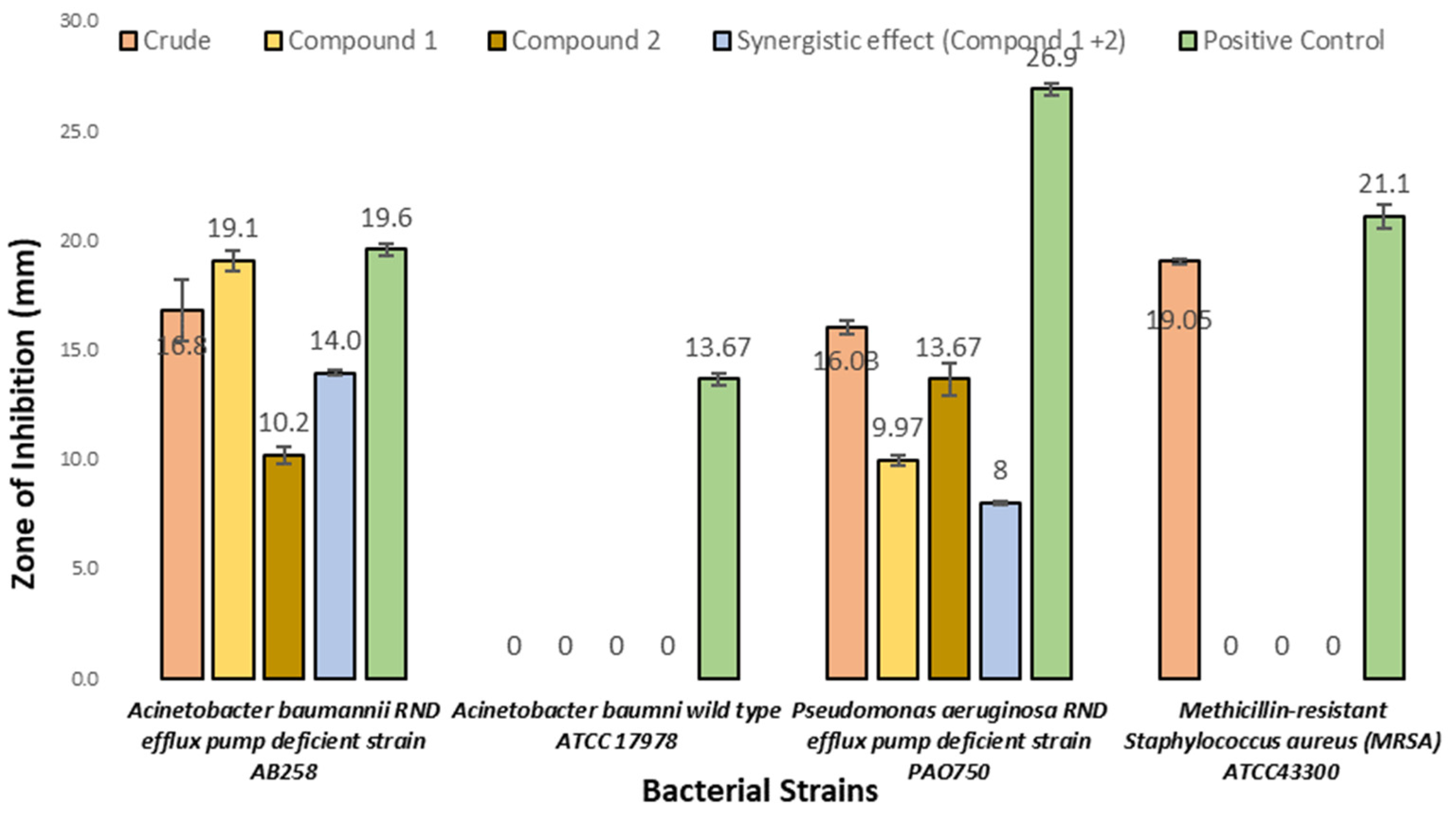
3.5.1. Antibacterial Activities
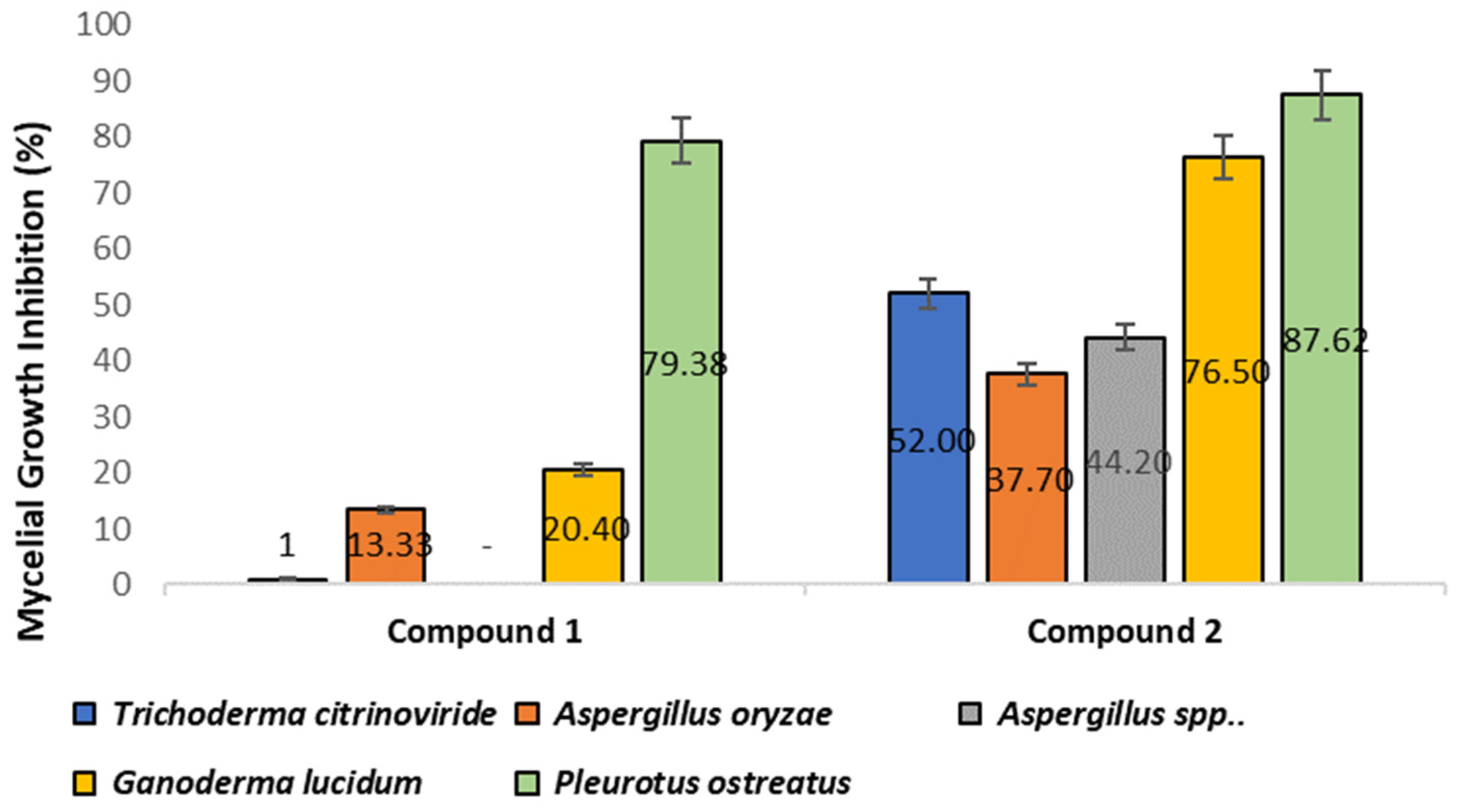
3.5.2. Antifungal Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bérdy, J. Bioactive Microbial Metabolites: A Personal View. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, G.; Balachandran, L. Biodiversity in Production of Antibiotics and Other Bioactive Compounds. Adv. Biochem. Eng. Biotechnol. 2015, 147, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Manganyi, M.C.; Ateba, C.N. Untapped Potentials of Endophytic Fungi: A Review of Novel Bioactive Compounds with Biological Applications. Microorganisms 2020, 8, 1934. [Google Scholar] [CrossRef]
- Zhao, W.-Y.; Yi, J.; Chang, Y.-B.; Sun, C.-P.; Ma, X.-C. Recent Studies on Terpenoids in Aspergillus Fungi: Chemical Diversity, Biosynthesis, and Bioactivity. Phytochemistry 2022, 193, 113011. [Google Scholar] [CrossRef]
- Blachowicz, A.; Chiang, A.J.; Romsdahl, J.; Kalkum, M.; Wang, C.C.C.; Venkateswaran, K. Proteomic Characterization of Aspergillus Fumigatus Isolated from Air and Surfaces of the International Space Station. Fungal Genet. Biol. 2019, 124, 39–46. [Google Scholar] [CrossRef]
- Knox, B.P.; Blachowicz, A.; Palmer, J.M.; Romsdahl, J.; Huttenlocher, A.; Wang, C.C.C.; Keller, N.P.; Venkateswaran, K. Characterization of Aspergillus Fumigatus Isolates from Air and Surfaces of the International Space Station. mSphere 2016, 1, e00227-16. [Google Scholar] [CrossRef]
- Tekaia, F.; Latgé, J.-P. Aspergillus Fumigatus: Saprophyte or Pathogen? Curr. Opin. Microbiol. 2005, 8, 385–392. [Google Scholar] [CrossRef]
- Dagenais, T.R.T.; Keller, N.P. Pathogenesis of Aspergillus Fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Guo, C.-J.; Palmer, J.M.; Sekonyela, R.; Wang, C.C.C.; Keller, N.P. Prototype of an Intertwined Secondary-Metabolite Supercluster. Proc. Natl. Acad. Sci. 2013, 110, 17065–17070. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Rank, C.; Nielsen, K.F.; Larsen, T.O. Metabolomics of Aspergillus Fumigatus. Med. Mycol. 2009, 47, S53–S71. [Google Scholar] [CrossRef]
- Lind, A.L.; Wisecaver, J.H.; Lameiras, C.; Wiemann, P.; Palmer, J.M.; Keller, N.P.; Rodrigues, F.; Goldman, G.H.; Rokas, A. Drivers of Genetic Diversity in Secondary Metabolic Gene Clusters within a Fungal Species. PLoS Biol. 2017, 15, e2003583. [Google Scholar] [CrossRef]
- Bignell, E.; Cairns, T.C.; Throckmorton, K.; Nierman, W.C.; Keller, N.P. Secondary Metabolite Arsenal of an Opportunistic Pathogenic Fungus. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160023. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Clardy, J. One Pathway, Many Products. Nat. Chem. Biol. 2007, 3, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Martinet, L.; Naômé, A.; Deflandre, B.; Maciejewska, M.; Tellatin, D.; Tenconi, E.; Smargiasso, N.; de Pauw, E.; van Wezel, G.P.; Rigali, S. A Single Biosynthetic Gene Cluster Is Responsible for the Production of Bagremycin Antibiotics and Ferroverdin Iron Chelators. mBio 2019, 10, e01230-19. [Google Scholar] [CrossRef]
- Alanjary, A.; Medema, M.H. Mining bacterial genomes to reveal secret synergy. J. Biol. Chem. 2018, 293, 19996–19997. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, S.K.; Kang, J.S.; Choi, H.D.; Son, B.W. Radical Scavenging Hydroxyphenyl Ethanoic Acid Derivatives from a Marine-Derived Fungus. J. Microbiol. Biotechnol. 2006, 16, 637–638. [Google Scholar]
- An, C.-Y.; Li, X.-M.; Li, C.-S.; Gao, S.-S.; Shang, Z.; Wang, B.-G. Triazoles and Other N-Containing Metabolites from the Marine-Derived Endophytic Fungus Penicillium Chrysogenum EN-118. Helv. Chim. Acta 2013, 96, 682–687. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Magaldi, S.; Mata-Essayag, S.; Hartung de Capriles, C.; Perez, C.; Colella, M.T.; Olaizola, C.; Ontiveros, Y. Well Diffusion for Antifungal Susceptibility Testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef]
- Performance Standards for Antimicrobial Susceptibility Testing: 20th Informational Supplement; CLSI Document M100-S20; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010.
- Sadeghian, M.; Shahidi Bonjar, G.H.; Sharifi Sirchi, G.R. Post Harvest Biological Control of Apple Bitter Rot by Soil-Borne Actinomycetes and Molecular Identification of the Active Antagonist. Postharvest Biol. Technol. 2016, 112, 46–54. [Google Scholar] [CrossRef]
- Mun, S.-K.; Kang, K.-Y.; Jang, H.-Y.; Hwang, Y.-H.; Hong, S.-G.; Kim, S.-J.; Cho, H.-W.; Chang, D.-J.; Hur, J.-S.; Yee, S.-T. Atraric Acid Exhibits Anti-Inflammatory Effect in Lipopolysaccharide-Stimulated RAW264.7 Cells and Mouse Models. Int. J. Mol. Sci. 2020, 21, 7070. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Gao, J. Chemistry and Biology of Secondary Metabolites from Aspergillus Genus. Nat. Prod. J. 2018, 8, 275–304. [Google Scholar] [CrossRef]
- Shi, Y.-S.; Zhang, Y.; Chen, X.-Z.; Zhang, N.; Liu, Y.-B. Metabolites Produced by the Endophytic Fungus Aspergillus Fumigatus from the Stem of Erythrophloeum Fordii Oliv. Molecules 2015, 20, 10793–10799. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Liu, X.; Guo, H.; Ren, B.; Chen, C.; Piggott, A.M.; Yu, K.; Gao, H.; Wang, Q.; Liu, M. Brevianamides with Antitubercular Potential from a Marine-Derived Isolate of Aspergillus Versicolor. Org. Lett. 2012, 14, 4770–4773. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Zhu, T.J.; Fan, G.T.; Liu, H.B.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Three New Dioxopiperazine Metabolites from a Marine-Derived Fungus Aspergillus Fumigatus Fres. Nat. Prod. Res. 2010, 24, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.-M.; Wang, B.-G. Anthraquinone Derivatives Produced by Marine-Derived Fungus Aspergillus Versicolor EN-7. Biosci. Biotechnol. Biochem. 2012, 76, 1774–1776. [Google Scholar] [CrossRef]
- Li, D.-H.; Han, T.; Guan, L.-P.; Bai, J.; Zhao, N.; Li, Z.-L.; Wu, X.; Hua, H.-M. New Naphthopyrones from Marine-Derived Fungus Aspergillus Niger 2HL-M-8 and Their in Vitro Antiproliferative Activity. Nat. Prod. Res. 2016, 30, 1116–1122. [Google Scholar] [CrossRef]
- Felix, S.; Sandjo, L.P.; Opatz, T.; Erkel, G. SF002-96-1, a New Drimane Sesquiterpene Lactone from an Aspergillus Species, Inhibits Survivin Expression. Beilstein J. Org. Chem. 2013, 9, 2866–2876. [Google Scholar] [CrossRef]
- Song, F.; Ren, B.; Chen, C.; Yu, K.; Liu, X.; Zhang, Y.; Yang, N.; He, H.; Liu, X.; Dai, H. Three New Sterigmatocystin Analogues from Marine-Derived Fungus Aspergillus Versicolor MF359. Appl. Microbiol. Biotechnol. 2014, 98, 3753–3758. [Google Scholar] [CrossRef]
- Boruta, T.; Bizukojc, M. Production of Lovastatin and Itaconic Acid by Aspergillus Terreus: A Comparative Perspective. World J. Microbiol. Biotechnol. 2017, 33, 34. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, Q.; Wang, J.; Liu, J.; Qi, C.; Lai, Y.; Zhu, H.; Xue, Y.; Hu, Z.; Zhang, Y. Anti-Inflammatory Butenolide Derivatives from the Coral-Derived Fungus Aspergillus Terreus and Structure Revisions of Aspernolides D and G, Butyrolactone VI and 4′, 8′′-Diacetoxy Butyrolactone VI. RSC Adv. 2018, 8, 13040–13047. [Google Scholar] [CrossRef] [PubMed]
- Cazar, M.E.; Schmeda-Hirschmann, G.; Astudillo, L. Antimicrobial Butyrolactone I Derivatives from the Ecuadorian Soil Fungus Aspergillus Terreus Thorn. Var Terreus. World J. Microbiol. Biotechnol. 2005, 21, 1067–1075. [Google Scholar] [CrossRef]
- Perrone, G.; Gallo, A. Aspergillus Species and Their Associated Mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols; Methods in Molecular Biology; Moretti, A., Susca, A., Eds.; Springer: New York, NY, USA, 2017; pp. 33–49. ISBN 978-1-4939-6707-0. [Google Scholar]
- Hessenkemper, W.; Roediger, J.; Bartsch, S.; Houtsmuller, A.B.; van Royen, M.E.; Petersen, I.; Grimm, M.-O.; Baniahmad, A. A Natural Androgen Receptor Antagonist Induces Cellular Senescence in Prostate Cancer Cells. Mol. Endocrinol. 2014, 28, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Güvenç, A.; Küpeli Akkol, E.; Süntar, İ.; Keleş, H.; Yıldız, S.; Çalış, İ. Biological Activities of Pseudevernia Furfuracea (L.) Zopf Extracts and Isolation of the Active Compounds. J. Ethnopharmacol. 2012, 144, 726–734. [Google Scholar] [CrossRef] [PubMed]

| S. No | Bacterial Test Organism | Source |
|---|---|---|
| 1 | Acinetobacter baumannii (RND) efflux pump-deficient strain | AB258 |
| 2 | Acinetobacter baumannii wild-type strain | ATCC17978 |
| 3 | Pseudomonas aeruginosa (RND) efflux pump-deficient strain | PAO750 |
| 4 | Staphylococcus aureus subsp. aureus Rosenach; methicillin-resistant | ATCC43300 |
| Fungal test organism | ||
| 1 | Trichoderma citrinoviride | Lab strain * |
| 2 | Aspergillus oryzae | NSAR1 |
| 3 | Aspergillus spp. | Lab strain * |
| 4 | Ganoderma lucidum | WIN(M)1804 |
| Pleurotus ostreatus | WIN(M)1803 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, H.; Sykes, E.M.E.; Kumar, A.; Sorensen, J.L. Isolation of Bioactive Metabolites from Soil Derived Fungus-Aspergillus fumigatus. Microorganisms 2023, 11, 590. https://doi.org/10.3390/microorganisms11030590
Gill H, Sykes EME, Kumar A, Sorensen JL. Isolation of Bioactive Metabolites from Soil Derived Fungus-Aspergillus fumigatus. Microorganisms. 2023; 11(3):590. https://doi.org/10.3390/microorganisms11030590
Chicago/Turabian StyleGill, Harman, Ellen M. E. Sykes, Ayush Kumar, and John L. Sorensen. 2023. "Isolation of Bioactive Metabolites from Soil Derived Fungus-Aspergillus fumigatus" Microorganisms 11, no. 3: 590. https://doi.org/10.3390/microorganisms11030590
APA StyleGill, H., Sykes, E. M. E., Kumar, A., & Sorensen, J. L. (2023). Isolation of Bioactive Metabolites from Soil Derived Fungus-Aspergillus fumigatus. Microorganisms, 11(3), 590. https://doi.org/10.3390/microorganisms11030590







