Listeria monocytogenes, Escherichia coli and Coagulase Positive Staphylococci in Cured Raw Milk Cheese from Alentejo Region, Portugal
Abstract
1. Introduction
2. Materials and Methods
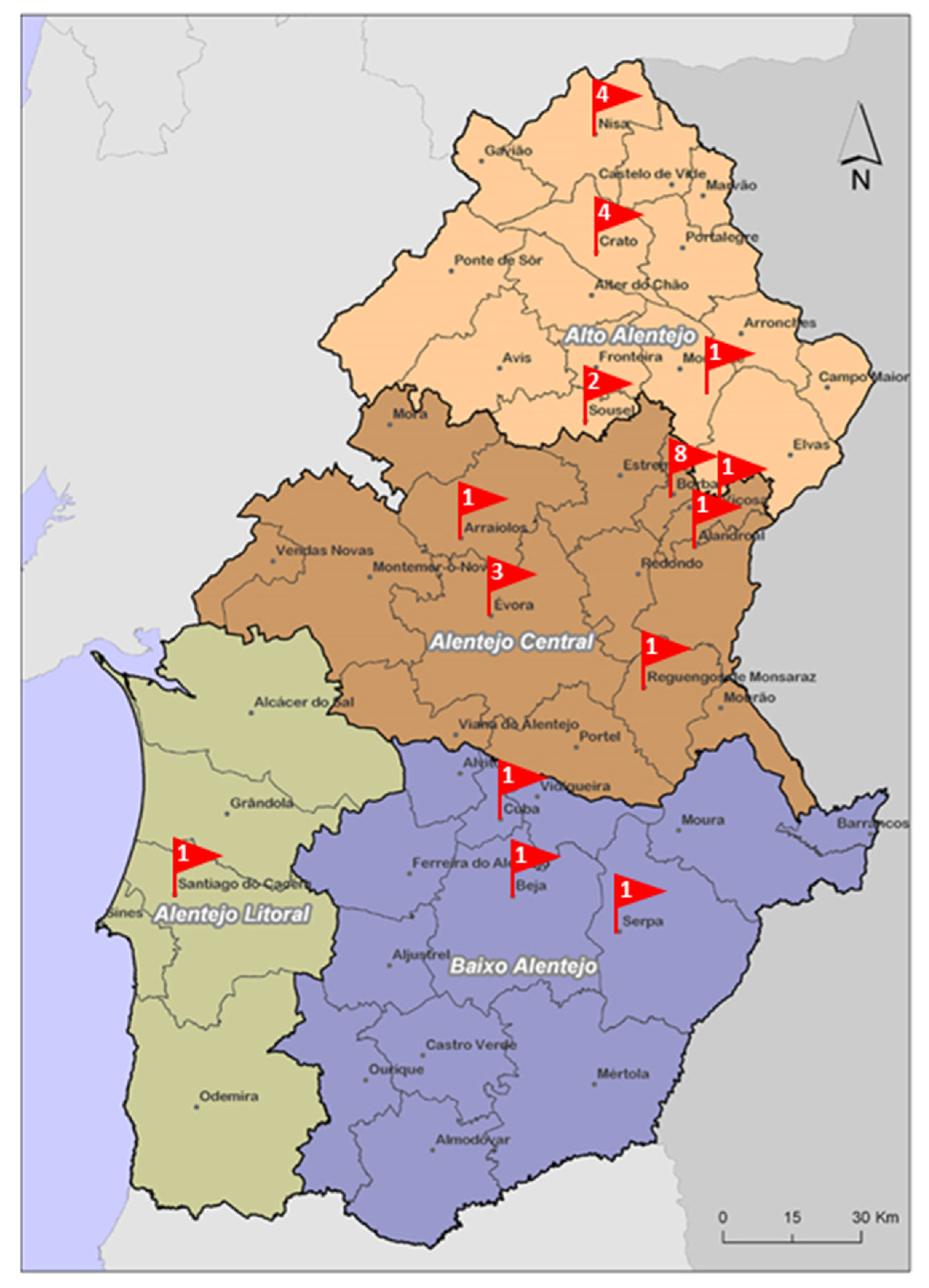
2.1. Sampling
2.2. Microbiological Analysis
2.2.1. E. coli and Coagulase Positive Staphylococci (CPS) Detection and Enumeration
2.2.2. L. monocytogenes and Listeria spp. (Not including L. monocytogenes) Detection and Enumeration
2.2.3. Staphylococcal Enterotoxins (SE) Detection
2.3. Interpretation of Microbiological Results
- Satisfactory, when the results of all the analyzed samples were classified as satisfactory;
- Borderline, when none of the samples were unsatisfactory and the results of at least one sample was classified as borderline;
- Unsatisfactory/potentially injurious to health, when at least one of the samples was classified as unsatisfactory/ potential injurious to health.
2.4. Pathogenic E. coli Identification and Antimicrobial Susceptibility Testing
2.5. Listeria spp. and E. coli Whole-Genome Sequencing, In Silico Typing and Screening of E. coli Virulence/AMR Genes
2.6. Core-Genome Clustering Analysis of Listeria spp. Isolates
3. Results
3.1. Microbiological Quality
3.2. Pathogenic E. coli Identification and Antimicrobial Susceptibility Testing
3.3. E. coli and Listeria Monocytogenes Typing
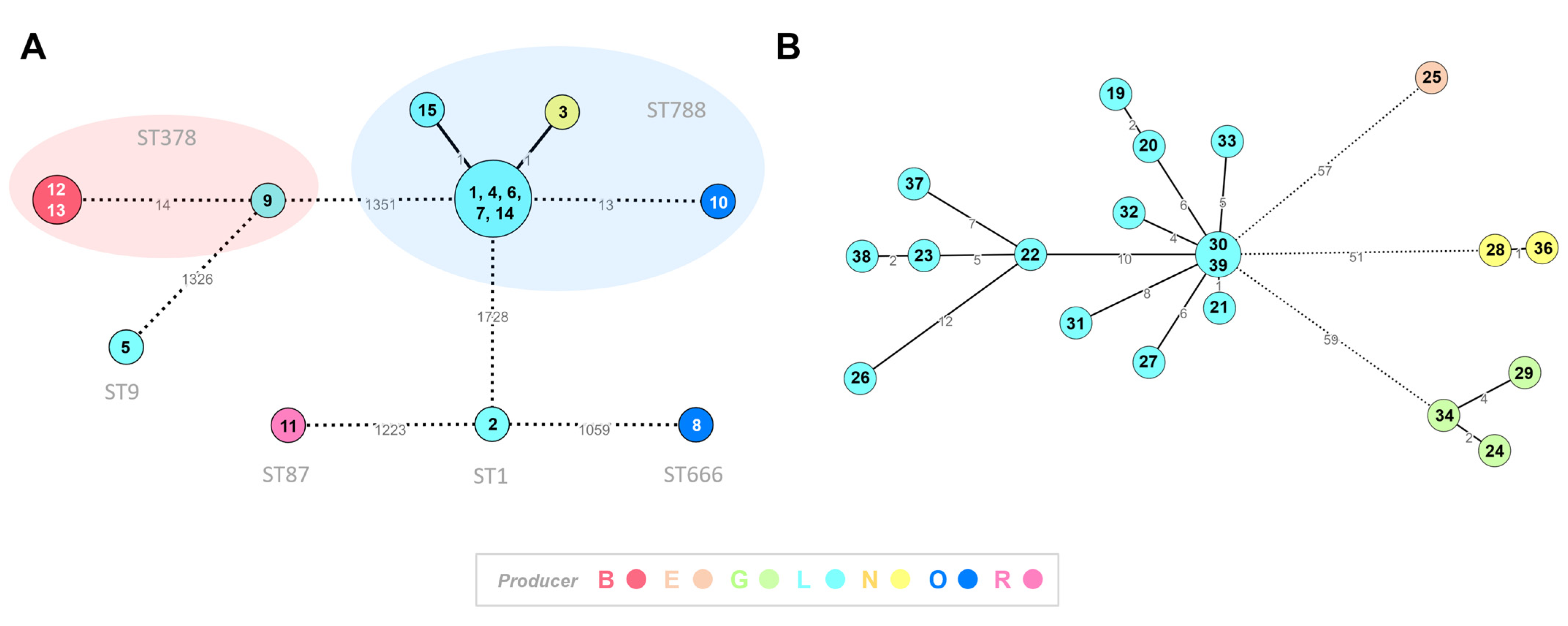
3.4. Core-Genome Clustering Analysis of Listeria spp. Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Regulation (EC) No 853/2004 of 30 April 2004 Laying Down Specific Hygiene Rules for on the Hygiene of Foodstuffs; L139/55; Official Journal of the European Communities: Luxembourg, 2004. [Google Scholar]
- European Food Safety Authority (EFSA) Panel on Biological Hazards (BIOHAZ). Scientific Opinion on the public health risks related to the consumption of raw drinking milk. EFSA J. 2015, 13, 3940. [Google Scholar] [CrossRef]
- Eurostat. Milk and Milk Product Statistics—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Milk_and_milk_product_statistics (accessed on 2 December 2022).
- Harboe, M.K.; Broe, M.L.; Qvist, K.B. The Production, Action and Application of Rennet and Coagulants. In Technology of Cheesemaking, 2nd ed.; Law, B.A., Tamime, A.Y., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 98–129. [Google Scholar]
- Dias, J. The use of cheese from Alentejo in Portuguese gastronomy: A travel through history. Int. J. Gastron. Food Sci. 2022, 29, 100579. [Google Scholar] [CrossRef]
- European Commission. Agriculture and Rural Development. Geographical Indications and Quality Schemes Explained. Available online: https://agriculture.ec.europa.eu/farming/geographical-indications-and-quality-schemes/geographical-indications-and-quality-schemes-explained_en (accessed on 19 December 2022).
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19(12), 6971. [Google Scholar]
- Nüesch-Inderbinen, M.; Bloemberg, G.V.; Müller, A.; Stevens, M.J.A.; Cernela, N.; Kollöffel, B.; Stephan, R. Listeriosis Caused by Persistence of Listeria monocytogenes Serotype 4b Sequence Type 6 in Cheese Production Environment. Emerg. Infect. Dis. 2021, 27, 284–288. [Google Scholar] [CrossRef]
- ISO 7218:2007; Microbiology of Food and Animal Feeding Stuffs—General Requirements and Guidance for Microbiological Examinations. ISO: Geneva, Switzerland, 2007.
- ISO 11290-1:2017; Microbiology of Food Chain—Horizontal Methos for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. ISO: Geneva, Switzerland, 2017.
- ISO 11290-2:2017; Microbiology of Food Chain—Horizontal Methos for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 2: Enumeration Method. ISO: Geneva, Switzerland, 2017.
- ISO 19020:2017; Microbiology of Food Chain—Horizontal Methos for the Immunoenzymatic Detection of Staphylococcal Enterotoxins in Foodstuffs. ISO: Geneva, Switzerland, 2017.
- European Commission. Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; Official Journal of the European Communities: Luxembourg, 2005. [Google Scholar]
- Luxembourg Ministère de la Santé. Critères Microbiologiques Applicables aux Denrées Alimentaires—Lignes Directrices pour L’Interprétation. Available online: https://securite-alimentaire.public.lu/dam-assets/fr/professionnel/Denrees-alimentaires/Qualite-microbiologique/recueil_criteres_microbiologiques/F-054-05.pdf (accessed on 19 December 2022).
- Health Protection Agency. Guidelines for Assessing the Microbiological Safety of Ready-to-eat Foods Placed on the Market. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/363146/Guidelines_for_assessing_the_microbiological_safety_of_ready-to-eat_foods_on_the_market.pdf (accessed on 19 December 2022).
- Persson, S.; Olsen, K.E.; Scheutz, F.; Krogfelt, K.A.; Gerner-Smidt, P. A method for fast and simple detection of major diarrhoeagenic Escherichia coli in the routine diagnostic laboratory. Clin. Microbiol. Infect. 2007, 13, 516–524. [Google Scholar] [CrossRef]
- Boisen, N.; Scheutz, F.; Rasko, D.A.; Redman, J.C.; Persson, S.; Simon, J.; Kotloff, K.L.; Levine, M.M.; Sow, S.; Tamboura, B.; et al. Genomic characterization of enteroaggregative Escherichia coli from children in Mali. J. Infect. Dis. 2012, 205, 431–444. [Google Scholar] [CrossRef]
- Fujioka, M.; Otomo, Y.; Ahsan, C.R. A novel single-step multiplex polymerase chain reaction assay for the detection of diarrheagenic Escherichia coli. J. Microbiol. Methods 2013, 92, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Scheutz, F.; Teel, L.D.; Beutin, L.; Piérard, D.; Buvens, G.; Karch, H.; Mellmann, A.; Caprioli, A.; Tozzoli, R.; Morabito, S.; et al. Multicenter Evaluation of a Sequence-Based Protocol for Subtyping Shiga Toxins and Standardizing Stx Nomenclature. J. Clin. Microbiol. 2012, 50, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Pista, A.; Silveira, L.; Ribeiro, S.; Fontes, M.; Castro, R.; Coelho, A.; Furtado, R.; Lopes, T.; Maia, C.; Mixão, V.; et al. Pathogenic Escherichia coli, Salmonella spp. and Campylobacter spp. in Two Natural Conservation Centers of Wildlife in Portugal: Genotypic and Phenotypic Characterization. Microorganisms 2022, 10, 2132. [Google Scholar] [CrossRef]
- Sora, V.M.; Meroni, G.; Martino, P.A.; Soggiu, A.; Bonizzi, L.; Zecconi, A. Extraintestinal Pathogenic Escherichia coli: Virulence Factors and Antibiotic Resistance. Pathogens 2021, 10, 1355. [Google Scholar] [CrossRef]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Available online: http://www.eucast.org (accessed on 19 December 2022).
- Llarena, A.-K.; Ribeiro-Gonçalves, B.F.; Silva, D.N.; Halkilahti, J.; Machado, M.P.; Da Silva, M.S.; Jaakkonen, A.; Isidro, J.; Hämäläinen, C.; Joenperä, J.; et al. INNUENDO: A Cross-sectoral Platform for the Integration of Genomics in the Surveillance of Food-borne Pathogens. EFSA Support. Publ. 2018, 15, 1498E. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Langmead, B. Aligning Short Sequencing Reads with Bowtie. Curr. Protoc. Bioinform. 2010, 32, 11.7.1–11.7.14. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PloS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast Metagenomic Sequence Classification Using Exact Alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Machado, M.P.; Silva, D.N.; Rossi, M.; Moran-Gilad, J.; Santos, S.; Ramirez, M.; Carriço, J.A. chewBBACA: A Complete Suite for Gene-by-Gene Schema Creation and Strain Identification. Microb. Genom. 2018, 4, e000166. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.; Criscuolo, A.; Pouseele, H.; Maury, M.M.; Leclercq, A.; Tarr, C.; Björkman, J.T.; Dallman, T.; Reimer, A.; Enouf, V.; et al. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbiol. 2016, 2, 16185. [Google Scholar] [CrossRef]
- Mamede, R.; Vila-Cerqueira, P.; Silva, M.; Carriço, J.A.; Ramirez, M. Chewie Nomenclature Server (chewie-NS): A Deployable Nomenclature Server for Easy Sharing of Core and Whole Genome MLST Schemas. Nucleic Acids Res. 2020, 49, D660–D666. [Google Scholar] [CrossRef]
- Mixão, V.; Pinto, M.; Sobral, D.; Di Pasquale, A.; Gomes, J.P.; Borges, V. ReporTree: A Surveillance-Oriented Tool to Strengthen the Linkage between Pathogen Genetic Clusters and Epidemiological Data. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Zhou, Z.; Alikhan, N.-F.; Sergeant, M.J.; Luhmann, N.; Vaz, C.; Francisco, A.P.; Carriço, J.A.; Achtman, M. GrapeTree: Visualization of Core Genomic Relationships among 100,000 Bacterial Pathogens. Genome Res. 2018, 28, 1395–1404. [Google Scholar] [CrossRef]
- Van Walle, I.; Björkman, J.T.; Cormican, M.; Dallman, T.; Mossong, J.; Moura, A.; Pietzka, A.; Ruppitsch, W.; Takkinen, J. European Listeria WGS typing group. Retrospective validation of whole genome sequencing-enhanced surveillance of listeriosis in Europe, 2010 to 2015. Eurosurveillance 2018, 23, 1700798. [Google Scholar] [CrossRef]
- Treangen, T.J.; Ondov, B.D.; Koren, S.; Phillippy, A.M. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014, 15, 524. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Foodborne Outbreaks—Dashboard. Available online: https://www.efsa.europa.eu/en/microstrategy/FBO-dashboard (accessed on 19 December 2022).
- Little, C.L.; Rhoades, J.R.; Sagoo, S.K.; Harris, J.; Greenwood, M.; Mithani, V.; Grant, K.; McLauchlin, J. Microbiological quality of retail cheeses made from raw, thermized or pasteurized milk in the UK. Food Microbiol. 2008, 25, 304–312. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Hunt, K.; McSweeney, S.; Jordan, K. Occurrence of foodborne pathogens in Irish farmhouse cheese. Food Microbiol. 2009, 26, 910–914. [Google Scholar] [CrossRef]
- Rudol, M.; Scherer, S. High incidence of Listeria monocytogenes in European red smear cheese. Int. J. Food Microbiol. 2001, 63, 91–98. [Google Scholar] [CrossRef]
- Almeida, G.; Figueiredo, A.; Rôla, M.; Barros, R.M.; Gibbs, P.; Hogg, T.; Teixeira, P. Microbiological characterization of randomly selected Portuguese raw milk cheeses with reference to food safety. J. Food Prot. 2007, 70, 1710–1716. [Google Scholar] [CrossRef]
- Kongo, J.M.; Malcata, F.X.; Ho, A.J.; Wiedmann, M. Detection and characterization of Listeria monocytogenes in Sao Jorge (Portugal) cheese production. J. Dairy Sci. 2006, 89, 4456–4461. [Google Scholar] [CrossRef]
- Rosengren, A.; Fabricius, A.; Guss, B.; Sylvén, S.; Lindqvist, R. Occurrence of foodborne pathogens and characterization of Staphylococcus aureus in cheese produced on farm-dairies. Int. J. Food Microbiol. 2010, 144, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, N.; Ceniti, C.; Santoro, A.; Clausi, M.T.; Casalinuovo, F. Foodborne Pathogen Assessment in Raw Milk Cheeses. Int. J. Food Sci. 2020, 2020, 3616713. [Google Scholar] [CrossRef]
- Giammanco, G.M.; Pepe, A.; Aleo, A.; D’Agostino, V.; Milone, S.; Mammina, C. Microbiological quality of Pecorino Siciliano “primosale” cheese on retail sale in the street markets of Palermo, Italy. New Microbiol. 2011, 34, 179–185. [Google Scholar]
- Coroneo, V.; Carraro, V.; Aissani, N.; Sanna, A.; Ruggeri, A.; Succa, S.; Meloni, B.; Pinna, A.; Sanna, C. Detection of Virulence Genes and Growth Potential in Listeria monocytogenes Strains Isolated from Ricotta Salata Cheese. J. Food Sci. 2016, 81, M114–M120. [Google Scholar] [CrossRef] [PubMed]
- Spanu, C.; Scarano, C.; Ibba, M.; Spanu, V.; De Santis, E.P.L. Occurrence and traceability of Listeria monocytogenes strains isolated from sheep’s milk cheese-making plants environment. Food Control 2015, 47, 318–325. [Google Scholar] [CrossRef]
- Cartwright, E.J.; Jackson, K.A.; Johnson, S.D.; Graves, L.M.; Silk, B.J.; Mahon, B.E. Listeriosis outbreaks and associated food vehicles, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Dworak, R.; Prager, R.; Becker, B.; Brockmann, S.; Wicke, A.; Wichmann- Schauer, H.; Hof, H.; Werber, D.; Stark, K. Large listeriosis outbreak linked to cheese made from pasteurized milk, Germany, 2006–2007. Foodborne Pathog. Dis. 2010, 7, 1581–1584. [Google Scholar] [CrossRef]
- Fretz, R.; Pichler, J.; Sagel, U.; Much, P.; Ruppitsch, W.; Pietzka, A.T.; Stöger, A.; Huhulescu, S.; Heuberger, S.; Appl, G.; et al. Update: Multinational listeriosis outbreak due to ‘Quargel’, a sour milk curd cheese, caused by two different L. monocytogenes serotype 1/2a strains, 2009–2010. Eurosurveillance 2010, 15, 19543. [Google Scholar] [CrossRef]
- Yde, M.; Naranjo, M.; Mattheus, W.; Stragier, P.; Pochet, B.; Beulens, K.; De Schrijver, K.; Van den Branden, D.; Laisnez, V.; Flipse, W.; et al. Usefulness of the European Epidemic Intelligence Information System in the management of an outbreak of listeriosis, Belgium, 2011. Eurosurveillance 2012, 17, 20279. [Google Scholar] [CrossRef]
- de Castro, V.; Escudero, J.; Rodriguez, J.; Muniozguren, N.; Uribarri, J.; Saez, D.; Vazquez, J. Listeriosis outbreak caused by Latin-style fresh cheese, Bizkaia, Spain, August 2012. Eurosurveillance 2012, 17, 20298. [Google Scholar] [CrossRef]
- Magalhaes, R.; Almeida, G.; Ferreira, V.; Santos, I.; Silva, J.; Mendes, M.M.; Pita, J.; Mariano, G.; Mancio, I.; Sousa, M.M.; et al. Cheese-related listeriosis outbreak, Portugal, March 2009 to February 2012. Eurosurveillance 2015, 20, 21104. [Google Scholar] [CrossRef]
- Ertas, N.; Gonulalan, Z.; Yildirim, Y.; Kum, E. Detection of Staphylococcus aureus enterotoxins in sheep cheese and dairy desserts by multiplex PCR technique. Int. J. Food Microbiol. 2010, 142, 74–77. [Google Scholar] [CrossRef]
- Caro, I.; García-Armesto, M.R. Occurrence of Shiga toxin-producing Escherichia coli in a Spanish raw ewe’s milk cheese. Int. J. Food Microbiol. 2007, 116, 410–413. [Google Scholar] [CrossRef]
- Marozzi, S.; De Santis, P.; Lovari, S.; Condoleo, R.; Bilei, S.; Marcianò, R.; Mezher, Z. Prevalence and Molecular Characterisation of Shiga Toxin-Producing Escherichia Coli in Raw Milk Cheeses from Lazio Region, Italy. Ital. J. Food Saf. 2016, 5, 4566. [Google Scholar] [CrossRef] [PubMed]
- Stephan, R.; Schumacher, S.; Corti, S.; Krause, G.; Danuser, J.; Beutin, L. Prevalence and characteristics of Shiga toxin-producing Escherichia coli in Swiss raw milk cheeses collected at producer level. J. Dairy Sci. 2008, 91, 2561–2565. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.; Sánchez, S.; Blanco, J.E.; Hermoso de Mendoza, J.; Hermoso de Mendoza, M.; García, A.; Gil, C.; Tejero, N.; Rubio, R.; Alonso, J.M. Prevalence, serotypes and virulence genes of Shiga toxin-producing Escherichia coli isolated from ovine and caprine milk and other dairy products in Spain. Int. J. Food Microbiol. 2006, 107, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Manges, A.R.; Johnson, J.R. Food-borne origins of Escherichia coli causing extraintestinal infections. Clin. Infect. Dis. 2012, 55, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Jeannot, K.; Xiao, Y.; Knapp, C.W. Editorial: Horizontal Gene Transfer Mediated Bacterial Antibiotic Resistance. Front. Microbiol. 2019, 10, 1933. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. Available online: https://apps.who.int/iris/handle/10665/112642 (accessed on 19 October 2022).
- Reid, C.J.; Cummins, M.L.; Börjesson, S.; Brouwer, M.S.M.; Hasman, H.; Hammerum, A.M.; Roer, L.; Hess, S.; Berendonk, T.; Nesporová, K.; et al. A role for ColV plasmids in the evolution of pathogenic Escherichia coli ST58. Nat. Commun. 2022, 13, 683. [Google Scholar] [CrossRef]
- Blanco, J.; Mora, A.; Mamani, R.; López, C.; Blanco, M.; Dahbi, G.; Herrera, A.; Blanco, J.E.; Alonso, M.P.; García-Garrote, F.; et al. National survey of Escherichia coli causing extraintestinal infections reveals the spread of drug-resistant clonal groups O25b:H4-B2-ST131, O15:H1-D-ST393 and CGA-D-ST69 with high virulence gene content in Spain. J. Antimicrob. Chemother. 2011, 66, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Painset, A.; Björkman, J.T.; Kiil, K.; Guillier, L.; Mariet, J.F.; Félix, B.; Amar, C.; Rotariu, O.; Roussel, S.; Perez-Reche, F.; et al. LiSEQ—Whole-genome sequencing of a cross-sectional survey of Listeria monocytogenes in ready-to-eat foods and human clinical cases in Europe. Microb. Genom. 2019, 5, e000257. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, P.; Cilla, G.; López-Olaizola, M.; Vicente, D.; Marimón, J.M. Epidemiology and Clinical Features of Listeriosis in Gipuzkoa, Spain, 2010–2020. Front. Microbiol. 2022, 13, 894334. [Google Scholar] [CrossRef]
| Interpretation | ||||
|---|---|---|---|---|
| Parameters | Satisfactory | Borderline | Unsatisfactory/Potential Injurious to Health | |
| Pathogens | L. monocytogenes | Not detected in 25 g | ≤102 cfu/g | >102 cfu/g |
| CPS | <10 cfu/g | 10–≤104 cfu/g | >104 cfu/g | |
| STEC and non-STEC | Not detected in 25 g | N/A | Detected in 25 g | |
| Indicator organisms | E. coli | <10 cfu/g | 10–≤104 cfu/g | >104 cfu/g |
| Listeriaspp. (notL. monocytogenes) | Not detectedin 25 g | ≤102 cfu/g | >102 cfu/g | |
| MQ | Results | # Samples |
| Unsatisfactory | E. coli > 104 cfu/g | 21 (4 also ExPEC) |
| CPS > 104 cfu/g | 11 (1 SE+) | |
| L. monocytogenes > 100 cfu/g | 3 | |
| ExPEC | 4 | |
| E. coli and CPS > 104 cfu/g | 4 (1SE+ and ExPEC) | |
| L. monocytogenes > 100 cfu/g and E. coli > 104 cfu/g | 1 | |
| Total | 44 | |
| Results | # Samples | |
| Borderline | E. coli > 10 and ≤104 cfu/g | 9 |
| CPS > 10 and ≤104 cfu/g | 2 | |
| Listeria ssp. ≠ L. monocytogenes Detected in 25 g and <100 cfu/g | 1 | |
| E. coli and CPS > 10 and ≤104 cfu/g | 8 | |
| Listeria spp. other than L. monocytogenes Detected in 25 g and <100 cfu/g; E. coli > 10 and ≤104 cfu/g | 3 | |
| L. monocytogenes Detected in 25 g and <100 cfu/g; E. coli > 10 and ≤104 cfu/g | 2 | |
| Listeria spp. ≠ L. monocytogenes Detected in 25 g and <100 cfu/g; E. coli and CPS > 10 and ≤104 cfu/g | 4 | |
| L. monocytogenes Detected in 25g and <100 cfu/g; Listeria spp. ≠ L. monocytogenes Detected in 25 g and <100 cfu/g; E. coli and CPS > 10 and ≤104 cfu/g | 1 | |
| Total | 30 |
| Prod | Brand-Milk Type | Region | # Samples * | L. monocytogenes | Other Listeria spp. | E. coli | CPS | MQ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D/25 g | >102 cfu/g | D/25 g | >10 <104 cfu/g | >104 cfu/g | Pathogenic | AMR | >10 <104 cfu/g | >104 cfu/g | Toxin | |||||
| A | 1-Ewe (with chili) | Alto Alentejo | 3/1/1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | U |
| 2-Ewe | 4/4/1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | B | ||
| B | 3-Ewe | 5/5/2 | 2 | 0 | 0 | 4 | 1 | 0 | 1 | 0 | 0 | 0 | U | |
| C | 4-Ewe, cow and goat | 3/3/3 | 0 | 0 | 0 | 1 | 2 | 1 (ExPEC) | 0 | 2 | 0 | 0 | U | |
| D | 5-Ewe | 3/3/0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | S | |
| 6-Ewe (PDO) | 3/3/0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | S | ||
| 7-Ewe and goat | 3/3/0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | S | ||
| E | 8-Ewe, cow and goat | 3/3/2 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 0 | U | |
| F | 9-Ewe | 3/3/3 | 0 | 0 | 2 | 0 | 3 | 0 | 0 | 2 | 1 | 0 | U | |
| G | 10-Ewe | 4/4/3 | 0 | 0 | 3 | 4 | 0 | 0 | 0 | 2 | 1 | 0 | U | |
| H | 11-Ewe (PDO) | 4/3/2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | U | |
| I | 12-Ewe and cow | Alentejo Central | 5/5/3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 1 | 1 | U |
| 13-Ewe (PDO) | 3/3/0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | B | ||
| 14-Ewe and cow | 3/3/2 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 3 | 0 | U | ||
| J | 15-Ewe | 2/2/2 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | U | |
| K | 16-Ewe | 2/2/1 | 0 | 0 | 0 | 2 | 0 | 1 (ExPEC) | 0 | 1 | 0 | 0 | U | |
| L | 17-Ewe and cow | 4/3/2 | 2 | 1 (103) | 2 | 3 | 0 | 1 (ExPEC) | 1 | 1 | 1 | 0 | U | |
| 18-Ewe and cow | 7/6/5 | 1 | 0 | 5 | 4 | 1 | 1 (ExPEC) | 2 | 2 | 0 | 0 | U | ||
| 19-Ewe and cow | 3/3/3 | 2 | 0 | 2 | 3 | 0 | 0 | 0 | 1 | 2 | 0 | U | ||
| 20-Ewe | 3/3/3 | 2 | 1 (103) | 3 | 0 | 3 | 0 | 1 | 3 | 0 | 0 | U | ||
| 21-Ewe | 3/3/3 | 2 | 0 | 3 | 0 | 3 | 2 (ExPEC) | 2 | 2 | 1 | 1 | U | ||
| M | 22-Ewe and cow | 4/3/3 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 2 | 1 | 0 | U | |
| N | 23-Ewe and cow | 3/3/2 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | U | |
| O | 24-Ewe | 3/3/1 | 2 | 2 (102; 103) | 0 | 2 | 0 | 0 | 1 | 2 | 0 | 0 | U | |
| 25-Ewe (PDO) | 1/1/0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | S | ||
| P | 26-Ewe and cow | 2/2/2 | 0 | 0 | 0 | 2 | 0 | 1 (ExPEC) | 1 | 0 | 0 | 0 | U | |
| Q | 27-Ewe | Alentejo Litoral | 2/1/1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | U |
| R | 28-Ewe (PDO) | Baixo Alentejo | 3/3/2 | 1 | 0 | 1 | 1 | 2 | 0 | 1 | 2 | 1 | 0 | U |
| S | 29-Ewe | 2/2/1 | 0 | 0 | 0 | 1 | 1 | 1 (ExPEC) | 1 | 0 | 0 | 0 | U | |
| T | 30-Ewe (PDO) | 3/3/2 | 0 | 0 | 0 | 1 | 2 | 1 (ExPEC) | 2 | 3 | 0 | 0 | U | |
| Total | 96/89/55 | 15/96 (15.6%) | 4/96 (4.2%) | 24/96 (25%) | 44/89 (49.4%) | 26/89 (29.2%) | 9/89 (10.1%) | 15/55 (27.3%) | 35/89 (39.3%) | 15/89 (16.9%) | 2/89 (2.2%) | |||
| E. coli | Listeria monocytogenes cfu/g | Coagulase Positive Staphylococci cfu/g | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMR | cfu/g | Pathogenic (ST) | ||||||||
| Brand | AMP | AMC | CHL | TET | TMP | SMX | ||||
| 3 | x | x | ++ | N | − | − | ||||
| 14 | x | + | N | − | ++ | |||||
| 15 | x | + | N | − | − | |||||
| 17 | x | x | + | N | + | ++ | ||||
| 18 | x | x | + | N | − | − | ||||
| 18 * | x | x | x | x | + | ExPEC (ST69) | + | + | ||
| 20 | x | x | x | ++ | N | + | + | |||
| 21 * | x | x | x | ++ | ExPEC (ST58) | − | ++ SE | |||
| 21 * | x | x | x | x | x | ++ | ExPEC (ST10) | + | + | |
| 24 | x | x | x | + | N | + | + | |||
| 26 * | x | x | x | x | + | ExPEC (ST10) | − | − | ||
| 28 | x | x | ++ | N | + | ++ | ||||
| 29 * | x | x | x | ++ | ExPEC (ST58) | − | − | |||
| 30 * | x | x | x | ++ | ExPEC (ST58) | − | + | |||
| 30 | x | x | x | + | N | − | + | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praça, J.; Furtado, R.; Coelho, A.; Correia, C.B.; Borges, V.; Gomes, J.P.; Pista, A.; Batista, R. Listeria monocytogenes, Escherichia coli and Coagulase Positive Staphylococci in Cured Raw Milk Cheese from Alentejo Region, Portugal. Microorganisms 2023, 11, 322. https://doi.org/10.3390/microorganisms11020322
Praça J, Furtado R, Coelho A, Correia CB, Borges V, Gomes JP, Pista A, Batista R. Listeria monocytogenes, Escherichia coli and Coagulase Positive Staphylococci in Cured Raw Milk Cheese from Alentejo Region, Portugal. Microorganisms. 2023; 11(2):322. https://doi.org/10.3390/microorganisms11020322
Chicago/Turabian StylePraça, Joana, Rosália Furtado, Anabela Coelho, Cristina Belo Correia, Vítor Borges, João Paulo Gomes, Angela Pista, and Rita Batista. 2023. "Listeria monocytogenes, Escherichia coli and Coagulase Positive Staphylococci in Cured Raw Milk Cheese from Alentejo Region, Portugal" Microorganisms 11, no. 2: 322. https://doi.org/10.3390/microorganisms11020322
APA StylePraça, J., Furtado, R., Coelho, A., Correia, C. B., Borges, V., Gomes, J. P., Pista, A., & Batista, R. (2023). Listeria monocytogenes, Escherichia coli and Coagulase Positive Staphylococci in Cured Raw Milk Cheese from Alentejo Region, Portugal. Microorganisms, 11(2), 322. https://doi.org/10.3390/microorganisms11020322







