Isolation and Identification of Aeromonas veronii in Sheep with Fatal Infection in China: A Case Report
Abstract
1. Introduction
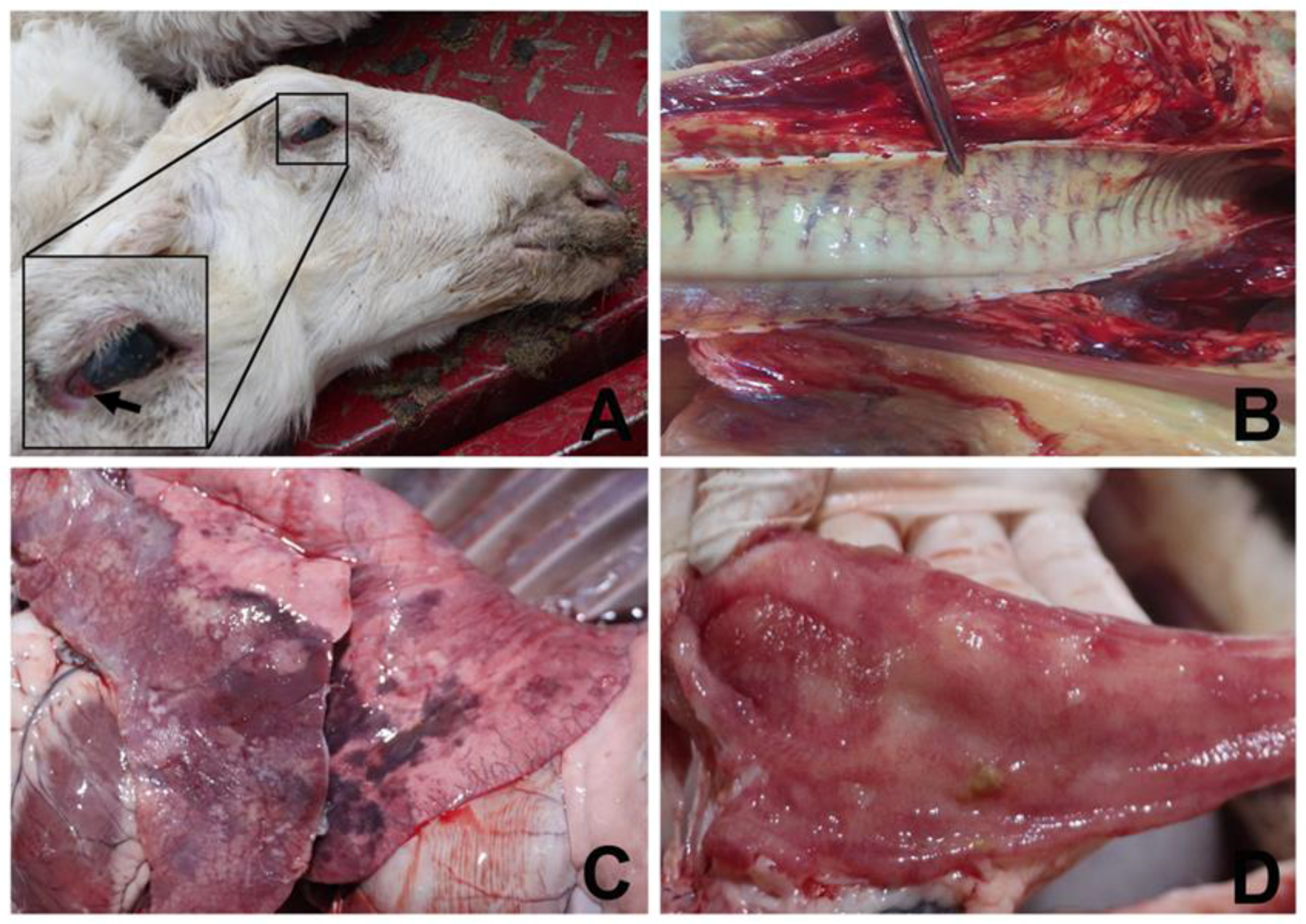
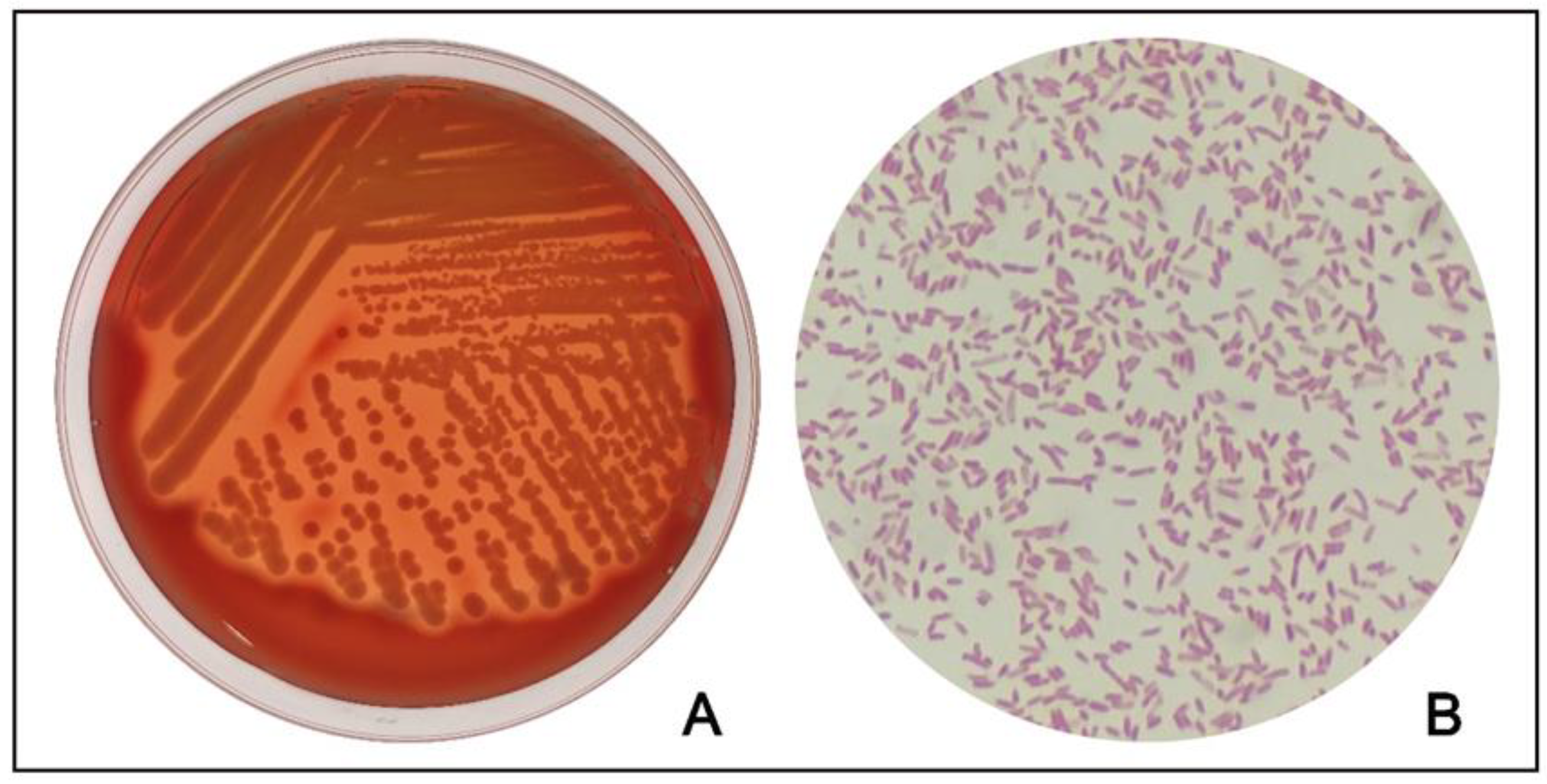
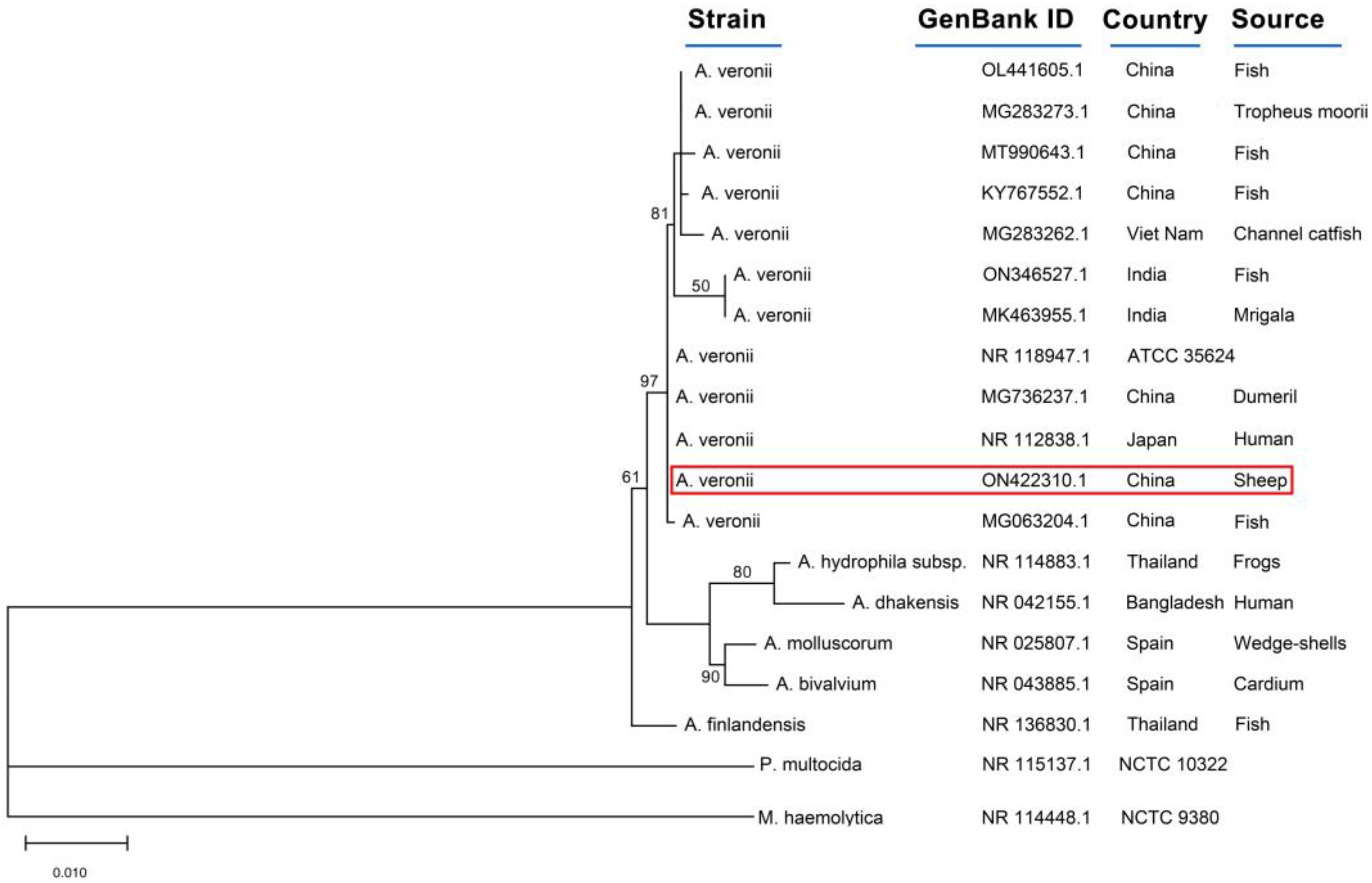
2. Case Description
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández, B.A.; Figueras, M.J. An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms. 2020, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.; Yu, W. Extensive community-acquired pneumonia with hemophagocytic syndrome caused by Aeromonas veronii in an immunocompetent patient. J. Microbiol. Immunol. Infect. 2017, 50, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lin, Q.; Zhao, Y.; Zhu, G.; Jiang, E.; Li, S.; Mi, Y. Clinical characteristics and risk factors of Aeromonas bloodstream infections in patients with hematological diseases. BMC Infect. Dis. 2022, 22, 303. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Torri, A.; Amadori, E.; Rossi, A.; Bartolini, G.; Casadei, C. Aeromonas veronii biovar veronii and sepsis-infrequent complication of biliary drainage placement: A case report. World. J. Clin. Cases 2019, 7, 759–764. [Google Scholar] [CrossRef]
- Raj, N.S.; Swaminathan, T.R.; Dharmaratnam, D.A. Aeromonas veronii caused bilateral exophthalmia and mass mortality in cultured Nile tilapia, Oreochromis niloticus in India. Aquaculture 2019, 51, 734278. [Google Scholar] [CrossRef]
- Wang, B.; Mao, C.; Feng, J.; Li, Y.; Hu, J.; Jiang, B. A First Report of Aeromonas veronii Infection of the Sea Bass, Lateolabrax maculatus in China. Front Vet. Sci. 2021, 7, 600587. [Google Scholar] [CrossRef]
- Su, X.; Zhong, Y.; Wang, X.; Qin, T.; Chen, S.J.; Peng, D.X. Isolation and identification of Aeromonas veronii from duck and preliminary study on its pathogenicity. Chin. J Pre-Vet. Med. 2020, 42, 1008–1589. [Google Scholar]
- LI, W.J.; Zhao, Y.; Liu, Y.; Qi, X.X.; Kang, K.; Chen, M. Isolation and identification of pathogenic Aeromonas veronii from fox. Chin. J Pre-Vet. Med. 2012, 34, 290–292. [Google Scholar]
- Getnet, K.; Abera, B.; Getie, H.; Molla, W.; Mekonnen, S.A.; Megistu, B.A.; Abat, A.S.; Dejene, H.; Birhan, M. Serotyping and Seroprevalence of Mannheimia haemolytica, Pasteurella multocida, and Bibersteinia trehalose and Assessment of Determinants of Ovine Pasteurellosis in West Amhara Sub-region, Ethiopia. Front. Vet. Sci. 2022, 9, 866206. [Google Scholar] [CrossRef]
- Bell, S. Respiratory disease in sheep: Differential diagnosis and epidemiology. In. Pract. 2008, 30, 200–207. [Google Scholar] [CrossRef]
- Besser, T.E.; Frances, C.E.; Highland, M.A. Bighorn sheep pneumonia: Sorting out the cause of a polymicrobial disease. Prev. Vet. Med. 2013, 108, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Y.; Lu, S.Y.; Si, T. DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice. Nat Commun. 2015, 6, 7415. [Google Scholar] [CrossRef]
- Calvi, L.M.; Bromberg, O.; Rhee, Y. Osteoblastic expansion induced by parathyroid hormone receptor signaling in murine osteocytes is not sufficient to increase hematopoietic stem cells. Blood. 2012, 119, 2489–2499. [Google Scholar] [CrossRef] [PubMed]
- McAuliffe, L.; Hatchell, F.M.; Ayling, R.D.; King, A.I.; Nicholas, R.A. Detection of Mycoplasma ovipneumoniae in Pasteurella-vaccinated sheep flocks with respiratory disease in England. Vet. Rec. 2003, 153, 687–688. [Google Scholar] [CrossRef]
- He, Y.P.; Zhang, Q.; Fu, M.Z.; Xu, X.G. Development of multiplex PCR for simultaneous detection and differentiation of six DNA and RNA viruses from clinical samples of sheep and goats. J. Virol. Methods 2017, 243, 44–49. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, P.; Singh, R. Pathology and polymerase chain reaction detection of ovine progressive pneumonia (maedi) cases in slaughtered sheep in India. Vet. World 2017, 10, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mao, L.; Cheng, S.; Wang, Q.; Huang, J.; Deng, J.; Wang, Z.; Zhang, W.; Yang, L.; Hao, F.; et al. A novel parainfluenza virus type 3 (PIV3) identified from goat herds with respiratory diseases in eastern China. Vet. Microbiol. 2014, 174, 100–106. [Google Scholar] [CrossRef]
- Eleraky, N.Z.; Kania, S.A.; Potgieter, L.N. The ovine respiratory syncytial virus F gene sequence and its diagnostic application. J. Vet. Diagn. Invest. 2001, 13, 455–461. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Holt, J.H.; Krieg, N.R.; Sneath, P.H. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams & Wilkins: Baltimore, MD, USA, 1994. [Google Scholar]
- Leblanc, D.; Mittal, K.R.; Olivier, G.; Lallier, R. Serogrouping of motile Aeromonas species isolated from healthy and moribund fish. Appl. Environ. Microbiol. 1987, 42, 56–60. [Google Scholar] [CrossRef]
- Lhan, Z.; Gülhan, T.; Aksakal, A. Aeromonas hydrophila associated with ovine abortion. Small Rumin. Res. 2006, 61, 73–78. [Google Scholar]
- Xia, Y.; Li, H.; Shen, Y. Antimicrobial Drug Resistance in Salmonella enteritidis Isolated from Edible Snakes with Pneumonia and Its Pathogenicity in Chickens. Front Vet Sci. 2020, 7, 463. [Google Scholar] [CrossRef] [PubMed]
- Lallier, R.; Higgins, R. Biochemical and toxigenic characteristics of Aeromonas spp. isolated from diseased mammals, moribund and healthy fish. Vet. Microbiol. 1988, 18, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.B.; Oliveira, W.F.; Correia, M.; Fontes, A.; Coelho, L. Aeromonas and Human Health Disorders: Clinical Approaches. Front. Microbiol. 2022, 13, 868–890. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Gao, X.; Jiang, Q.; Wen, Y.; Lin, L. Characterization of Virulence Properties of Aeromonas veronii Isolated from Diseased Gibel Carp (Carassius gibelio). Int. J. Mol. Sci. 2016, 17, 496. [Google Scholar] [CrossRef]
- Skwor, T.A.; Shinko, J.; Augustyniak, A.; Gee, C.; Andraso, G. Aeromonas hydrophila and Aeromonas veronii Predominate among Potentially Pathogenic Ciprofloxacin- and Tetracycline-Resistant Aeromonas Isolates from Lake Erie. Appl. Environ. Microbiol. 2014, 80, 841–848. [Google Scholar] [CrossRef]
- Vila, J.; Gallardo, F.; Vargas, M.; Solerk, L.; Figueras, M.J.; Gascon, J. Aeromonas spp. and traveler’s diarrhea: Clinical features and antimicrobial resistance. Emerg. Infect. Dis. 2003, 9, 552–555. [Google Scholar] [CrossRef]
- Kumar, A.; Tikoo, S.K.; Malik, P.; Kumar, A.T. Respiratory diseases of small ruminants. Vet. Med. Int. 2014, 2014, 373642. [Google Scholar] [CrossRef]
- Brogden, K.A.; Lehmkuhl, H.D.; Cutlip, R.C. Pasteurella haemolytica complicated respiratory infections in sheep and goats. Vet. Res. 1998, 29, 233–254. [Google Scholar]
- Kearney, G.D.; Shaw, R.; Prentice, M.; Tutor-Marcom, R. Evaluation of respiratory symptoms and respiratory protection behavior among poultry workers in small farming operations. J. Agromed. 2014, 19, 162–170. [Google Scholar] [CrossRef]
- Wang, T.; He, Q.; Yao, W.; Shao, Y.; Li, J.; Huang, F. The Variation of Nasal Microbiota Caused by Low Levels of Gaseous Ammonia Exposure in Growing Pigs. Front. Microbiol. 2019, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, M.; Liu, Q.; Feng, J. The alterations of tracheal microbiota and inflammation caused by different levels of ammonia exposure in broiler chickens. Poult. Sci. 2021, 100, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Lavoy, E.C.P.; McFarlin, B.K.; Simpson, R.J. Immune Responses to Exercising in a Cold Environment. Wild. Environ. Med. 2011, 22, 343–351. [Google Scholar]
- Belay, T. Cold-induced stress impairs the production of Th1 protective cytokines during co-culturing of dendritic cells and naive T helper cells of Chlamydia muridarum infected mice. J. Immunol. 2018, 200, 117–135. [Google Scholar] [CrossRef]
- Makarova, O.V.; Trunova, G.V.; Diatroptov, M.E.; Serebryakov, S.N.; Kondashevskaya, M.V.; Malaitsev, V.V. Comparative characterization of cytokine production by concanavalin A-activated splenocytes from BALB/c and C57BL/6 mice after cold exposure. Bull. Exp. Biol. Med. 2005, 139, 220–222. [Google Scholar] [CrossRef]
- Borsoi, A.; Quinteiro-Filho, W.M.; Calefi, A.S. Effects of cold stress and Salmonella Heidelberg infection on bacterial load and immunity of chickens. Avian. Pathol. 2015, 44, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhou, G.; Tian, G. Changes in Rumen Microbiota Affect Metabolites, Immune Responses and Antioxidant Enzyme Activities of Sheep under Cold Stimulation. Animals 2021, 11, 712. [Google Scholar] [CrossRef]
- Goldstein, E.; Lippert, W.; Warshauer, D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J. Clin. Investig. 1974, 54, 519–528. [Google Scholar] [CrossRef]
- Gomes, A.V.; Quinteiro-Filho, W.M.; Ribeiro, A. Overcrowding stress decreases macrophage activity and increases Salmonella Enteritidis invasion in broiler chickens. Avian. Pathol. 2014, 43, 82–90. [Google Scholar] [CrossRef]

| Group | Dose | Numbers (n) | Death Number (n) | Mortality (P) | P2 | |
|---|---|---|---|---|---|---|
| CFU/Mouse | Log | |||||
| G1 | 1.1 × 107 | 7.04 | 8 | 8 | 1 | 1 |
| G2 | 1.1 × 106 | 6.04 | 8 | 6 | 0.75 | 0.5625 |
| G3 | 1.1 × 105 | 5.04 | 8 | 2 | 0.25 | 0.0625 |
| G4 | 1.1 × 104 | 4.04 | 8 | 0 | 0 | 0 |
| lgLD50 = X − d(∑Pi − 0.5); Standard error SlogLD50 = d√((∑P − ∑P2)/(n − 1)) | ∑P = 2.00 | ∑P2 = 1.625 | ||||
| Drugs | Diameter (mm) | Sensitivity | Judging Criteria (mm) | Dose (ug/disc) | ||
|---|---|---|---|---|---|---|
| R | I | S | ||||
| Amp | 0 | R | ≤18 | 19–21 | ≥22 | 10 |
| Cef | 13 | R | ≤14 | 15–22 | ≥23 | 30 |
| Cet | 17 | S | ≤14 | 15–22 | ≥23 | 30 |
| Amo | 0 | R | ≤13 | 14–17 | ≥18 | 10 |
| Kan | 12 | R | ≤13 | 14–17 | ≥18 | 30 |
| Gen | 19 | S | ≤14 | - | >14 | 10 |
| Neo | 10 | R | ≤12 | 13–16 | ≥17 | 30 |
| Str | 9 | R | ≤11 | 12–14 | ≥15 | 10 |
| Tet | 13 | R | ≤14 | 15–18 | ≥19 | 30 |
| Dox | 13 | I | ≤12 | 13–15 | ≥16 | 30 |
| Flo | 12 | R | ≤12 | 13–17 | ≥18 | 30 |
| Enr | 23 | S | ≤12 | 13–16 | ≥17 | 10 |
| Tyl | 13 | R | ≤14 | 14–15 | ≥16 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Y.; Zhao, X.; Adam, F.E.A.; Xie, Q.; Feng, H.; Ding, J.; Bai, X.; Wang, J.; Yang, Z. Isolation and Identification of Aeromonas veronii in Sheep with Fatal Infection in China: A Case Report. Microorganisms 2023, 11, 333. https://doi.org/10.3390/microorganisms11020333
Miao Y, Zhao X, Adam FEA, Xie Q, Feng H, Ding J, Bai X, Wang J, Yang Z. Isolation and Identification of Aeromonas veronii in Sheep with Fatal Infection in China: A Case Report. Microorganisms. 2023; 11(2):333. https://doi.org/10.3390/microorganisms11020333
Chicago/Turabian StyleMiao, Yongqiang, Xueliang Zhao, Fathalrhman Eisa Addoma Adam, Qingfang Xie, Hang Feng, Jingru Ding, Xindong Bai, Juan Wang, and Zengqi Yang. 2023. "Isolation and Identification of Aeromonas veronii in Sheep with Fatal Infection in China: A Case Report" Microorganisms 11, no. 2: 333. https://doi.org/10.3390/microorganisms11020333
APA StyleMiao, Y., Zhao, X., Adam, F. E. A., Xie, Q., Feng, H., Ding, J., Bai, X., Wang, J., & Yang, Z. (2023). Isolation and Identification of Aeromonas veronii in Sheep with Fatal Infection in China: A Case Report. Microorganisms, 11(2), 333. https://doi.org/10.3390/microorganisms11020333








