Changes to Soil Microbiome Resulting from Synergetic Effects of Fungistatic Compounds Pyrimethanil and Fluopyram in Lowbush Blueberry Agriculture, with Nine Fungicide Products Tested
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Management
2.2. StemPlant Analyses
2.3. Soil Sampling, DNA Isolation, and Sequencing
2.4. Sequence Processing and Data Analysis
3. Results
3.1. Phenotype Expression, Crop Outcomes, and Disease Incidence
3.2. Data Characteristics
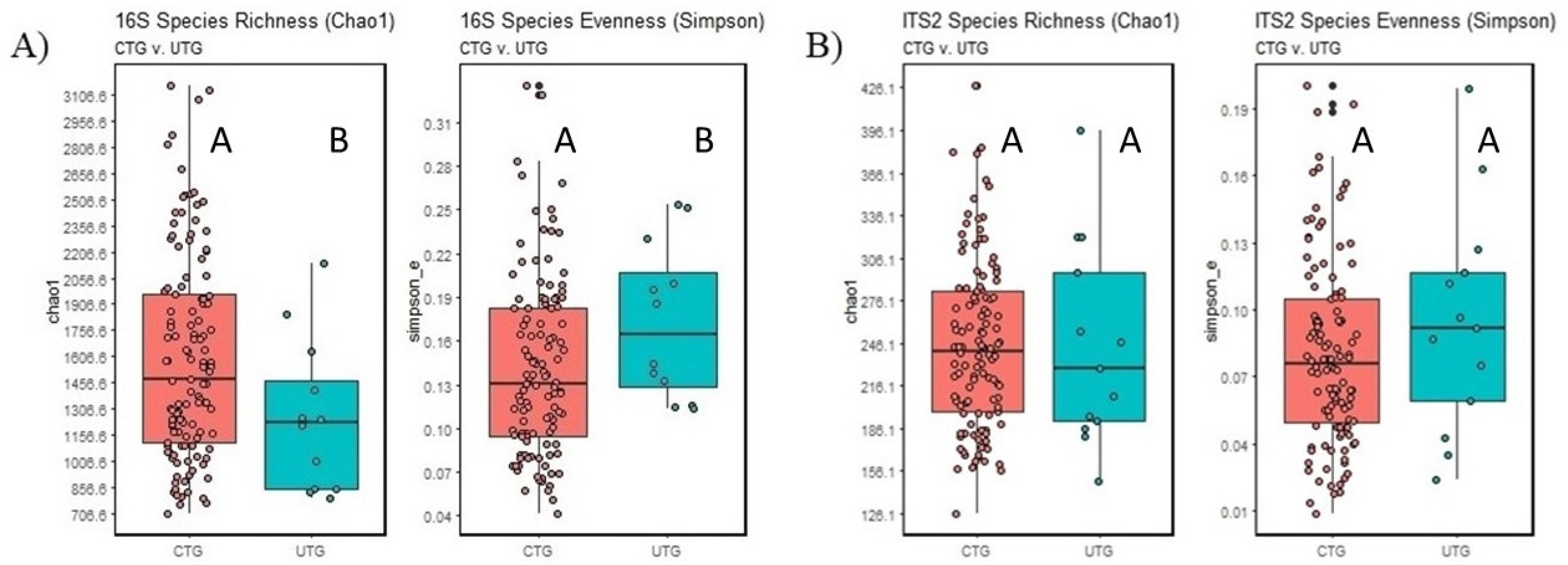
3.3. Alpha Diversity
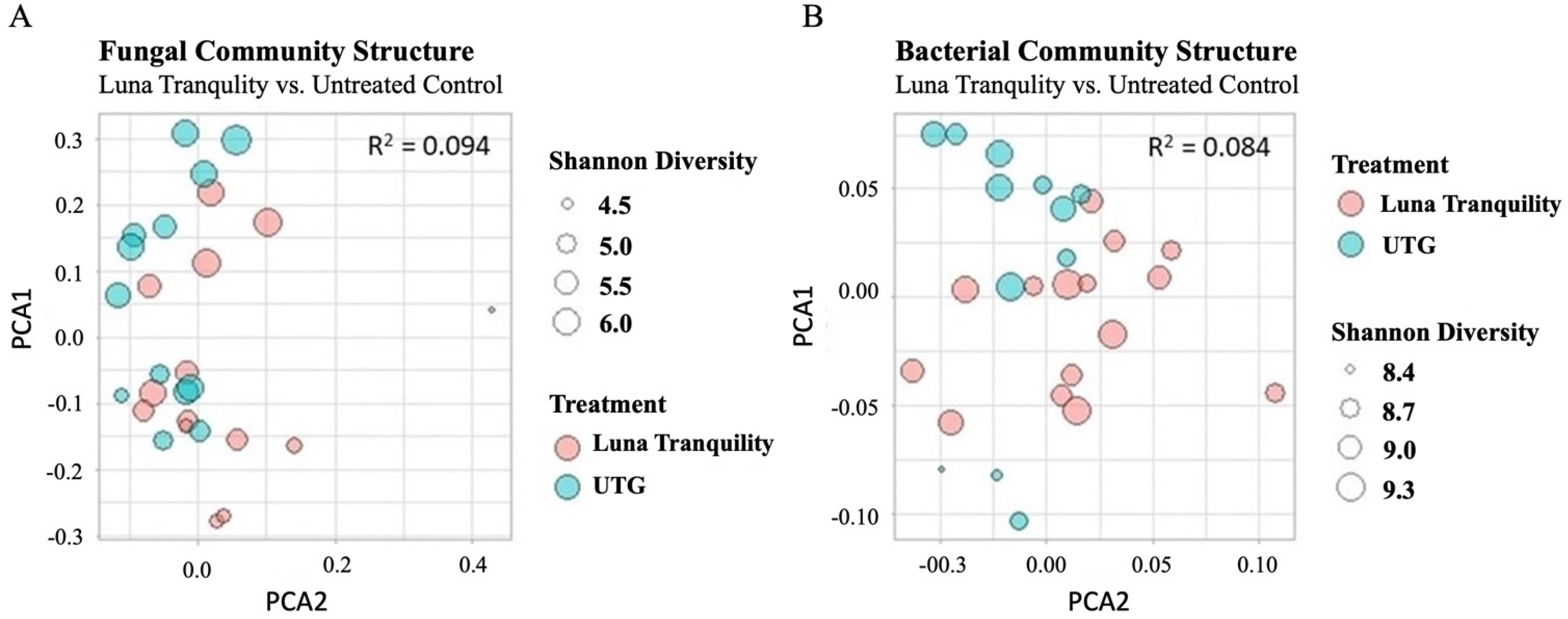
3.4. Beta Diversity
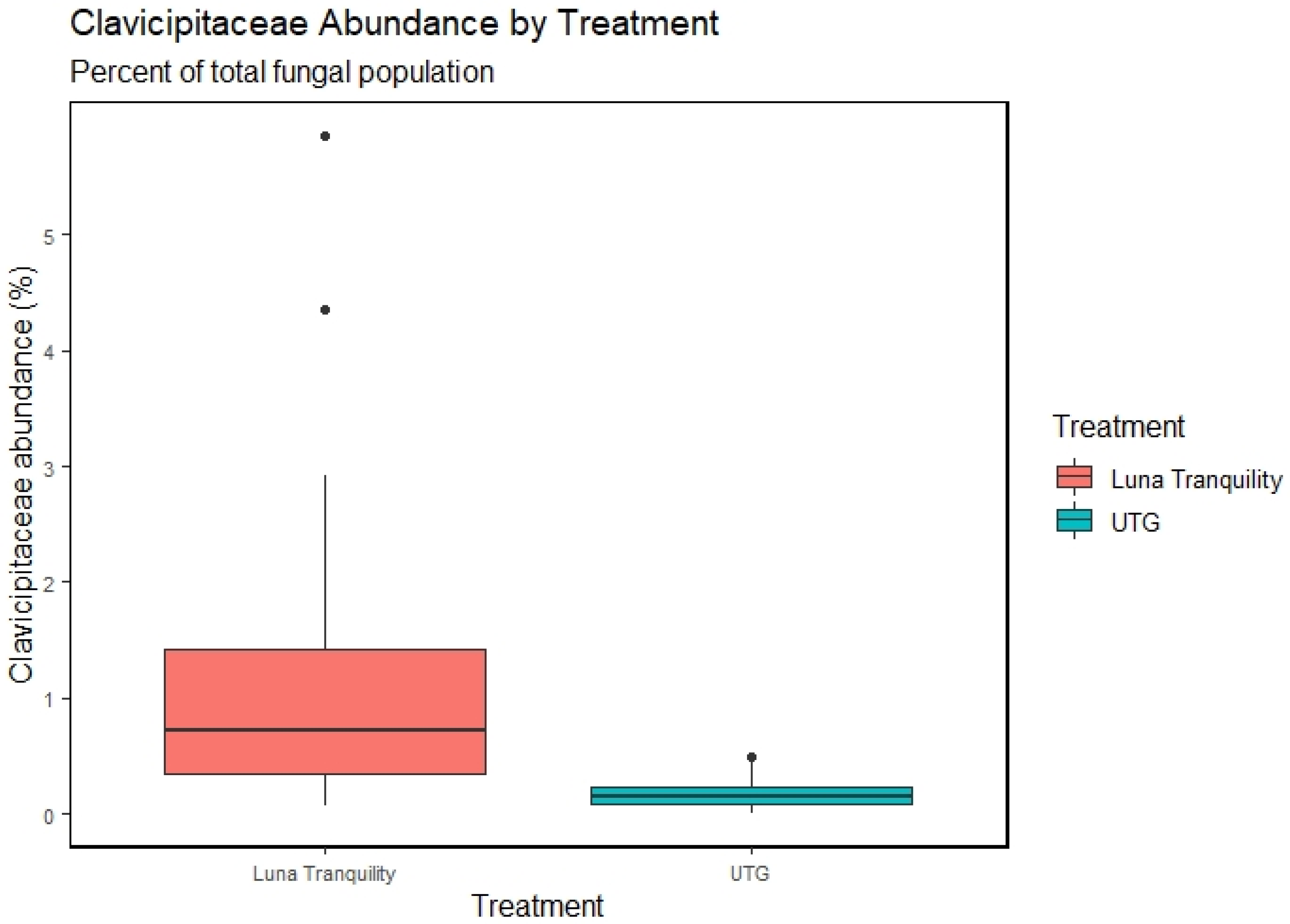
3.5. Effect of Fungicides Application on Soil Community Structure
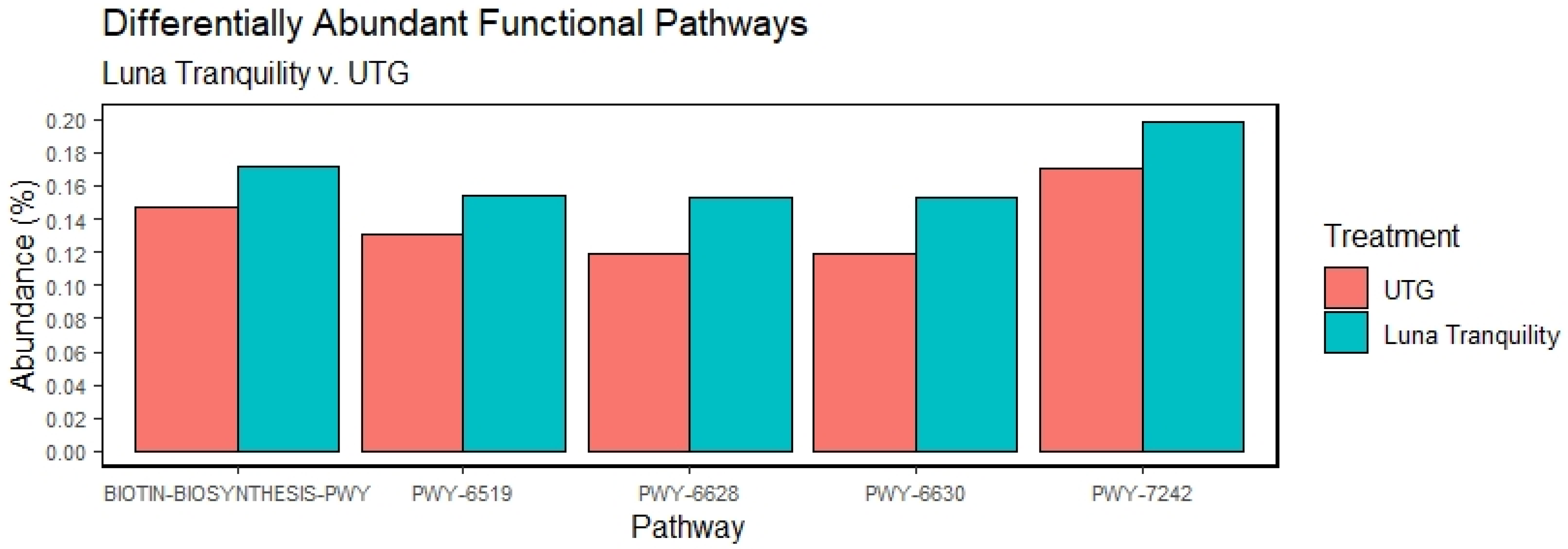
3.6. Effect of Fungicides Application on Bacterial Functional Potentials
4. Discussion
4.1. Effects of Fungicides on Taxonomic Profiles
4.2. Fungicides and Functional Potential
4.3. Plant Outcomes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zilber-Rosenberg, I.; Rosenberg, E. Role of Microorganisms in the Evolution of Animals and Plants: The Hologenome Theory of Evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef]
- Mitchell, D.T.; Gibson, B.R. Ericoid Mycorrhizal Association: Ability to Adapt to a Broad Range of Habitats. Mycologist 2006, 20, 2–9. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; ISBN 978-0-12-370526-6. [Google Scholar]
- Yang, H.; Zhao, X.; Liu, C.; Long, B.; Zhao, M.; Li, L. Link to external site, this link will open in a new window Diversity and Characteristics of Colonization of Root-Associated Fungi of Vaccinium Uliginosum. Sci. Rep. 2018, 8, 15283. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Tedersoo, L. Evolutionary History of Mycorrhizal Symbioses and Global Host Plant Diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Vrålstad, T.; Myhre, E.; Schumacher, T. Molecular Diversity and Phylogenetic Affinities of Symbiotic Root-Associated Ascomycetes of the Helotiales in Burnt and Metal Polluted Habitats. New Phytol. 2002, 155, 131–148. [Google Scholar] [CrossRef]
- Morvan, S.; Meglouli, H.; Sahraoui, A.L.-H.; Hijri, M. Into the Wild Blueberry (Vaccinium Angustifolium) Rhizosphere Microbiota. Environ. Microbiol. 2020, 22, 3803–3822. [Google Scholar] [CrossRef]
- Selosse, M.-A.; Setaro, S.; Glatard, F.; Richard, F.; Urcelay, C.; Weiß, M. Sebacinales Are Common Mycorrhizal Associates of Ericaceae. New Phytol. 2007, 174, 864–878. [Google Scholar] [CrossRef]
- Yurgel, S.N.; Douglas, G.M.; Comeau, A.M.; Mammoliti, M.; Dusault, A.; Percival, D.; Langille, M.G.I. Variation in Bacterial and Eukaryotic Communities Associated with Natural and Managed Wild Blueberry Habitats. Phytobiomes J. 2017, 1, 102–113. [Google Scholar] [CrossRef]
- Lloyd, A.W.; Percival, D.; Yurgel, S.N. Effect of Fungicide Application on Lowbush Blueberries Soil Microbiome. Microorganisms 2021, 9, 1366. [Google Scholar] [CrossRef]
- Comeau, A.M.; Douglas, G.M.; Langille, M.G.I. Microbiome Helper: A Custom and Streamlined Workflow for Microbiome Research. mSystems 2017, 2, e00127-16. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A Fast and Accurate Illumina Paired-End ReAd MergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Xu, Z.Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns. mSystems 2017, 2, e00191-16. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact Sequence Variants Should Replace Operational Taxonomic Units in Marker-Gene Data Analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2′s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.D.; Macklaim, J.M.; Linn, T.G.; Reid, G.; Gloor, G.B. ANOVA-Like Differential Expression (ALDEx) Analysis for Mixed Population RNA-Seq. PLoS ONE 2013, 8, e67019. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019.
- Zhang, Y.; Xu, J.; Dong, F.; Liu, X.; Wu, X.; Zheng, Y. Response of Microbial Community to a New Fungicide Fluopyram in the Silty-Loam Agricultural Soil. Ecotoxicol. Environ. Saf. 2014, 108, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.L.; Bandow, C.; Proença, D.N.; Santos, S.; Guilherme, R.; Morais, P.V.; Römbke, J.; Sousa, J.P. Does Altered Rainfall Regime Change Pesticide Effects in Soil? A Terrestrial Model Ecosystem Study from Mediterranean Portugal on the Effects of Pyrimethanil to Soil Microbial Communities under Extremes in Rainfall. Appl. Soil Ecol. 2014, 84, 245–253. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, T.; Zhu, L.; Juhasz, A.; Du, Z.; Li, B.; Wang, J.; Wang, J.; Sun, Y. Response of Soil Microbes after Direct Contact with Pyraclostrobin in Fluvo-Aquic Soil. Environ. Pollut. 2019, 255, 113164. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, J.; Liu, Y.; Dong, F.; Liu, X.; Zhang, W.; Zheng, Y. Impact of Fluxapyroxad on the Microbial Community Structure and Functional Diversity in the Silty-Loam Soil. J. Integr. Agric. 2015, 14, 114–124. [Google Scholar] [CrossRef]
- Hunter, D.M.; Milner, R.J.; Spurgin, P.A. Aerial Treatment of the Australian Plague Locust, Chortoicetes Terminifera (Orthoptera: Acrididae) with Metarhizium Anisopliae (Deuteromycotina: Hyphomycetes). Bull. Entomol. Res. 2001, 91, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sasan, R.K.; Bidochka, M.J. The Insect-Pathogenic Fungus Metarhizium Robertsii (Clavicipitaceae) Is Also an Endophyte That Stimulates Plant Root Development. Am. J. Bot. 2012, 99, 101–107. [Google Scholar] [CrossRef]
- Peres, C.M.; Naveau, H.; Agathos, S.N. Cross Induction of 4-Nitrobenzoate and 4-Aminobenzoate Degradation by Burkholderia Cepacia Strain PB4. In Novel Approaches for Bioremediation of Organic Pollution; Fass, R., Flashner, Y., Reuveny, S., Eds.; Springer: Boston, MA, USA, 1999; pp. 71–81. ISBN 978-1-4615-4749-5. [Google Scholar]
- Bhat, A.P.; Gogate, P.R. Degradation of Nitrogen-Containing Hazardous Compounds Using Advanced Oxidation Processes: A Review on Aliphatic and Aromatic Amines, Dyes, and Pesticides. J. Hazard. Mater. 2021, 403, 123657. [Google Scholar] [CrossRef] [PubMed]
- Arbeli, Z.; Fuentes, C.L. Accelerated Biodegradation of Pesticides: An Overview of the Phenomenon, Its Basis and Possible Solutions; and a Discussion on the Tropical Dimension. Crop Prot. 2007, 26, 1733–1746. [Google Scholar] [CrossRef]
- Sun, T.; Li, M.; Saleem, M.; Zhang, X.; Zhang, Q. The Fungicide “Fluopyram” Promotes Pepper Growth by Increasing the Abundance of P-Solubilizing and N-Fixing Bacteria. Ecotoxicol. Environ. Saf. 2020, 188, 109947. [Google Scholar] [CrossRef]
| Trade Name | Active Ingredients | Application Rate | Mode of Action |
|---|---|---|---|
| Cabrio | pyraclostrobin | 1.95 g/100 m2 | inhibits mitochondrial cytochrome bc-1 complex |
| Miravis Bold | pydiflumetofen | 0.84 mL/100 m2 | inhibits succinate dehydrogenase |
| Sercadis | fluxapyroxad | 1.30 mL/100 m2 | inhibits succinate dehydrogenase |
| Scala | pyrimethanil | 11.25 mL/100 m2 | inhibits methionine production |
| Scholar | fludioxonil | 2.04 mL/100 m2 | inhibits a catalytic enzyme that breaks down methylglyoxal |
| Velum Prime | fluopyram | 3.0 mL/100 m2 | inhibits succinate dehydrogenase |
| Luna Tranquility | fluopyram + pyrimethanil | 12 mL/100 m2 | inhibits succinate dehydrogenase + inhibits methionine production |
| Miravis Prime | pydiflumetofen + fludioxonil | 8.77 mL/ 100 m2 | inhibits a catalytic enzyme that breaks down methylglyoxal + inhibits succinate dehydrogenase |
| Merivon | fluxapyroxad + pyraclostrobin | 7.31 mL/100 m2 | inhibits succinate dehydrogenase + inhibits mitochondrial cytochrome bc-1 complex |
| Treatment | Mean Floral Node Development Stage (n = 28) | Standard Deviation | Fisher’s LSD 1 (p < 0.05) |
|---|---|---|---|
| UTG | 3.86 | 0.76 | AC |
| Miravis Prime | 3.46 | 0.51 | B |
| Cabrio | 3.61 | 0.74 | BC |
| Scala | 3.61 | 0.69 | BC |
| Miravis Bold | 3.68 | 0.61 | ABC |
| Sercadis | 3.79 | 0.42 | AC |
| Luna Tranquility | 3.79 | 0.5 | AC |
| Scholar | 3.86 | 0.52 | AC |
| Velum Prime | 3.96 | 0.74 | A |
| Merivon | 3.96 | 0.43 | C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lloyd, A.W.; Percival, D.; Langille, M.G.I.; Yurgel, S.N. Changes to Soil Microbiome Resulting from Synergetic Effects of Fungistatic Compounds Pyrimethanil and Fluopyram in Lowbush Blueberry Agriculture, with Nine Fungicide Products Tested. Microorganisms 2023, 11, 410. https://doi.org/10.3390/microorganisms11020410
Lloyd AW, Percival D, Langille MGI, Yurgel SN. Changes to Soil Microbiome Resulting from Synergetic Effects of Fungistatic Compounds Pyrimethanil and Fluopyram in Lowbush Blueberry Agriculture, with Nine Fungicide Products Tested. Microorganisms. 2023; 11(2):410. https://doi.org/10.3390/microorganisms11020410
Chicago/Turabian StyleLloyd, Austin W., David Percival, Morgan G. I. Langille, and Svetlana N. Yurgel. 2023. "Changes to Soil Microbiome Resulting from Synergetic Effects of Fungistatic Compounds Pyrimethanil and Fluopyram in Lowbush Blueberry Agriculture, with Nine Fungicide Products Tested" Microorganisms 11, no. 2: 410. https://doi.org/10.3390/microorganisms11020410
APA StyleLloyd, A. W., Percival, D., Langille, M. G. I., & Yurgel, S. N. (2023). Changes to Soil Microbiome Resulting from Synergetic Effects of Fungistatic Compounds Pyrimethanil and Fluopyram in Lowbush Blueberry Agriculture, with Nine Fungicide Products Tested. Microorganisms, 11(2), 410. https://doi.org/10.3390/microorganisms11020410





