Role of Probiotics in Preventing Carbapenem-Resistant Enterobacteriaceae Colonization in the Intensive Care Unit: Risk Factors and Microbiome Analysis Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stool Bacterial Analysis for Metagenomics Analysis
2.2. Statistical Analysis
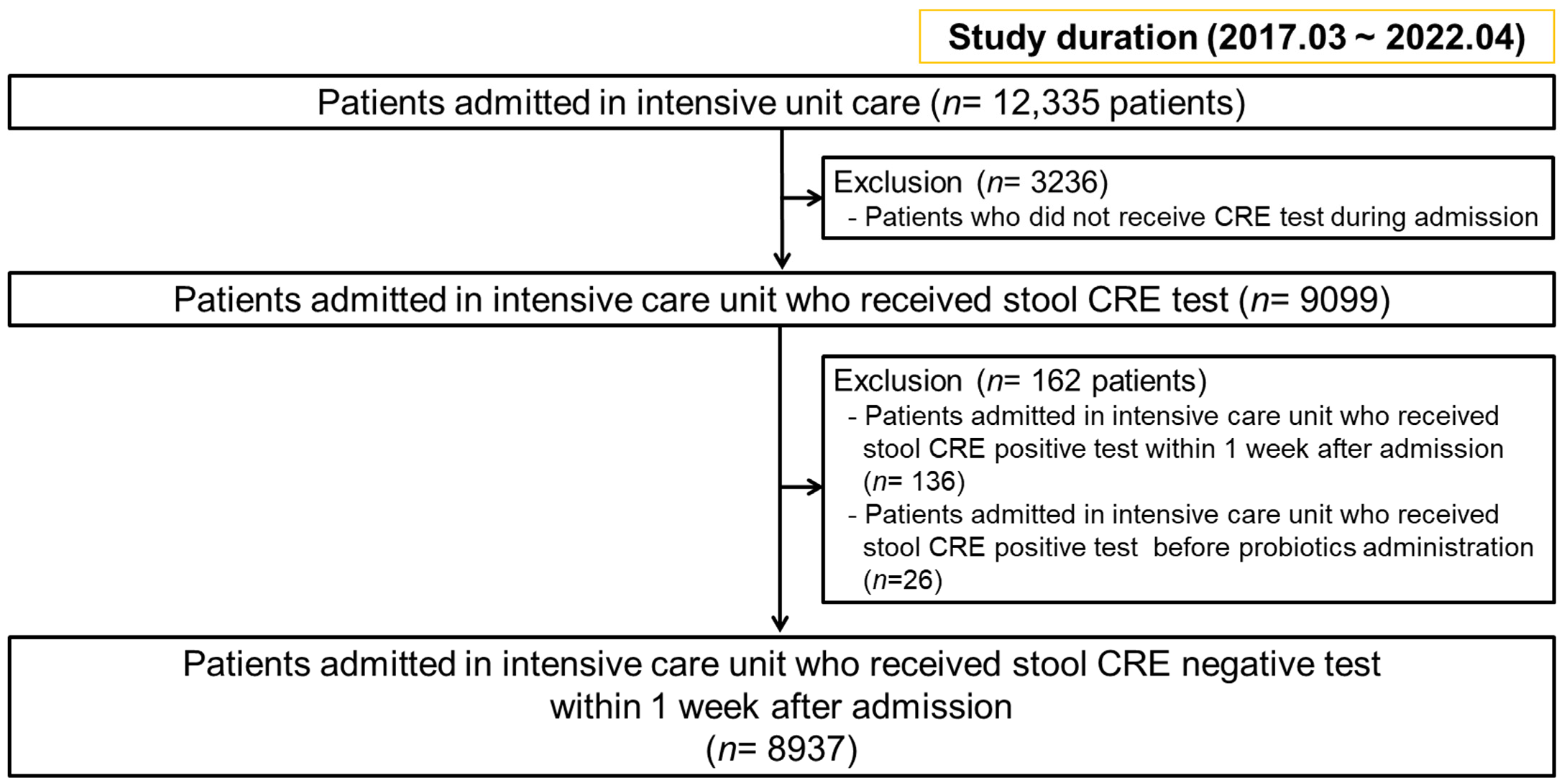
3. Results
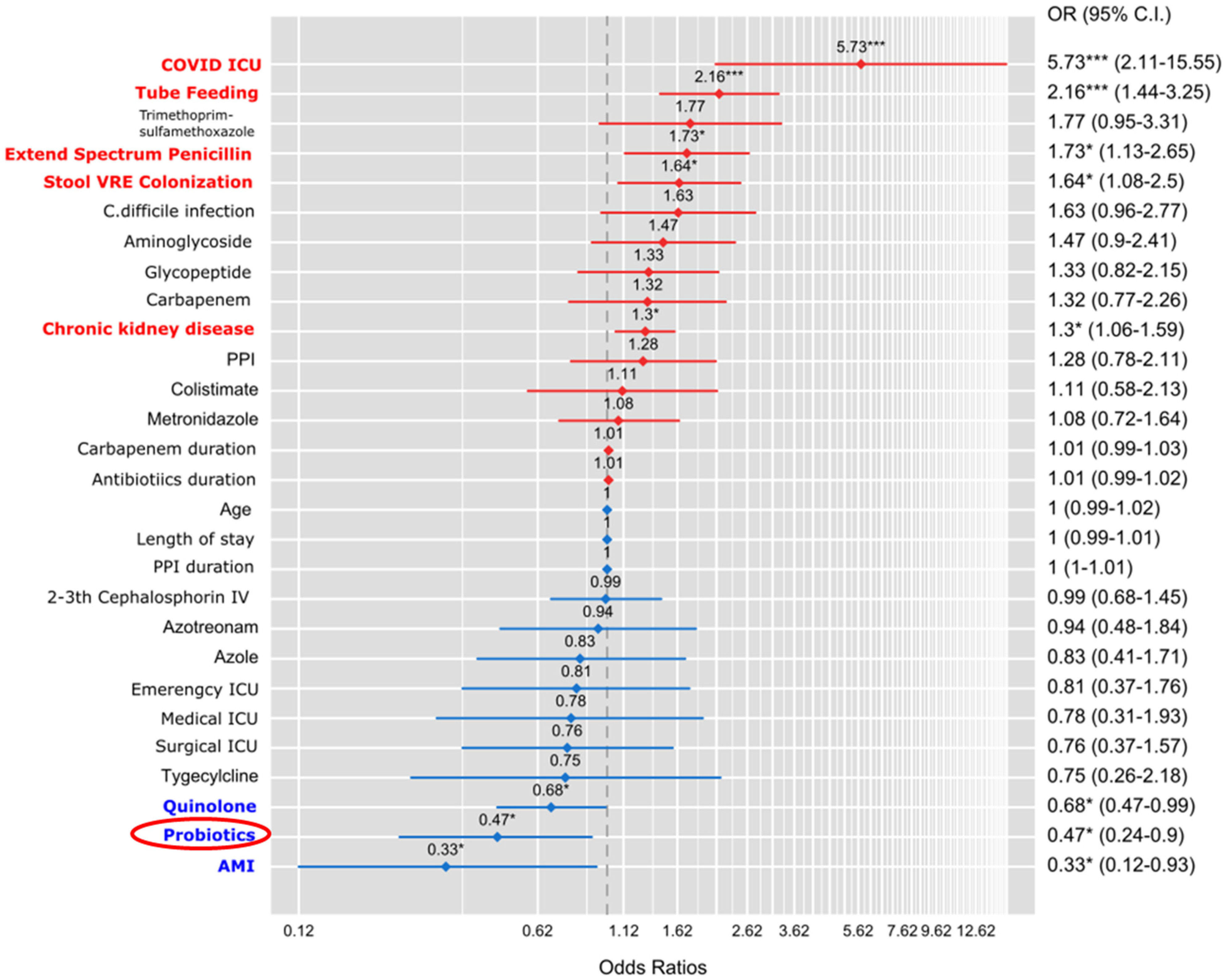
3.1. Multivariable Analysis of Risk Factors Affecting New CRE Colonization
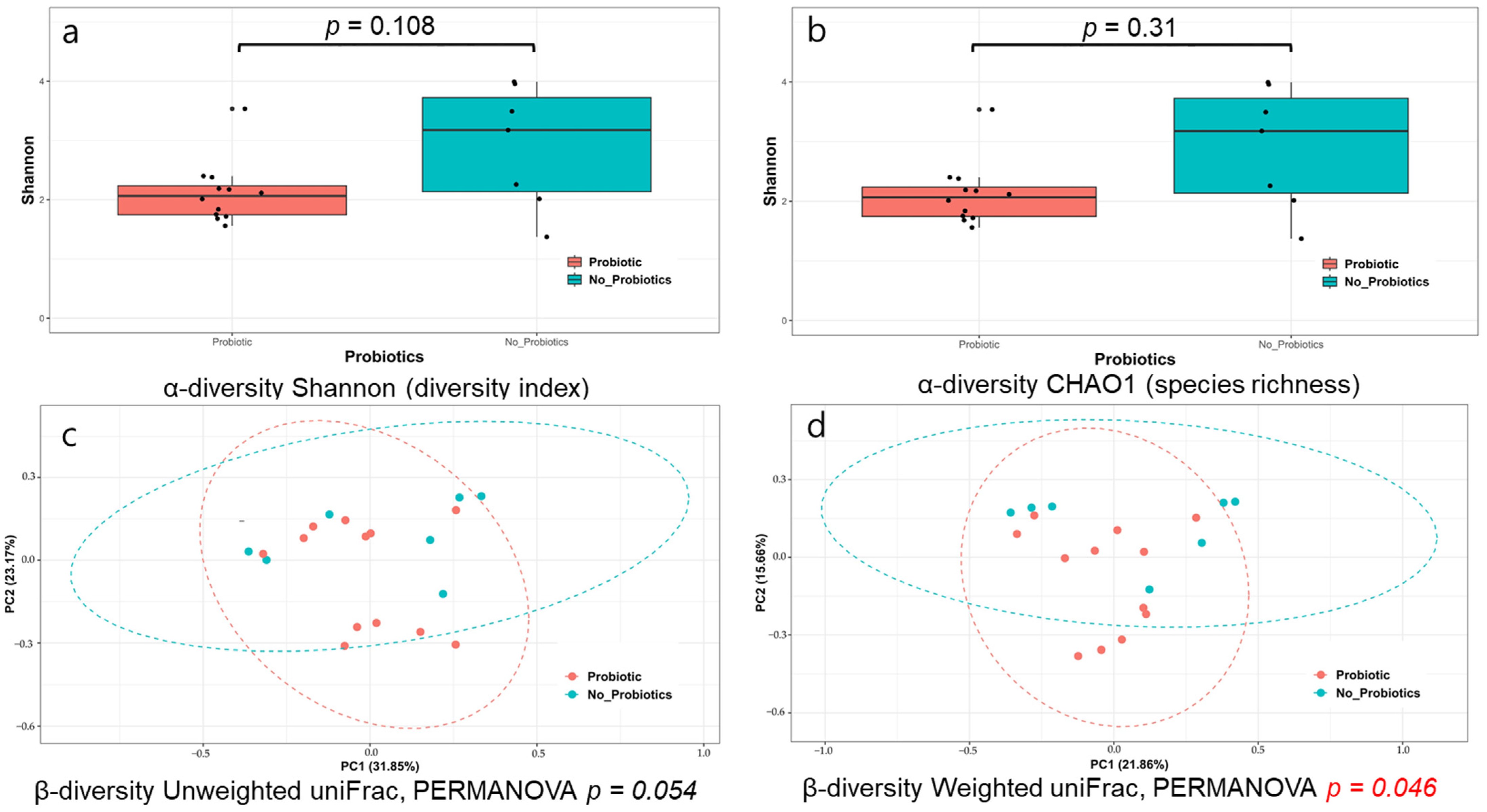
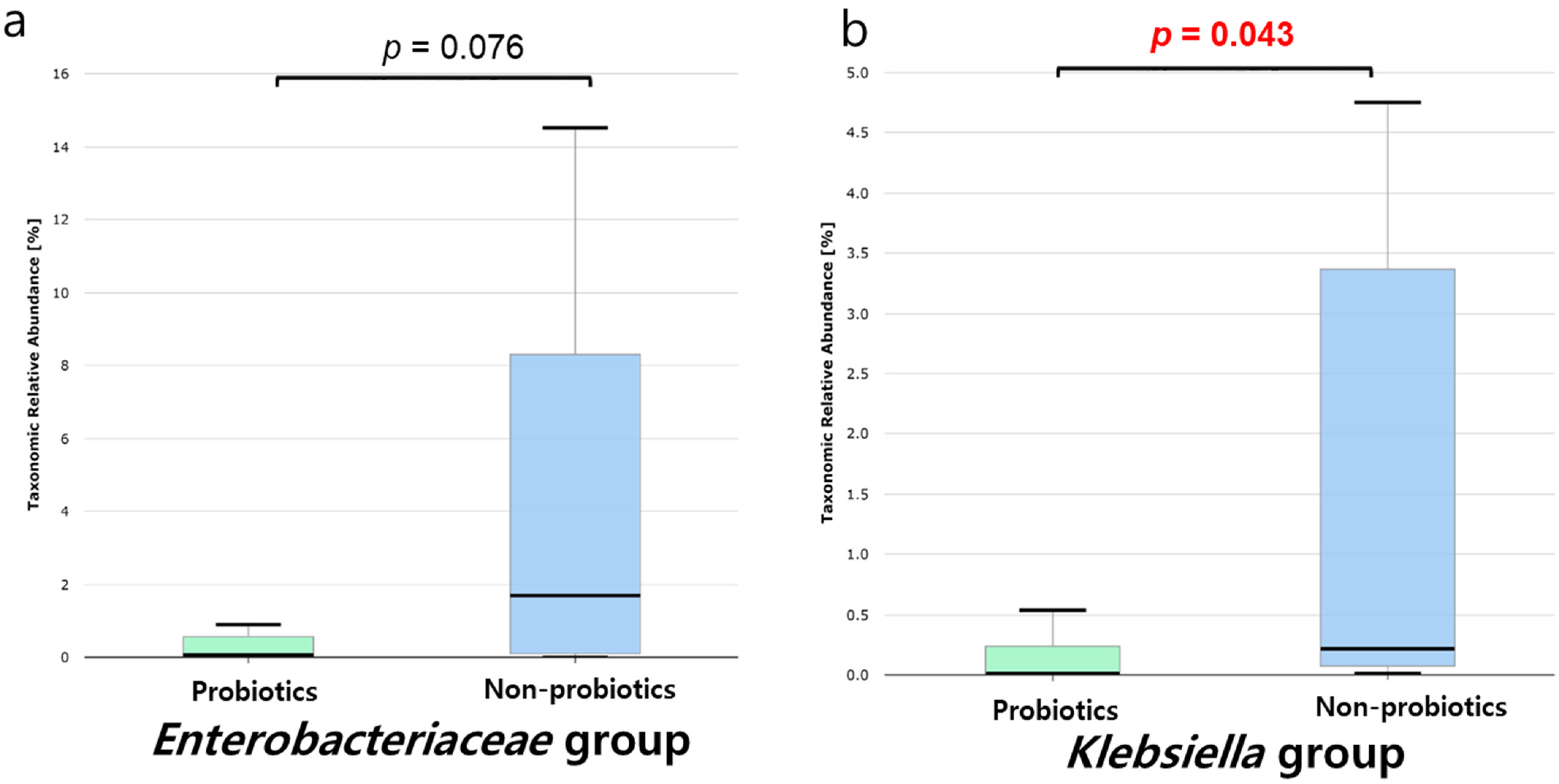
3.2. Analysis of the Microbiome’s Response to Probiotics in Patients with CRE Colonization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antibiotic Resistance Threats in the United States, 2019, US Department of Health Human Service: Washington, DC, USA, 2019.
- Yi, J.; Kim, K.H. Identification and infection control of carbapenem-resistant Enterobacterales in intensive care units. Acute Crit. Care 2021, 36, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Tischendorf, J.; de Avila, R.A.; Safdar, N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: A systematic review. Am. J. Infect. 2016, 44, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.; Lee, S.K.; Cheon, J.H.; Yong, D.E.; Koh, H.; Kang, Y.K.; Jeong, W.Y.; Lee, W.J.; Sohn, Y.; Cho, Y.; et al. Fecal Microbiota Transplantation for multidrug-resistant organism: Efficacy and Response prediction. J. Infect. 2020, 81, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, J.H.; Park, S.H.; Cha, B.; Kwon, K.S.; Kim, H.; Shin, Y.W. Efficacy and Safety of Fecal Microbiota Transplantation for Clearance of Multidrug-Resistant Organisms under Multiple Comorbidities: A Prospective Comparative Trial. Biomedicines 2022, 10, 2404. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Lee, S.K.; Cheon, J.H.; Yong, D.E.; Koh, H.; Kang, Y.K.; Kim, M.H.; Sohn, Y.; Cho, Y.; Baek, Y.J.; et al. Faecal microbiota transplantation reduces amounts of antibiotic resistance genes in patients with multidrug-resistant organisms. Antimicrob. Resist. Infect. Control 2022, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Arshad, M. The Role of Probiotics, Prebiotics and Synbiotics in Combating Multidrug-Resistant Organisms. Clin. Ther. 2020, 42, 1637–1648. [Google Scholar] [CrossRef]
- Campos-Madueno, E.I.; Moradi, M.; Eddoubaji, Y.; Shahi, F.; Moradi, S.; Bernasconi, O.J.; Moser, A.I.; Endimiani, A. Intestinal colonization with multidrug-resistant Enterobacterales: Screening, epidemiology, clinical impact, and strategies to decolonize carriers. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 229–254. [Google Scholar] [CrossRef]
- Resat Sipahi, O.; Quliyeva, G.; Cilli, F.; Deniz Kucukler, N.; Dikis, D.; Bilgili-Korkmaz, N.; Barik-Aksit, S.; Kepeli, N.; Arda, B.; Ulusoy, S. 532. Can Saccharomyces boulardii Therapy Be Effective in Decolonizing Rectal Carbapenem-Resistant Enterobacteriaceae (CRE) Colonization? Open Forum Infect Dis. 2019, 6 (Suppl. S2), S255–S256. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Li, W.; Fu, L.; Niu, B.; Wu, S.; Wooley, J. Ultrafast clustering algorithms for metagenomic sequence analysis. Brief. Bioinform. 2012, 13, 656–668. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Aleidan, F.A.S.; Alkhelaifi, H.; Alsenaid, A.; Alromaizan, H.; Alsalham, F.; Almutairi, A.; Alsulaiman, K.; Abdel Gadir, A.G. Incidence and risk factors of carbapenem-resistant Enterobacteriaceae infection in intensive care units: A matched case-control study. Expert. Rev. Anti Infect. Ther. 2021, 19, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Yi, J.; Ko, M.K.; Lee, S.O.; Lee, J.E.; Kim, K.H. Prevalence and Risk Factors of Carbapenem-resistant Enterobacteriaceae Acquisition in an Emergency Intensive Care Unit in a Tertiary Hospital in Korea: A Case-Control Study. J. Korean Med. Sci. 2019, 34, e140. [Google Scholar] [CrossRef] [PubMed]
- Porretta, A.D.; Baggiani, A.; Arzilli, G.; Casigliani, V.; Mariotti, T.; Mariottini, F.; Scardina, G.; Sironi, D.; Totaro, M.; Barnini, S.; et al. Increased Risk of Acquisition of New Delhi Metallo-Beta-Lactamase-Producing Carbapenem-Resistant Enterobacterales (NDM-CRE) among a Cohort of COVID-19 Patients in a Teaching Hospital in Tuscany, Italy. Pathogens 2020, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef]
- Vlad, N.D.; Cernat, R.C.; Carp, S.; Mitan, R.; Dumitru, A.; Nemet, C.; Voidazan, S.; Rugina, S.; Dumitru, I.M. Predictors of carbapenem-resistant Enterobacteriaceae (CRE) strains in patients with COVID-19 in the ICU ward: A retrospective case-control study. J. Int. Med. Res. 2022, 50, 3000605221129154. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Son, E.; Choe, Y.J.; Kang, C.R.; Roh, S.; Hwang, Y.O.; Cho, S.; Bang, J. Outbreak of carbapenem-resistant Enterobacterales at a long-term care facility in Seoul, Korea: Surveillance and intervention mitigation strategies. Epidemiol. Health 2023, 45, e2023057. [Google Scholar] [CrossRef]
- Lowman, W.; Etheredge, H.R.; Gaylard, P.; Fabian, J. The novel application and effect of an ultraviolet light decontamination strategy on the healthcare acquisition of carbapenem-resistant Enterobacterales in a hospital setting. J. Hosp. Infect. 2022, 121, 57–64. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Spiliopoulou, I.; Christofidou, M.; Logothetis, D.; Manolopoulou, P.; Dodou, V.; Fligou, F.; Marangos, M.; Anastassiou, E.D. Co-colonization by multidrug-resistant bacteria in two Greek intensive care units. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1947–1955. [Google Scholar] [CrossRef]
- Patel, R.; DuPont, H.L. New approaches for bacteriotherapy: Prebiotics, new-generation probiotics, and synbiotics. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S108–S121. [Google Scholar] [CrossRef]
- Korach-Rechtman, H.; Hreish, M.; Fried, C.; Gerassy-Vainberg, S.; Azzam, Z.S.; Kashi, Y.; Berger, G. Intestinal Dysbiosis in Carriers of Carbapenem-Resistant Enterobacteriaceae. mSphere 2020, 5, e00173-20. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E.; McDonald, D.; Knight, R. Role of the microbiome, probiotics, and ‘dysbiosis therapy’ in critical illness. Curr. Opin. Crit. Care 2016, 22, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Dall, L.B.; Lausch, K.R.; Gedebjerg, A.; Fuursted, K.; Storgaard, M.; Larsen, C.S. Do probiotics prevent colonization with multi-resistant Enterobacteriaceae during travel? A randomized controlled trial. Travel. Med. Infect. Dis. 2019, 27, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Bommarito, K.M.; Reske, K.A.; Seiler, S.M.; Hink, T.; Babcock, H.M.; Kollef, M.H.; Fraser, V.J.; Burnham, C.A.; Dubberke, E.R.; et al. Randomized Controlled Trial to Determine the Impact of Probiotic Administration on Colonization With Multidrug-Resistant Organisms in Critically Ill Patients. Infect. Control Hosp. Epidemiol. 2015, 36, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Buyukeren, M.; Yigit, S.; Buyukcam, A.; Kara, A.; Celik, H.T.; Yurdakok, M. A new use of Lactobacillus rhamnosus GG administration in the NICU: Colonized vancomycin-resistant enterococcus eradication in the gastrointestinal system. J. Matern. Fetal Neonatal Med. 2022, 35, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.T.; Tang, J.; Mu, D.Z. Effect of oral administration of probiotics on intestinal colonization with drug-resistant bacteria in preterm infants. Chin. J. Contemp. Pediatr. 2014, 16, 606–609. [Google Scholar]
- Manley, K.J.; Fraenkel, M.B.; Mayall, B.C.; Power, D.A. Probiotic treatment of vancomycin-resistant enterococci: A randomised controlled trial. Med. J. Aust. 2007, 186, 454–457. [Google Scholar] [CrossRef]
- Szachta, P.; Ignys, I.; Cichy, W. An evaluation of the ability of the probiotic strain Lactobacillus rhamnosus GG to eliminate the gastrointestinal carrier state of vancomycin-resistant enterococci in colonized children. J. Clin. Gastroenterol. 2011, 45, 872–877. [Google Scholar] [CrossRef]
- Ljungquist, O.; Kampmann, C.; Resman, F.; Riesbeck, K.; Tham, J. Probiotics for intestinal decolonization of ESBL-producing Enterobacteriaceae: A randomized, placebo-controlled clinical trial. Clin. Microbiol. Infect. 2020, 26, 456–462. [Google Scholar] [CrossRef]
- Nouvenne, A.; Ticinesi, A.; Meschi, T. Carbapenemase-producing Klebsiella pneumoniae in elderly frail patients admitted to medical wards. Ital. J. Med. 2015, 9, 116–119. [Google Scholar] [CrossRef]
- Sharif, S.; Greer, A.; Skorupski, C.; Hao, Q.; Johnstone, J.; Dionne, J.C.; Lau, V.; Manzanares, W.; Eltorki, M.; Duan, E.; et al. Probiotics in Critical Illness: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2022, 50, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, A.; Ruelle, L.; Konopnicki, D.; Miendje Deyi, V.Y.; Dauby, N. Saccharomyces cerevisiae fungemia: Risk factors, outcome and links with S. boulardii-containing probiotic administration. Infect. Dis. Now 2021, 51, 293–295. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | CRE Colonization (n = 157) | No CRE Colonization (n = 8780) | p-Value |
|---|---|---|---|
| Age, years, median (IQR) | 73.0 (61.0–80.0) | 69.0 (56.0–79.0) | 0.006 |
| Sex | >0.999 | ||
| Male | 91 (58.0) | 5119(58.3) | |
| Female | 73 (42.0) | 3661 (41.7) | |
| ICU Category | <0.001 | ||
| Cardiology ICU (CCU) | 9 (5.2) | 770 (8.8) | |
| Emergency ICU (EA and EB) | 88 (56.1) | 5778 (65.8) | |
| Medical ICU (MA and MB) | 36 (22.9) | 1491 (17.0) | |
| Surgical ICU (SB) | 12 (7.6) | 681 (7.8) | |
| COVID ICU (IICU) | 12 (7.6) | 58 (0.7) | |
| Length of stay, days, median (IQR) | 33.0 (21.0–63.0) | 12.0 (6.0–21.0) | <0.001 |
| Stool VRE Positive | 725 (8.3) | 41 (26.1) | <0.001 |
| Clostridium difficile infection | 29 (18.5) | 397 (4.5) | <0.001 |
| CRE-colonized genus | |||
| Citrobacter | 3 (1.9) | ||
| Enterobacter | 9 (5.7) | ||
| Escherichia | 25 (15.9) | ||
| Klebsiella | 120 (76.3) | ||
| Tube feeding | 116 (66.7) | 2558 (29.2) | <0.001 |
| Probiotics | 13 (8.3) | 461 (5.3) | 0.264 |
| Probiotics duration, days, median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.165 |
| Saccharomyces boulardii | 10 (6.4) | 340 (3.9) | 0.455 |
| Lactobacillus rhamnosus | 3 (1.7) | 121 (1.4) | 0.955 |
| PPI | 135 (86.0) | 6236 (71.0) | <0.001 |
| PPI duration, days, median (IQR) | 17.0 (2.0–42.0) | 3.0 (0.0–10.0) | <0.001 |
| Carbapenem | 55 (35.0) | 883 (10.1) | <0.001 |
| Carbapenem duration, days, median (IQR) | 14.0 (8.0–26.5) | 9.0 (5.0–18.0) | <0.001 |
| Antibiotics | |||
| Aminoglycoside | 32 (20.4) | 548 (6.2) | <0.001 |
| Penicillin | 6 (3.8) | 178 (2.0) | 0.116 |
| Extend Spectrum Penicillin | 115 (73.2) | 3675 (41.9) | <0.001 |
| Macrolide | 9 (5.7) | 466 (5.3) | 0.931 |
| Glycopeptide | 53 (33.8) | 742 (8.5) | <0.001 |
| Cephalosphorin PO | 7 (4.5) | 330 (3.8) | >0.999 |
| 1st Cephalosphorin IV | 16 (10.2) | 685 (7.8) | 0.167 |
| 2nd–3rd Cephalosphorin IV | 106 (67.5) | 4662 (53.1) | <0.001 |
| Quinolone | 75 (47.8) | 2967 (33.8) | <0.001 |
| Metronidazole | 75 (47.8) | 2317 (26.4) | <0.001 |
| Clindamycin | 5 (3.2) | 357 (4.1) | 0.726 |
| Azotreonam | 16 (10.2) | 264 (3.0) | <0.001 |
| Colistimate | 18 (11.5) | 158 (1.8) | <0.001 |
| Tygecylcline | 8 (5.1) | 76 (0.9) | <0.001 |
| Trimethoprim–Sulfamethoxazole | 20 (12.7) | 181 (2.1) | <0.001 |
| Rifamycin | 6 (3.8) | 169 (1.9) | 0.248 |
| Azole | 13 (7.5) | 193 (2.2) | <0.001 |
| Antibiotics duration, days, median (IQR) | 8 (1–16) | 27 (17–50) | <0.001 |
| Comorbidity | |||
| Diabetes | 4 (2.3) | 351 (4.0) | 0.344 |
| Diabetes complication | 1 (0.6) | 73 (0.8) | 0.484 |
| Liver disease (Mild) | 2 (1.3) | 276 (3.1) | 0.269 |
| Liver disease (moderate to severe) | 3 (1.9) | 225 (2.6) | 0.125 |
| Acute myocardial infarction | 4 (2.3) | 908 (10.3) | 0.002 |
| Congestive heart failure | 15 (9.6) | 932 (10.6) | 0.766 |
| PAOD | 6 (3.8) | 229 (2.6) | 0.490 |
| Cerebral vascular attack | 28 (17.8) | 1842 (21.0) | 0.389 |
| Hemiplegia | 22 (14.0) | 1249 (14.2) | >0.999 |
| Dementia | 2 (1.3) | 75 (0.9) | 0.898 |
| COPD | 2 (1.3) | 289 (3.3) | 0.236 |
| Rheumatic disease | 3 (1.9) | 46 (0.5) | 0.074 |
| Peptic ulcer | 6 (3.8) | 450 (5.1) | 0.580 |
| Chronic kidney disease | 37 (23.6) | 967 (11.0) | <0.001 |
| Localized solid tumor | 16 (10.2) | 836 (9.5) | 0.884 |
| Lymphoma or Leukemia | 2 (1.3) | 60 (0.7) | 0.690 |
| Metastasis of tumor | 2 (1.3) | 243 (2.8) | 0.374 |
| HIV | 0 (0.0) | 7 (0.1) | >0.999 |
| Charlson comorbidity index, median (IQR) | 1 (0–2) | 1 (0–2) | 0.894 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Shin, J.; Park, S.-H.; Cha, B.; Hong, J.-T.; Lee, D.-H.; Kwon, K.S. Role of Probiotics in Preventing Carbapenem-Resistant Enterobacteriaceae Colonization in the Intensive Care Unit: Risk Factors and Microbiome Analysis Study. Microorganisms 2023, 11, 2970. https://doi.org/10.3390/microorganisms11122970
Lee J-H, Shin J, Park S-H, Cha B, Hong J-T, Lee D-H, Kwon KS. Role of Probiotics in Preventing Carbapenem-Resistant Enterobacteriaceae Colonization in the Intensive Care Unit: Risk Factors and Microbiome Analysis Study. Microorganisms. 2023; 11(12):2970. https://doi.org/10.3390/microorganisms11122970
Chicago/Turabian StyleLee, Jung-Hwan, Jongbeom Shin, Soo-Hyun Park, Boram Cha, Ji-Taek Hong, Don-Haeng Lee, and Kye Sook Kwon. 2023. "Role of Probiotics in Preventing Carbapenem-Resistant Enterobacteriaceae Colonization in the Intensive Care Unit: Risk Factors and Microbiome Analysis Study" Microorganisms 11, no. 12: 2970. https://doi.org/10.3390/microorganisms11122970
APA StyleLee, J.-H., Shin, J., Park, S.-H., Cha, B., Hong, J.-T., Lee, D.-H., & Kwon, K. S. (2023). Role of Probiotics in Preventing Carbapenem-Resistant Enterobacteriaceae Colonization in the Intensive Care Unit: Risk Factors and Microbiome Analysis Study. Microorganisms, 11(12), 2970. https://doi.org/10.3390/microorganisms11122970




