Perspectives on the Use of Biopolymeric Matrices as Carriers for Plant-Growth Promoting Bacteria in Agricultural Systems
Abstract
1. Introduction
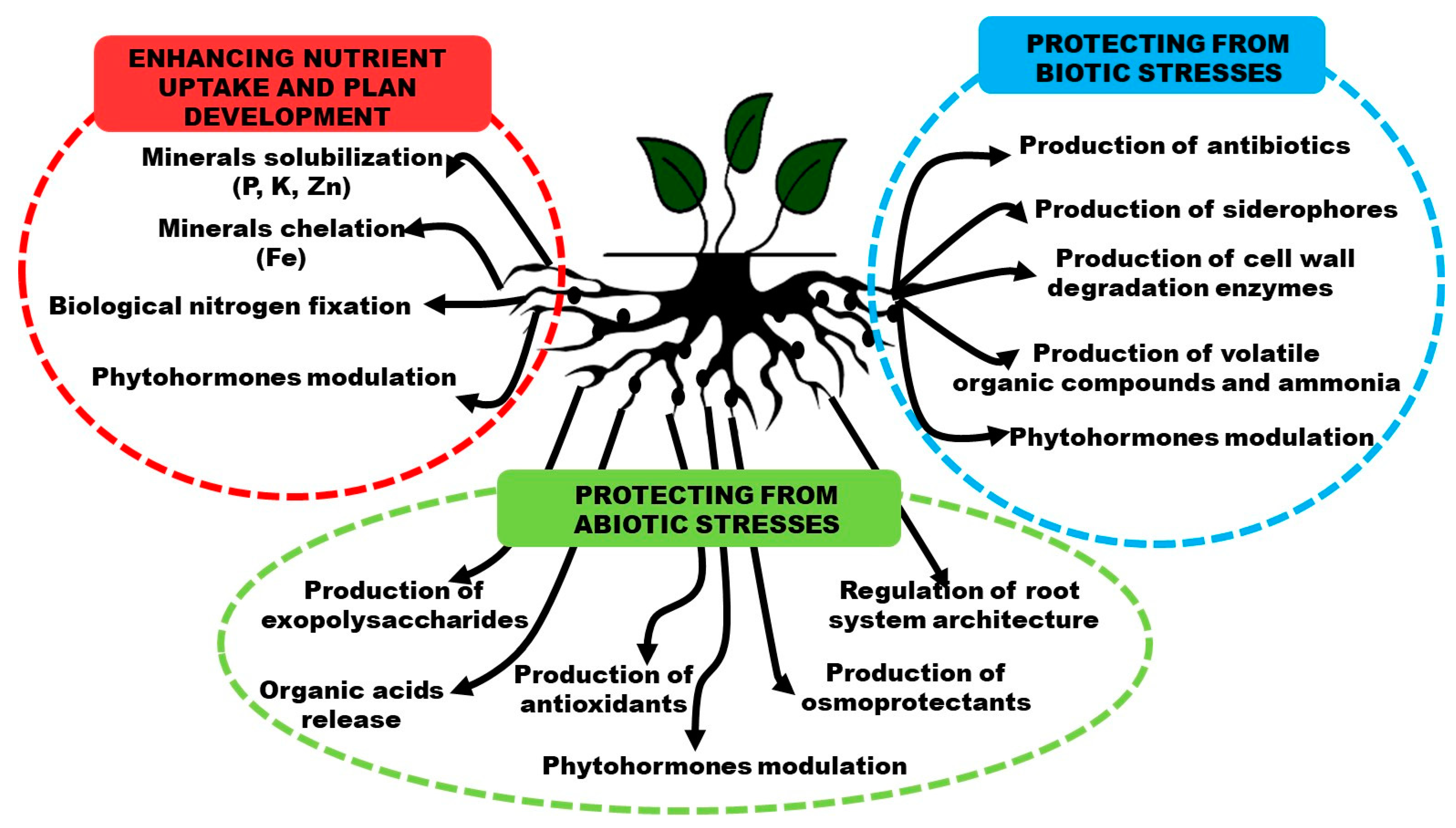
2. Plant Growth-Promoting Bacteria (PGPB) and Their Mechanism of Action
3. Biopolymeric Matrices as Carriers for PGPB
3.1. Polymeric Matrices Based on Alginate
3.2. Polymeric Matrices Based on Starch
3.3. Polymeric Matrices Based on Chitosan
3.4. Polymeric Matrices Based on Gelatin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2022. In Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable; FAO: Rome, Italy, 2022; 231p. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, S.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Kumar, M.; Hasanuzzaman, M. Mechanistic Insights of Plant Growth Promoting Bacteria Mediated Drought and Salt Stress Tolerance in Plants for Sustainable Agriculture. Int. J. Mol. Sci. 2022, 23, 3741. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Optimistic Contributions of Plant Growth-Promoting Bacteria for Sustainable Agriculture and Climate Stress Alleviation. Environ. Res. 2023, 217, 114924. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group ii to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022; 3056p. [Google Scholar]
- Sohail, M.T.; Mustafa, S.; Ali, M.M.; Riaz, S. Agricultural Communities’ Risk Assessment and the Effects of Climate Change: A Pathway toward Green Productivity and Sustainable Development. Front. Environ. Sci. 2022, 10, 948016. [Google Scholar] [CrossRef]
- Muhie, S.H. Novel Approaches and Practices to Sustainable Agriculture. J. Agric. Food Res. 2022, 10, 100446. [Google Scholar] [CrossRef]
- Cernev, T.; Fenner, R. The Importance of Achieving Foundational Sustainable Development Goals in Reducing Global Risk. Futures 2020, 115, 102492. [Google Scholar] [CrossRef]
- Jaiswar, A.; Varshney, D.; Kaushik, V.; Sharma, N.; Bedi, A. Plant-Associated Bacteria in Ecosystems Functioning and Sustainability. In Microbial Bioremediation; Bhat, R.A., Butnariu, M., Dar, G.H., Hakeem, K.R., Eds.; Springer: New York, NY, USA, 2023; pp. 265–281. [Google Scholar]
- Gamalero, E.; Bona, E.; Glick, B.R. Current Techniques to Study Beneficial Plant-Microbe Interactions. Microorganisms 2022, 10, 1380. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Pirzada, T.; Farias, B.V.; Mathew, R.; Guenther, R.H.; Byrd, M.V.; Sit, T.L.; Khan, S.A. Recent Advances in Biodegradable Matrices for Active Ingredient Release in Crop Protection: Towards Attaining Sustainability in Agriculture. COCIS 2020, 48, 121–136. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying Mechanics of Plant Growth Promoting Rhizobacteria (PGPR): A Review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Ebrahimi-Zarandi, M.; Gholizadeh Vazvani, M.; Skorik, Y.A. Reducing drought stress in plants by Encapsulating Plant Growth-Promoting Bacteria with Polysaccharides. Int. J. Mol. Sci. 2021, 22, 12979. [Google Scholar] [CrossRef] [PubMed]
- Elnahal, A.S.M.; Saadoney, M.T.; Saad, A.M.; Desoky, E.S.M.; Tahan, A.M.; Rady, M.M.; Abuqamar, S.; Tarabily, K.A. The Use of Microbial Inoculants for Biological Control, Plant Growth Promotion, and Sustainable Agriculture: A Review. Eur. J. Plant. Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Alenezi, F.N.; Belbahri, L. Recent Advances in Encapsulation Techniques of Plant Growth-Promoting Microorganisms and their Prospects in the Sustainable Agriculture. Appl. Sci. 2022, 12, 9020. [Google Scholar] [CrossRef]
- Marcelino, P.R.F.; Milani, K.M.; Mali, S.; Santos, O.J.A.P.; de Oliveira, A.L.M. Formulations of Polymeric Biodegradable Low-Cost Foam by Melt Extrusion to Deliver Plant Growth-Promoting Bacteria in Agricultural Systems. Appl. Microbiol. Biotechnol. 2016, 100, 7323–7338. [Google Scholar] [CrossRef] [PubMed]
- Vercelheze, A.E.S.; Marim, B.M.; Oliveira, A.L.M.; Mali, S. Development of Biodegradable Coatings for Maize Seeds and their Application for Azospirillum brasilense Immobilization. Appl. Microbiol. Biotechnol. 2019, 103, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Juric, S.; Ðermic, E.; Topolovec-Pintaric, S.; Bedek, M.; Vincekovic, M. Physicochemical Properties and Release Characteristics of Calcium Alginate Microspheres Loaded with Trichoderma viride spores. J. Integr. Agric. 2019, 18, 2534–2548. [Google Scholar] [CrossRef]
- Bashan, Y.; Hernandez, J.P.; Leyva, L.A.; Bacilio, M. Alginate Microbeads as Inoculant Carriers for Plant Growth-Promoting Bacteria. Biol. Fertil. Soils 2002, 35, 359–368. [Google Scholar] [CrossRef]
- Young, C.C.; Rekha, P.D.; Lai, W.A.; Arun, A.B. Encapsulation of Plant Growth-Promoting Bacteria in Alginate Beads Enriched with Humic Acid. Biotechnol. Bioeng. 2006, 95, 76–83. [Google Scholar] [CrossRef]
- Perez, J.J.; Francois, N.J.; Maroniche, G.A.; Borrajo, M.P.; Pereyra, M.A.; Creus, C.M. A Novel, Green, Low-Cost Chitosan-Starch Hydrogel as Potential Delivery System for Plant Growth-Promoting Bacteria. Carbohydr. Polym. 2018, 202, 409–417. [Google Scholar] [CrossRef]
- Lobo, C.B.; Tomás, M.S.J.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of Low-Cost Formulations of Plant Growth-Promoting Bacteria to Be Used as Inoculants in Beneficial Agricultural Technologies. Microbiol. Res. 2018, 219, 12–25. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts Towards Overcoming Drought Stress in Crops: Revisiting the Mechanisms Employed by Plant Growth-Promoting Bacteria. Front. Microbiol. 2022, 13, 962427. [Google Scholar] [CrossRef]
- Sati, D.; Pande, V.; Pandey, S.C.; Samant, M. Recent Advances in PGPR and Molecular Mechanisms Involved in Drought Stress Resistance. J. Soil Sci. Plant Nutr. 2022, 1–19. [Google Scholar] [CrossRef]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The Role of Plant Growth-Promoting Bacteria in Alleviating the Adverse Effects of Drought on Plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Ortiz-Castro, R.; Campos-García, J.; López-Bucio, J. Pseudomonas putida and Pseudomonas fluorescens influence Arabidopsis Root System Architecture through an Auxin Response Mediated by Bioactive Cyclodipeptides. J. Plant Growth Regul. 2020, 39, 254–265. [Google Scholar] [CrossRef]
- Glick, B.R. The Enhancement of Plant Growth by Free-Living Bacteria. Can. J. Microbiol. 1995, 41, 109–111. [Google Scholar] [CrossRef]
- Stegelmeier, A.A.; Rose, D.M.; Joris, B.R.; Glick, B.R. The Use of PGPB to Promote Plant Hydroponic Growth. Plants 2022, 11, 2783. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Qadir, M.; Alataway, A.; Dewidar, A.Z.; Elansary, H.O.; Lee, I. Phytohormones Producing Rhizobacteria Alleviate Heavy Metals Stress in Soybean through Multilayered Response. Microbiol. Res. 2023, 266, 127237. [Google Scholar]
- Mishra, P.; Mishra, J.; Arora, N.K. Plant Growth Promoting Bacteria for Combating Salinity Stress in Plants—Recent Developments and Prospects: A Review. Microbiol. Res. 2021, 252, 126861. [Google Scholar] [CrossRef] [PubMed]
- Ngalimat, M.S.; Mohd Hata, E.; Zulperi, D.; Ismail, S.I.; Ismail, M.R.; Mohd Zainudin, N.A.I.; Saidi, N.B.; Yusof, M.T. Plant Growth-Promoting Bacteria as an Emerging Tool to Manage Bacterial Rice Pathogens. Microorganisms 2021, 9, 682. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenge for Sustainable Crop Improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Javed, M.A.; Afridi, M.S.; Abbasi, H.A.; Qayyum, A.; Batool, T.; Ullah, A.; Marc, R.A.; Jaouni, S.K.; et al. Role of Endophytic Bacteria in Salinity Stress Amelioration by Physiological and Molecular Mechanisms of Defense: A Comprehensive Review. S. Afri. J. Bot. 2022, 151, 33–46. [Google Scholar] [CrossRef]
- Morales, C.L.R.; Mosqueda, O.M.C.; Lara, L.P.D.; Cota, P.F.I.; Villalobos, S.S.; Santoyo, G. Plant Growth-Promoting Bacterial Endophytes as Biocontrol Agents of Pre- and Post-Harvest Diseases: Fundamentals, Methods of Application and Future Perspectives. Microbiol. Res. 2020, 242, 126612. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in Plant Growth-Promoting Bacterial Inoculant Technology: Formulations and Practical Perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Santos, M.S.; Rodrigues, T.F.; Nogueira, M.A.; Hungria, M. The Challenge of Combining High Yields with Environmentally Friendly Bioproducts: A Review on the Compatibility of Pesticides with Microbial Inoculants. Agronomy 2021, 11, 870. [Google Scholar] [CrossRef]
- Hungria, M.; Mendes, I.C. Nitrogen Fixation with Soybean: The Perfect Symbiosis? In Biological Nitrogen Fixation; Wiley: Hoboken, NJ, USA, 2015; pp. 1009–1024. [Google Scholar]
- Freitas, V.F.; Cerezini, P.; Hungria, M.; Nogueira, M.A. Strategies to Deal with Drought-Stress in Biological Nitrogen Fixation in Soybean. Appl. Soil Ecol. 2022, 172, 104352. [Google Scholar] [CrossRef]
- Martins, M.R.; Jantalia, C.P.; Reis, V.M. Impact of Plant Growth-Promoting Bacteria on Grain Yield, Protein Content, and Urea-15 N Recovery by Maize in a Cerrado Oxisol. Plant Soil 2018, 422, 239–250. [Google Scholar] [CrossRef]
- Furlan, F.; Saatkamp, K.; Volpiano, C.G.; De Assis Franco, F.; Dos Santos, M.F.; Vendruscolo, E.C.G. Plant Growth-Promoting Bacteria Effect in Withstanding Drought in Wheat Cultivars. Sci. Agrar. 2017, 18, 104–113. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Express 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Aquino, J.P.A.; Macedo, F.B.J.; Antunes, J.E.L.; Figueiredo, M.V.B.; Neto, F.A.; Araujo, A.S.F. Plant Growth-Promoting Endophytic Bacteria on Maize and Sorghum|1. Pesqui. Agropecu. Trop. 2019, 49, 56241. [Google Scholar] [CrossRef]
- Lee, K.J.; Kamala-Kannan, S.; Sub, H.S.; Seong, C.K.; Lee, G.W. Biological Control of Phytophthora Blight in Red Pepper (Capsicum annuum L.) using Bacillus subtilis. World J. Microbiol. Biotechnol. 2008, 24, 1139–1145. [Google Scholar] [CrossRef]
- Abadi, V.A.J.M.; Sepehri, M.; Rahmani, H.A.; Zarei, M.; Ronaghi, A.; Taghavi, S.M.; Shamshiripour, M. Role of Dominant Phyllosphere Bacteria with Plant Growth–Promoting Characteristics on Growth and Nutrition of Maize (Zea mays L.). J. Soil Sci. Plant Nutr. 2020, 20, 2348–2363. [Google Scholar] [CrossRef]
- Kang, S.M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.J.; Park, J.M.; Kim, B.R.; Shin, D.H.; Lee, I.J. Gibberellin Secreting Rhizobacterium, Pseudomonas putida H-2-3 Modulates the Hormonal and Stress Physiology of Soybean to Improve the Plant Growth under Saline and Drought Conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar] [CrossRef]
- Sandhya, V.Z.A.S.; SK, Z.A.; Grover, M.; Reddy, G.; Venkateswarlu, B. Alleviation of Drought Stress Effects in Sunflower Seedlings by the Exopolysaccharides Producing Pseudomonas putida strain GAP-p45. Biol. Fertil. Soils 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Shintu, P.V.; Jayaram, K.M. Phosphate Solubilising Bacteria (Bacillus polymyxa)—An Effective Approach to Mitigate Drought in Tomato (Lycopersicon esculentum Mill.). Trop. Plant Res. 2015, 2, 17–22. [Google Scholar]
- Timmusk, S.; El-Daim, I.A.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-Tolerance of Wheat Improved by Rhizosphere Bacteria from Harsh Environments: Enhanced Biomass Production and Reduced Emissions of Stress Volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef]
- Armada, E.; Probanza, A.; Roldán, A.; Azcón, R. Native Plant Growth Promoting Bacteria Bacillus thuringiensis and Mixed or Individual Mycorrhizal Species Improved Drought Tolerance and Oxidative Metabolism in Lavandula dentata Plants. J. Plant Physiol. 2016, 192, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, Y.; Sheng, H.; Li, H.; Liu, X. Effects of the Inoculations Using Bacteria Producing ACC Deaminase on Ethylene Metabolism and Growth of Wheat Grown under Different Soil Water Contents. Plant Physiol. Biochem. 2018, 125, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Khan, A.L.; Waqas, M.; You, Y.H.; Kim, J.H.J.G.; Kim, J.H.J.G.; Hamayun, M.; Lee, I.J. Plant Growth-Promoting Rhizobacteria Reduce Adverse Effects of Salinity and Osmotic Stress by Regulating Phytohormones and Antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Zuluaga, M.Y.A.; Milani, K.M.L.; Miras-Moreno, B.; Lucini, L.; Valentinuzzi, F.; Mimmo, T.; Pii, Y.; Cesco, S.; Rodrigues, E.P.; de Oliveira, A.L.M. Inoculation with Plant Growth-Promoting Bacteria Alters the Rhizosphere Functioning of Tomato Plants. Appl. Soil Ecol. 2020, 158, 103784. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, Y.; Yuan, Q.; Shi, G.; Wu, L.; Lou, Z.; Huo, R.; Wu, H.; Borriss, R.; Gao, X. Bacillomycin D Produced by Bacillus amyloliquefaciens is Involved in the Antagonistic Interaction with the Plant-Pathogenic Fungus Fusarium graminearum. Appl. Environ. Microbiol. 2017, 83, e01075. [Google Scholar] [CrossRef]
- Hungria, M.; Rondina, A.B.L.; Nunes, A.L.P.; Araújo, R.S.; Nogueira, M.A. Seed and Leaf-Spray Inoculation of PGPR in Brachiarias (Urochloa spp.) as an Economic and Environmental Opportunity to Improve Plant Growth, Forage Yield and Nutrient Status. Plant Soil 2021, 463, 171–186. [Google Scholar] [CrossRef]
- Ghadamgahi, F.; Tarighi, S.; Taheri, P.; Saripella, G.V.; Anzalone, A.; Kalyandurg, P.B.; Catara, V.; Ortiz, R.; Vetukuri, R.R. Plant Growth-Promoting Activity of Pseudomonas Aeruginosa FG106 and its Ability to Act as a Biocontrol Agent against Potato, Tomato and Taro Pathogens. Biology 2022, 11, 140. [Google Scholar] [CrossRef]
- Choi, S.I.; Ajuna, H.B.; Won, S.J.; Choub, V.; Kim, C.W.; Moon, J.H.; Ahn, Y.S. The Insecticidal Potential of Bacillus velezensis CE 100 against Dasineura jujubifolia Jiao & Bu (Diptera: Cecidomyiidae) Larvae Infestation and its Role in the Enhancement of Yield and Fruit Quality of Jujube (Zizyphus jujuba Miller var. inermis Rehder). Crop Protec. 2023, 163, 106098. [Google Scholar]
- Chamekh, A.; Kharbech, O.; Fersi, C.; Liman, R.D.; Brandt, K.K.; Djebali, W.; Chouari, R. Insights on Strain 115 Plant Growth-Promoting Bacteria Traits and its Contribution in Lead Stress Alleviation in Pea (Pisum sativum L.) Plants. Arch. Microbiol. 2023, 205, 1–12. [Google Scholar] [CrossRef]
- Gohil, R.B.; Raval, V.H.; Panchal, R.R.; Rajput, K.N. Plant Growth Promoting Activities and Effect of Fermented Panchagavya Isolate Klebsiella sp. PG-64 on Vigna radiata. World J. Microbiol. Biotechnol. 2023, 39, 41. [Google Scholar] [CrossRef]
- Tyagia, J.; Mishra, A.; Kumari, S.; Singh, S.; Agarwal, H.; Pudake, R.M.; Varma, A.; Joshi, N.C. Deploying a Microbial Consortium of Serendipita indica, Rhizophagus intraradices, and Azotobacter chroococcum to Boost Drought Tolerance in Maize. Environ. Exp. Bot. 2023, 206, 105142. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Outstanding Impact of Azospirillum brasilense Strains Ab-V5 and Ab-V6 on the Brazilian Agriculture: Lessons that Farmers are Receptive to Adopt New Microbial Inoculants. Rev. Bras. Ciênc. Solo 2021, 45, 0200128. [Google Scholar] [CrossRef]
- Farquharson, E.A.; Ballard, R.A.; Herridge, D.F.; Ryder, M.H.; Denton, M.D.; Webster, A.; Yates, R.J.; Seymour, N.P.; Deaker, R.J.; Hartley, E.; et al. Inoculating Legumes: Practice and Science; Grains Research and Development Corporation: Barton, Australia, 2022. [Google Scholar]
- MAPA—Ministério da Agricultura, Pecuária e Abastecimento. Instrução Normativa No 5, de 6 de Agosto de 2004; Diário Oficial da União da República Federativa do Brasil: Brasília; Brasil, 2004. [Google Scholar]
- MAPA—Ministério da Agricultura, Pecuária e Abastecimento. Instrução Normativa No 13, de 24 de Março de 2011; Diário Oficial da União da República Federativa do Brasil: Brasília; Brasil, 2011. [Google Scholar]
- Hungria, M.; Campo, R.J. Inoculantes Microbianos: Situação no Brasil. In Biofertilizantes en Iberoamérica: Visión Técnica, Científica y Empresarial; Izaguirre-Mayoral, M.L., Labandera, C., Sanjuan, J., Eds.; Cyted/Biofag: Montevideo, Uruguay, 2007; pp. 22–31. [Google Scholar]
- Fusco, G.M.; Nicastro, R.; Rouphael, Y.; Carillo, P. The effects of the microbial biostimulants approved by EU regulation 2019/1009 on yield and quality of vegetable crops. Foods 2022, 11, 2656. [Google Scholar] [CrossRef] [PubMed]
- EC. REGULATION (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 12 November 2022).
- Schoebitz, M.; López, M.D.; Roldán, A. Bioencapsulation of Microbial Inoculants for Better Soil–Plant Fertilization. A Review. Agron. Sustain. Dev. 2013, 33, 751–765. [Google Scholar] [CrossRef]
- Malik, R.; Saxena, R.; Warkar, S.G. Biopolymer-Based Biomatrices—Organic Strategies to Combat Micronutrient Deficit for Dynamic Agronomy. ChemistrySelect 2022, 7, e202200006. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Surampalli, R.Y.; Prévost, D. Bio-Encapsulation of Microbial Cells for Targeted Agricultural Delivery. Crit. Rev. Biotechnol. 2011, 31, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Vemmer, M.; Patel, A.V. Review of Encapsulation Methods Suitable for Microbial Biological Control Agents. Biol. Control. 2013, 67, 380–389. [Google Scholar] [CrossRef]
- Chaudhary, T.; Dixit, M.; Gera, R.; Shukla, A.K.; Prakash, A.; Gupta, G.; Shukla, P. Techniques for Improving Formulations of Bioinoculants. 3Biotech 2020, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Karmakar, S.; Manna, S.; Kabiraj, S.; Jana, S. Recent progress in alginate-based carriers for ocular targeting of therapeutics. FHFH 2022, 2, 100071. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J. Introductory Chapter: Alginates—A General Overview. In Alginates—Recent Uses of This Natural Polymer; Pereira, L., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Martínez-Cano, B.; Mendoza-Meneses, C.J.; García-Trejo, J.F.; Macías-Bobadilla, G.; Aguirre-Becerra, H.; Soto-Zarazúa, G.M.; Feregrino-Pérez, A.A. Review and Perspectives of the Use of Alginate as a Polymer Matrix for Microorganisms Applied in Agro-Industry. Molecules 2022, 27, 4248. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y. Alginate Beads as Synthetic Inoculant Carriers for Slow Release of Bacteria that Affect Plant Growth. Appl. Environ. Microbiol. 1986, 51, 1089–1098. [Google Scholar] [CrossRef]
- Bashan, Y.; Gonzalez, L.E. Long-Term Survival of the Plant-Growth-Promoting Bacteria Azospirillum brasilense and Pseudomonas fluorescens in Dry Alginate Inoculant. Appl. Microbiol. Biotechnol. 1999, 51, 262–266. [Google Scholar] [CrossRef]
- Russo, A.; Basaglia, M.; Tola, E.; Casella, S. Survival, Root Colonization and Biocontrol Capacities of Pseudomonas fluorescens F113 LacZY in Dry Alginate Microbeads. J. Ind. Microbiol. Biotechnol. 2001, 27, 337–342. [Google Scholar] [CrossRef]
- Joe, M.M.; Saravanan, V.S.V.; Islam, M.R.; Sa, T. Development of Alginate-Based Aggregate Inoculants of Methylobacterium sp. and Azospirillum brasilense Tested Under in vitro Conditions to Promote Plant Growth. J. Appl. Microbiol. 2014, 116, 408–423. [Google Scholar] [CrossRef]
- El-Komy, H. Coimmobilization of Azospirillum lipoferum and Bacillus megaterium for Successful Phosphorus and Nitrogen Nutrition of Wheat Plants. Food Technol. Biotechnol. 2005, 43, 19–27. [Google Scholar]
- Yabur, R.; Bashan, Y.; Hernández-Carmona, G. Alginate from the Macroalgae Sargassum sinicola as a Novel Source for Microbial Immobilization Material in Wastewater Treatment and Plant Growth Promotion. J. Appl. Phycol. 2007, 19, 43–53. [Google Scholar] [CrossRef]
- Trivedi, P.; Pandey, A. Recovery of Plant Growth-Promoting Rhizobacteria from Sodium Alginate Beads after 3 Years following Storage at 4 °C. J. Ind. Microbiol. Biotechnol. 2008, 35, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, Y.; Kaleem, I.; Li, C. Preparation of Calcium–Alginate Microcapsuled Microbial Fertilizer Coating Klebsiella oxytoca Rs-5 and its Performance under Salinity Stress. Eur. J. Soil Biol. 2011, 47, 152–159. [Google Scholar] [CrossRef]
- Schoebitz, M.; Simonin, H.; Poncelet, D. Starch Filler and Osmoprotectants Improve the Survival of Rhizobacteria in Dried Alginate Beads. J. Microencapsul. 2012, 29, 532–538. [Google Scholar] [CrossRef]
- Reetha, D.; Kumaresan, G.; John Milton, D. Studies to Improve the Shelf Life of Azospirillum lipoferum Immobilized in Alginate Beads. Int. J. Recent Sci. Res. 2014, 5, 2178–2182. [Google Scholar]
- Wang, H.Q.; Hua, F.; Zhao, Y.C.; Li, Y.; Wang, X. Immobilization of Pseudomonas sp. DG17 onto Sodium Alginate–Attapulgite–Calcium Carbonate. Biotechnol. Biotechnol. Equip. 2014, 28, 834–842. [Google Scholar] [CrossRef]
- Farhat, M.B.; Taktek, S.; Chouayekh, H. Encapsulation in Alginate Enhanced the Plant Growth Promoting Activities of Two Phosphate Solubilizing Bacteria Isolated from the Phosphate Mine of Gafsa. Net J. Agric. Sci. 2014, 2, 131–139. [Google Scholar]
- Forestier, S.; Alvarado, G.; Badjel Badjel, S.; Lesueur, D. Effect of Rhizobium Inoculation Methodologies on Nodulation and Growth of Leucaena leucocephala. World J. Microbiol. Biotechnol. 2001, 17, 359–362. [Google Scholar] [CrossRef]
- Gurley, H.G.; Zdor, R.E. Differential Rhizosphere Establishment and Cyanide Production by Alginate-Formulated Weed-Deleterious Rhizobacteria. Curr. Microbiol. 2005, 50, 167–171. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Moradi-Pour, M. The Effect of Bacillus subtilis Vru1 Encapsulated in Alginate–Bentonite Coating Enriched with Titanium Nanoparticles against Rhizoctonia solani on Bean. Int. J. Biol. Macromol. 2020, 152, 1089–1097. [Google Scholar] [CrossRef]
- Panichikkal, J.; Prathap, G.; Nair, R.A.; Krishnankutty, R.E. Evaluation of Plant Probiotic Performance of Pseudomonas sp. Encapsulated in Alginate Supplemented with Salicylic Acid and Zinc Oxide Nanoparticles. Int. J. Biol. Macromol. 2021, 166, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Souza-Alonso, P.; Rocha, M.; Rocha, I.; Ma, Y.; Freitas, H.; Oliveira, R.S. Encapsulation of Pseudomonas libanensis in Alginate Beads to Sustain Bacterial Viability and Inoculation of Vigna unguiculata under Drought Stress. Biotech 2021, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, L.; Lang, D.; Zhang, X.; Ma, X.; Li, X.; Zhang, X. Eco-friendly Bio-Encapsulation from Sodium Alginate-Trehalose-Kaolin and its Performance Evaluation in Improving Plant Growth under Salt or/and Drought Conditions. Int. J. Biol. Macromol. 2023, 225, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Hernandéz-Montiel, L.G.; Contreras, C.J.C.; Amador, B.M.; Hernández, L.V.; Aguilar, E.E.Q. Contreras, R.G.C. Efficiency of Two Inoculation Methods of Pseudomonas putida on Growth and Yield of Tomato Plants. J. Soil Sci. Plant Nutr. 2017, 17, 1003–1012. [Google Scholar] [CrossRef]
- Zago, S.L.; dos Santos, M.F.; Konrad, D.; Fiorini, A.; Rosado, F.R.; Missio, R.F.; Vendruscolo, E.C.G. Shelf Life of Azospirillum brasilense in Alginate Beads Enriched with Trehalose and Humic Acid. J. Agric. Sci. 2019, 11, 269–280. [Google Scholar] [CrossRef]
- Wiwattanapatapee, R.; Chumthong, A.; Pengnoo, A.; Kanjanamaneesathian, M. Preparation and Evaluation of Bacillus megaterium-Alginate Microcapsules for Control of Rice Sheath Blight Disease. World J. Microbiol. Biotechnol. 2013, 29, 1487–1497. [Google Scholar] [CrossRef]
- Pour, M.M.; Riseh, R.S.; Mohammadinejad, R.; Hosseini, A. Investigating the Formulation of Alginate-Gelatin Encapsulated Pseudomonas fluorescens (VUPF5 and T17–4 Strains) for Controlling Fusarium solani on Potato. Int. J. Biol. Macromol. 2019, 133, 603–613. [Google Scholar] [CrossRef]
- Jung, G.; Mugnier, J.; Diem, H.G. Polymer-Entrapped Rhizobium as an Inoculant for Legumes. Plant Soil 1982, 65, 219–231. [Google Scholar] [CrossRef]
- Fages, J. An industrial view of Azospirillum inoculants: Formulation and Application Technology. Symbiosis 1992, 13, 15–26. [Google Scholar]
- Szopa, D.; Mielczarek, M.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Chojnacka, K.; Krowiak, A.W. Encapsulation Efficiency and Survival of Plant Growth-Promoting Microorganisms in an Alginate-Based Matrix—A Systematic Review and Protocol for a Practical Approach. Ind. Crops Prod. 2022, 181, 114846. [Google Scholar] [CrossRef]
- Rohman, S.; Kaewtatip, K.; Kantachote, D.; Tantirungkij, M. Encapsulation of Rhodopseudomonas palustris KTSSR54 Using Beads from Alginate/Starch Blends. J. Appl. Polym. Sci. 2021, 138, 50084. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Hassanisaadi, M.; Vatankhaha, M.; Kennedy, J.F. Encapsulating Biocontrol Bacteria with Starch as a Safe and Edible Biopolymer to Alleviate Plant Diseases: A Review. Carbohydr. Polym. 2023, 302, 120384. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ismail, S.; Dadrasnia, A. Encapsulation of Plant Growth Promoting Rhizobacteria—Prospects and Potential in Agricultural Sector: A Review. J. Plant Nutr. 2019, 42, 2600–2623. [Google Scholar] [CrossRef]
- Falcão, L.d.S.; Coelho, D.B.; Veggi, P.C.; Campelo, P.H.; Albuquerque, P.M.; de Moraes, M.A. Starch as a Matrix for Incorporation and Release of Bioactive Compounds: Fundamentals and Applications. Polymers 2022, 14, 2361. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, Fine Structure and Architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Minakawa, A.F.K.; Faria-Tischer, P.C.S.; Mali, S. Simple Ultrasound Method to Obtain Starch Micro- and Nanoparticles from Cassava, Corn and Yam Starches. Food Chem. 2019, 283, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.; Grossmann, M.V.S.; Yamashita, F. Filmes de Amido: Produção, Propriedades e Potencial de Utilização. Semin. Cienc. Agrar. 2010, 31, 137–156. [Google Scholar] [CrossRef]
- Sueiro, A.C.; Faria-Tischer, P.C.S.; Lonni, A.A.S.G.; Mali, S. Filmes Biodegradáveis de Amido de Mandioca, Pululana e Celulose Bacteriana. Quim. Nova 2016, 39, 1059–1064. [Google Scholar]
- Mali, S.; Sakanaka, L.S.; Yamashita, F.; Grossmann, M.V.E. Water Sorption and Mechanical Properties of Cassava Starch Films and their Relation to Plasticizing Effect. Carbohydr. Polym. 2005, 60, 283–289. [Google Scholar] [CrossRef]
- Luo, K.; Adra, H.J.; Kim, Y.-R. Preparation of Starch-Based Drug Delivery System through the Self-Assembly of Short Chain Glucans and Control of its Release Property. Carbohydr. Polym. 2020, 243, 116385. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, C.; Huang, Q. Combining in vitro Digestion Model with Cell Culture Model: Assessment of Encapsulation and Delivery of Curcumin in Milled Starch Particle Stabilized Pickering Emulsions. Int. J. Biol. Macromol. 2019, 139, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Yan, X.; Cui, Y.; Han, M.; Wang, Y.; Wang, J.; Zhang, R.; Wang, X. Characterization and Release Kinetics Study of Active Packaging Films Based on Modified Starch and Red Cabbage Anthocyanin Extract. Polymers 2022, 14, 1214. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.D.M.; Filgueiras, C.T.; Garcia, V.A.D.S.; De Carvalho, R.A.; Velasco, J.I.; Fakhouri, F.M. Antimicrobial Activity and GC-MS Profile of Copaiba Oil for Incorporation into Xanthosoma Mafaffa Schott Starch-Based Films. Polymers 2020, 12, 2883. [Google Scholar] [CrossRef]
- Tomsone, L.; Galoburda, R.; Kruma, Z.; Durrieu, V.; Cinkmanis, I. Microencapsulation of Horseradish (Armoracia rusticana L.) Juice Using Spray-Drying. Foods 2020, 9, 1332. [Google Scholar] [CrossRef]
- Ravichai, K.; Muangrat, R. Effect of Different Coating Materials on Freeze-Drying Encapsulation of Bioactive Compounds from Fermented Tea Leaf Wastewater. J. Food Process. Preserv. 2019, 43, e14145. [Google Scholar] [CrossRef]
- Ferreira, D.C.M.; Souza, A.L.; Silveira, J.V.W.; Marim, B.M.; Giraldo, G.A.G.; Mantovan, J.; Mali, S.; Pelissari, F.M. Chitosan Nanocomposites for Food Packaging Applications. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 393–434. [Google Scholar]
- Ramírez, M.Á.; Rodriguez, A.T.; Alfonso, L.; Peniche, C. Chitin and its Derivatives as Biopolymers with Potential Agricultural Applications. Biotecnol. Aplic. 2010, 27, 270–276. [Google Scholar]
- Saberi-Riseh, R.; Tamanadar, E.; Hajabdollahi, N.; Vatankhah, M.; Thakur, V.K.; Skorik, Y.A. Chitosan Microencapsulation of Rhizobacteria for Biological Control of Plant Pests and Diseases: Recent Advances and Applications. Rhizosphere 2022, 23, 100565. [Google Scholar] [CrossRef]
- Chanratana, M.; Han, G.H.; Joe, M.M.; Choudhury, A.R.; Sundaram, S.; Halim, M.A.; Sa, T. Evaluation of Chitosan and Alginate Immobilized Methylobacterium oryzae CBMB20 on Tomato Plant Growth. Arch. Agro. Soil Sci. 2018, 64, 1–14. [Google Scholar]
- Chanratana, M.; Joe, M.M.; Roy Choudhury, A.; Anandham, R.; Krishnamoorthy, R.; Kim, K.; Jeon, S.; Choi, J.; Choi, J.; Sa, T.M. Physiological Response of Tomato Plant to Chitosan-Immobilized Aggregated Methylobacterium oryzae CBMB20 Inoculation under Salinity Stress. 3Biotech 2019, 9, 397. [Google Scholar] [CrossRef]
- Panichikkal, J.; Puthiyattil, N.; Raveendran, A.; Nair, R.A.; Krishnankutty, R.E. Application of Encapsulated Bacillus licheniformis Supplemented with Chitosan Nanoparticles and Rice Starch for the Control of Sclerotium rolfsii in Capsicum annuum (L.) Seedlings. Curr. Microbiol. 2021, 78, 911–919. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Moradi-Pour, M. A Novel Encapsulation of Streptomyces fulvissimus Uts22 by Spray Drying and its Biocontrol Efficiency against Gaeumannomyces graminis, the Causal Agent of Take-All Disease in Wheat. Pest Manag. Sci. 2021, 77, 4357–4364. [Google Scholar] [CrossRef]
- Urena-Saborío, H.; Madrigal-Carballo, S.; Sandoval, J.; Vega-Baudrit, J.R.; Rodríguez-Morales, A. Encapsulation of Bacterial Metabolic Infiltrates Isolated from Different Bacillus Strains in Chitosan Nanoparticles as Potential Green Chemistry based Biocontrol Agents against Radopholus similis. J. Renew. Mater. 2017, 5, 290–299. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Andrews, E.H.; Martínez, C.V.; Carvajal, Z.Y.G. Composite Hydrogels Based on Gelatin, Chitosan and Polyvinyl Alcohol to Biomedical Applications: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Laffleur, F.; Strasdat, B. Gelatin-based formulations for dermal application. Eur. Polym. J. 2019, 118, 542–550. [Google Scholar] [CrossRef]
- Wang, C.S.; Natale, G.; Virgilio, N.; Heuzey, M.C. Synergistic Gelation of Gelatin B with Xanthan Gum. Food Hidrocoll. 2016, 60, 374–383. [Google Scholar] [CrossRef]
- Rojas-Sánchez, B.; Guzmán-Guzmán, P.; Morales-Cedeño, L.R.; Orozco-Mosqueda, M.d.C.; Saucedo-Martínez, B.C.; Sánchez-Yáñez, J.M.; Fadiji, A.E.; Babalola, O.O.; Glick, B.R.; Santoyo, G. Bioencapsulation of Microbial Inoculants: Mechanisms, Formulation Types and Application Techniques. Appl. Biosci. 2022, 1, 198–220. [Google Scholar] [CrossRef]
- Pereira, J.F.; Lonni, A.A.S.G.; Mali, S. Development of Biopolymeric Films with Addition of Vitamin C and Catuaba Extract as Natural Antioxidants. Prep. Biochem. Biotechnol. 2021, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakova, E.N.; Shcherbakov, A.V.; Rots, P.Y.; Gonchar, L.N.; Mulina, S.A.; Yahina, L.M.; Lactionov, Y.V.; Chebotar, V.K. Inoculation Technology for Legumes Based on Alginate Encapsulation. Agron. Res. 2018, 16, 2156–2168. [Google Scholar]
- Tu, L.; He, Y.; Yang, H.; Wu, Z.; Yi, L. Preparation and Characterization of Alginate-Gelatin Microencapsulated Bacillus subtilis SL-13 by Emulsification/Internal Gelation. J. Biomater. Sci. Polym. 2015, 26, 735–749. [Google Scholar] [CrossRef] [PubMed]
| Mechanism of Action | Microorganism | Plant | Stress Condition | Reference |
|---|---|---|---|---|
| Production of phytohormones 1,2 Production of antioxidants Production of siderophores | Acinetobacter beijerinckii | Soybean | Heavy metal (Cr) | [30] |
| Production of phytohormones 1,2 Production of antioxidants Production of siderophores | Raoultella planticola | Soybean | Heavy metal (Cr) | [30] |
| Biological nitrogen fixation | Bradyrhizobium spp | Soybean | - | [38,39] |
| Biological nitrogen fixation | Azospirillum brasilense | Maize | - | [40] |
| Production of phytohormones Improvement of water content | A. brasilense | Wheat | Drought | [41] |
| Biological nitrogen fixation Production of phytohormones 2 | A. brasilense | Maize, wheat and rice | - | [42] |
| Biological nitrogen fixation | Bacillus subtilis | Maize and sorghum | - | [43] |
| Production of siderophores Production of antibiotics | B. subtillis | Pepper | Biotic | [44] |
| Biological nitrogen fixation Production of phytohormones 2 Production of exopolysaccharides Production of siderophores | B. subtilis B. paralicheniformis Pseudomonas putida | Maize | - | [45] |
| Production of phytohormones 3 Production of antioxidants | P. putida | Soybean | Salinity and drought | [46] |
| Production of exopolysaccharides | P.putida | Sunflower | Drought | [47] |
| Phosphate solubilization | B. polymyxa | Tomato | Drought | [48] |
| Phosphate solubilization ACC deaminase production | B. thuringiensis | Wheat | Drought | [49] |
| Production of antioxidants | B. thuringiensis | Lavender | Drought | [50] |
| ACC deaminase production | Enterobacter mori E. asburiae E. ludwigii | Wheat | Drought | [51] |
| Production of phytohormones 3 | Burkholdera cepacia Acinetobacter calcoaceticus | Cucumber | Salinity | [52] |
| Production of phytohormones 2 Production of siderophores Phosphate solubilization | Enterobacter sp. Pseudomonas sp. | Tomato | - | [53] |
| Production of antibiotics | B. amyloliquefaciens | Wheat | Biotic Fusarium graminearum | [54] |
| Improvements in root architecture ACC deaminase production | P. fluorescens | Signalgrass | - | [55] |
| Phosphate solubilization Production of phytohormones 2 Production of siderophores Production of antibiotics Production of volatile organic compounds and ammonia | P. aeruginosa | Tomato, potato, taro, and strawberry | Biotic | [56] |
| Biological nitrogen fixation Phosphate solubilization Production of phytohormones 2 Production of chitinases | B. velezensis | Jujube fruits | Biotic | [57] |
| Production of antioxidants Production of siderophores | B. simplex | Pea | Heavy metal (Pb) | [58] |
| Phosphate solubilization Production of phytohormones 2,3 ACC deaminase production Production of exopolysaccharides | Klebsiella sp. | Mung bean | Salinity pH | [59] |
| Production of antioxidants | Azotobacter chroococcum | Maize | Drought | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.F.; Oliveira, A.L.M.; Sartori, D.; Yamashita, F.; Mali, S. Perspectives on the Use of Biopolymeric Matrices as Carriers for Plant-Growth Promoting Bacteria in Agricultural Systems. Microorganisms 2023, 11, 467. https://doi.org/10.3390/microorganisms11020467
Pereira JF, Oliveira ALM, Sartori D, Yamashita F, Mali S. Perspectives on the Use of Biopolymeric Matrices as Carriers for Plant-Growth Promoting Bacteria in Agricultural Systems. Microorganisms. 2023; 11(2):467. https://doi.org/10.3390/microorganisms11020467
Chicago/Turabian StylePereira, Jéssica F., André Luiz M. Oliveira, Daniele Sartori, Fabio Yamashita, and Suzana Mali. 2023. "Perspectives on the Use of Biopolymeric Matrices as Carriers for Plant-Growth Promoting Bacteria in Agricultural Systems" Microorganisms 11, no. 2: 467. https://doi.org/10.3390/microorganisms11020467
APA StylePereira, J. F., Oliveira, A. L. M., Sartori, D., Yamashita, F., & Mali, S. (2023). Perspectives on the Use of Biopolymeric Matrices as Carriers for Plant-Growth Promoting Bacteria in Agricultural Systems. Microorganisms, 11(2), 467. https://doi.org/10.3390/microorganisms11020467







