Motility in Periweissella Species: Genomic and Phenotypic Characterization and Update on Motility in Lactobacillaceae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Info and Culture Conditions
2.2. Motility Test
2.3. Transmission Electron Microscopy
2.4. Comparative Analysis of Flagellar Locus and Motility Proteins
2.4.1. Identification of Motility Protein Genes in Some Periweissella Type Strains
2.4.2. In Silico Identification of Motile Species in the Lactobacillaceae Family
2.4.3. Phylogenetic Analysis
2.5. Brightfield Microscopy
3. Results
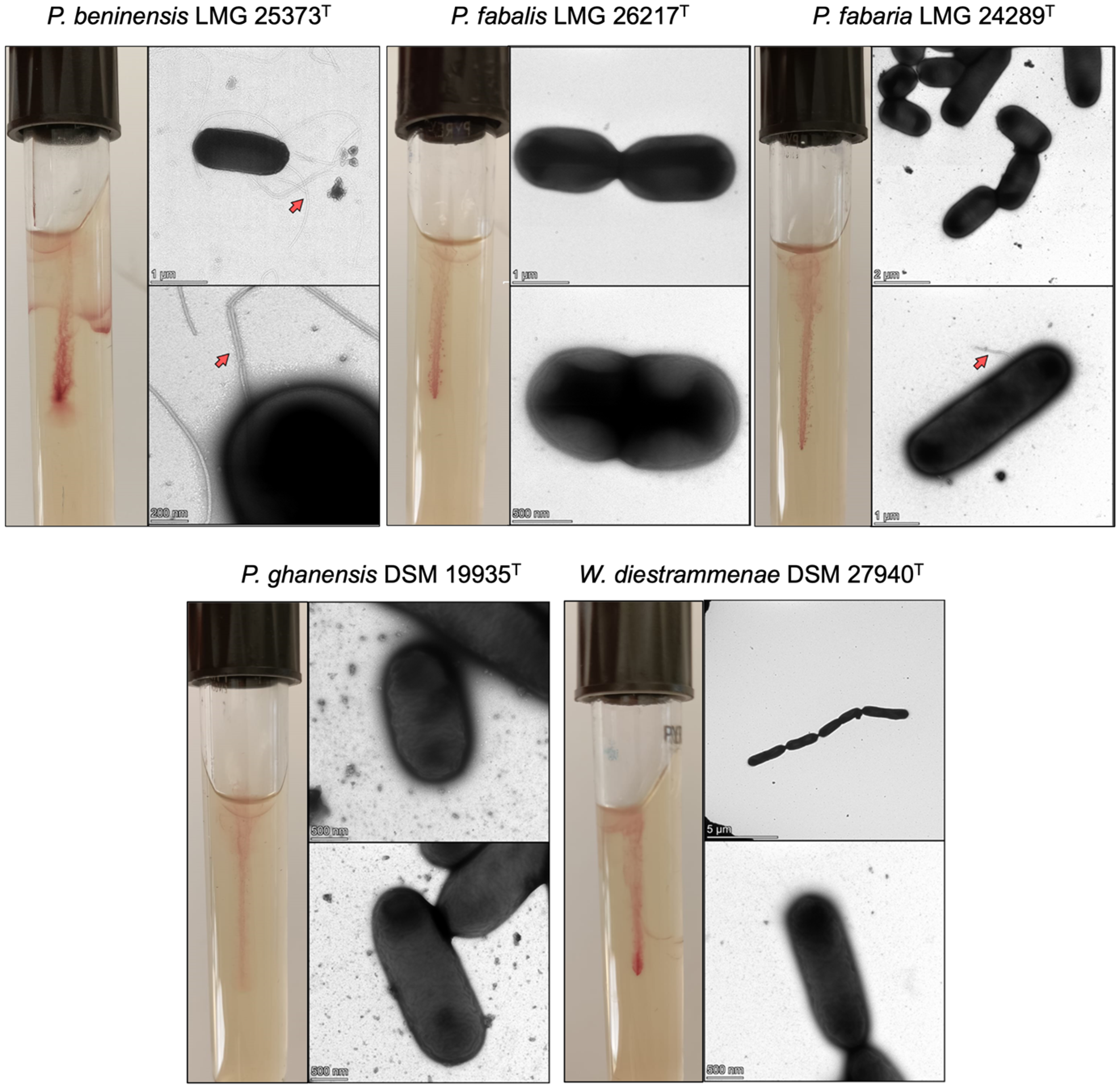
3.1. Motility Behavior
3.2. Transmission Electron Microscopy
3.3. Comparative Analysis of Flagellar Locus and Motility Proteins
3.3.1. Presence of Motility Proteins in Lactobacillaceae
3.3.2. Comparative Analysis of Motility Locus in Periweissella Species
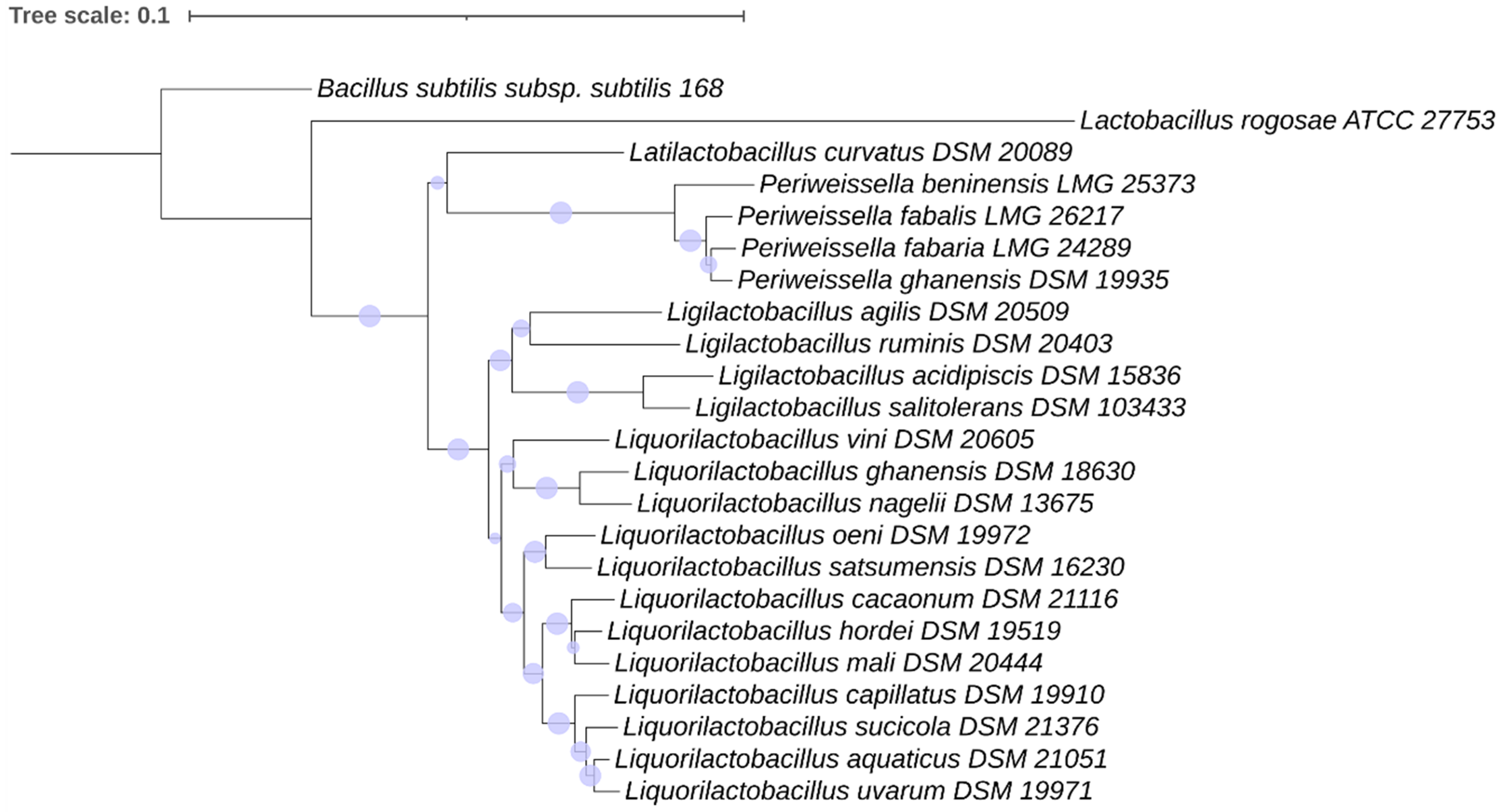
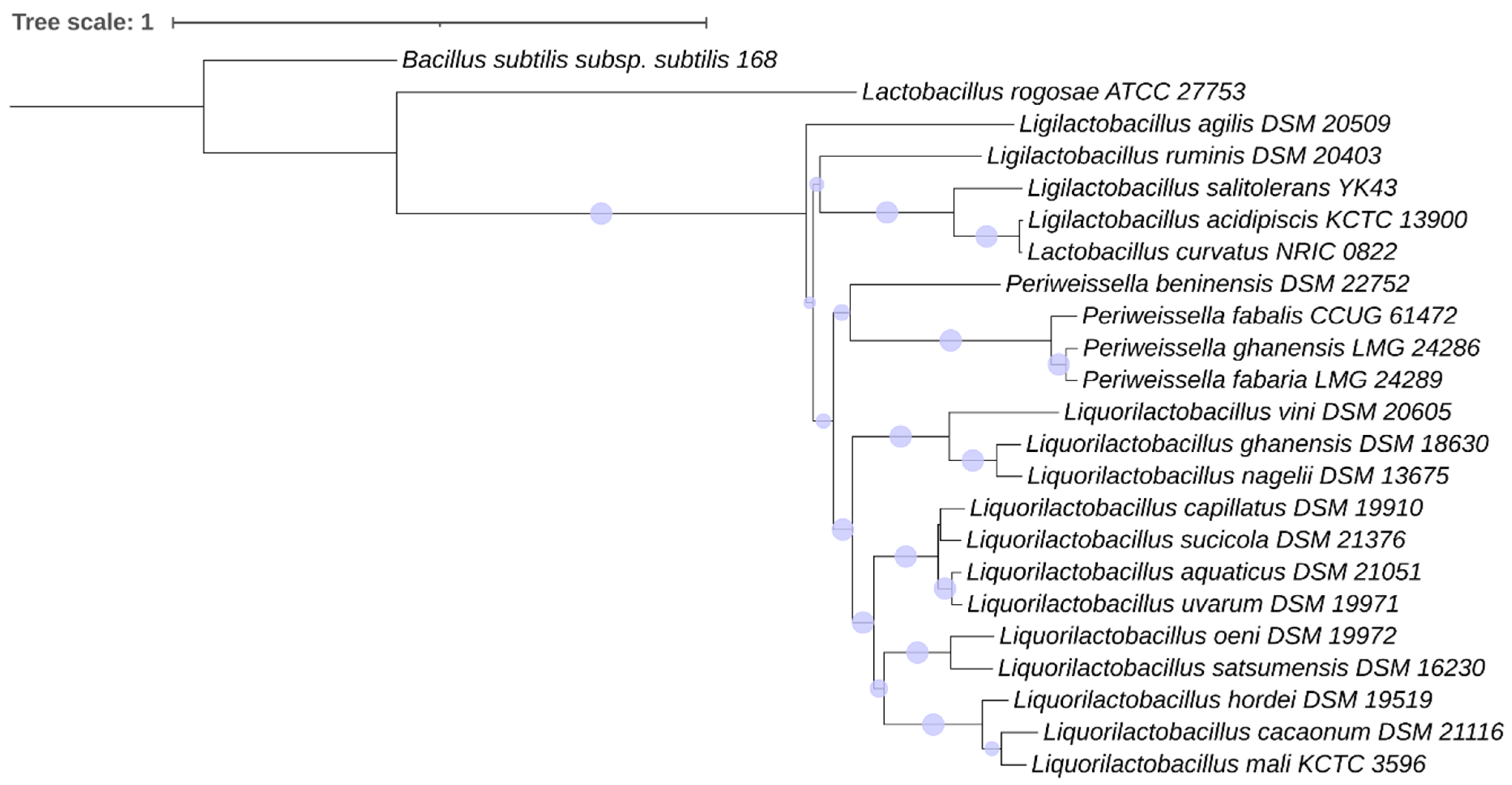
3.3.3. Phylogeny of the Motility Proteins in the Lactobacillaceae Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bello, S.; Rudra, B.; Gupta, R.S. Phylogenomic and comparative genomic analyses of Leuconostocaceae species: Identification of molecular signatures specific for the genera Leuconostoc, Fructobacillus and Oenococcus and proposal for a novel genus Periweissella gen. nov. Int. J. Syst. Evol. Microbiol. 2022, 72, 005284. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Hamada, M.; Cho, H.; Weon, H.Y.; Kim, J.S.; Hong, S.B.; Kim, S.J.; Kwon, S.W. Weissella cryptocerci sp. nov., isolated from gut of the insect Cryptocercus kyebangensis. Int. J. Syst. Evol. Microbiol. 2019, 69, 2801–2806. [Google Scholar] [CrossRef] [PubMed]
- Padonou, S.W.; Schillinger, U.; Nielsen, D.S.; Franz, C.M.A.P.; Hansen, M.; Hounhouigan, J.D.; Nago, M.C.; Jakobsen, M. Weissella beninensis sp. nov., a motile lactic acid bacterium from submerged cassava fermentations, and emended description of the genus Weissella. Int. J. Syst. Evol. Microbiol. 2009, 60, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Snauwaert, I.; Papalexandratou, Z.; De Vuyst, L.; Vandamme, P. Characterization of strains of Weissella fabalis sp. nov. and Fructobacillus tropaeoli from spontaneous cocoa bean fermentations. Int. J. Syst. Evol. Microbiol. 2013, 63, 1709–1716. [Google Scholar] [CrossRef]
- De Bruyne, K.; Camu, N.; De Vuyst, L.; Vandamme, P. Weissella fabaria sp. nov., from a Ghanaian cocoa fermentation. Int. J. Syst. Evol. Microbiol. 2010, 60, 1999–2005. [Google Scholar] [CrossRef]
- De Bruyne, K.; Camu, N.; Lefebvre, K.; De Vuyst, L.; Vandamme, P. Weissella ghanensis sp. nov., isolated from a Ghanaian cocoa fermentation. Int. J. Syst. Evol. Microbiol. 2008, 58, 2721–2725. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Cho, G.S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M.A.P. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef]
- Fusco, V.; Chieffi, D.; Fanelli, F.; Montemurro, M. The Weissella and Periweissella genera: Up to date taxonomy, ecology, safety, biotechnological and probiotic potential. Front. Microbiol. 2023, 14, 2023. [Google Scholar] [CrossRef]
- Lakra, A.K.; Domdi, L.; Hanjon, G.; Tilwani, Y.M.; Arul, V. Some probiotic potential of Weissella confusa MD1 and Weissella cibaria MD2 isolated from fermented batter. LWT 2020, 125, 109261. [Google Scholar] [CrossRef]
- Fanelli, F.; Montemurro, M.; Verni, M.; Garbetta, A.; Bavaro, A.R.; Chieffi, D.; Cho, G.-S.; Franz, C.M.A.P.; Rizzello, C.G.; Fusco, V. Probiotic Potential and Safety Assessment of Type Strains of Weissella and Periweissella Species. Microbiol. Spectr. 2023, 11, e0304722. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Bechtner, J.; Cnockaert, M.; Depoorter, E.; Díaz-Muñoz, C.; Vandamme, P.; De Vuyst, L.; Gänzle, M.G. Comparative genomic analysis of Periweissella and the characterization of novel motile species. Appl. Environ. Microbiol. 2023, 89, e0103423. [Google Scholar] [CrossRef] [PubMed]
- Haiko, J.; Westerlund-Wikström, B. The role of the bacterial flagellum in adhesion and virulence. Biology 2013, 2, 1242–1267. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Shields, P.; Cathcart, L. Motility Test Medium Protocol; American Society for Microbiology: Washington, DC, USA, 2011. [Google Scholar]
- Sørensen, M.C.; Gencay, Y.E.; Birk, T.; Baldvinsson, S.B.; Jäckel, C.; Hammerl, J.A.; Vegge, C.S.; Neve, H.; Brøndsted, L. Primary isolation strain determines both phage type and receptors recognised by Campylobacter jejuni bacteriophages. PLoS ONE 2015, 10, e0116287. [Google Scholar] [CrossRef]
- Fanelli, F.; Montemurro, M.; Chieffi, D.; Cho, G.S.; Franz, C.M.A.P.; Dell’Aquila, A.; Rizzello, C.G.; Fusco, V. Novel Insights into the Phylogeny and Biotechnological Potential of Weissella Species. Front. Microbiol. 2022, 13, 914036. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Cousin, F.J.; Lynch, S.M.; Harris, H.M.; McCann, A.; Lynch, D.B.; Neville, B.A.; Irisawa, T.; Okada, S.; Endo, A.; O’Toole, P.W. Detection and genomic characterization of motility in Lactobacillus curvatus: Confirmation of motility in a species outside the Lactobacillus salivarius clade. Appl. Environ. Microbiol. 2015, 81, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, S.-I. The Flagellar World: Electron Microscopic Images of Bacterial Flagella and Related Surface Structures from More than 30 Species; Academic Press: New York, NY, USA; Oxford, UK, 2014. [Google Scholar]
- Chen, Y.F.; Helmann, J.D. The Bacillus subtilis flagellar regulatory protein sigma D: Overproduction, domain analysis and DNA-binding properties. J. Mol. Biol. 1995, 249, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Yokoseki, T.; Kutsukake, K.; Ohnishi, K.; Iino, T. Functional analysis of the flagellar genes in the fliD operon of Salmonella typhimurium. Microbiology 1995, 141 Pt 7, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Kinoshita, M.; Inoue, Y.; Morimoto, Y.V.; Ihara, K.; Koya, S.; Hara, N.; Nishioka, N.; Kojima, S.; Homma, M.; et al. FliH and FliI ensure efficient energy coupling of flagellar type III protein export in Salmonella. Microbiologyopen 2016, 5, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.A.; Forde, B.M.; Claesson, M.J.; Darby, T.; Coghlan, A.; Nally, K.; Ross, R.P.; O’toole, P.W. Characterization of Pro-Inflammatory Flagellin Proteins Produced by Lactobacillus ruminis and Related Motile Lactobacilli. PLoS ONE 2012, 7, e40592. [Google Scholar] [CrossRef]
- Scharf, B.; Schuster-Wolff-Bühring, H.; Rachel, R.; Schmitt, R. Mutational analysis of the Rhizobium lupini H13-3 and Sinorhizobium meliloti flagellin genes: Importance of flagellin A for flagellar filament structure and transcriptional regulation. J. Bacteriol. 2001, 183, 5334–5342. [Google Scholar] [CrossRef]
- Tambalo, D.D.; Bustard, D.E.; Del Bel, K.L.; Koval, S.F.; Khan, M.F.; Hynes, M.F. Characterization and functional analysis of seven flagellin genes in Rhizobium leguminosarum bv. viciae. Characterization of R. leguminosarum flagellins. BMC Microbiol. 2010, 10, 219. [Google Scholar] [CrossRef]
- Mohari, B.; Thompson, M.A.; Trinidad, J.C.; Setayeshgar, S.; Fuqua, C. Multiple Flagellin Proteins Have Distinct and Synergistic Roles in Agrobacterium tumefaciens Motility. J. Bacteriol. 2018, 200, e00327-18. [Google Scholar] [CrossRef] [PubMed]
- Faulds-Pain, A.; Birchall, C.; Aldridge, C.; Smith, W.D.; Grimaldi, G.; Nakamura, S.; Miyata, T.; Gray, J.; Li, G.; Tang, J.; et al. Flagellin redundancy in Caulobacter crescentus and its implications for flagellar filament assembly. J. Bacteriol. 2011, 193, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Postel, S.; Deredge, D.; Bonsor, D.A.; Yu, X.; Diederichs, K.; Helmsing, S.; Vromen, A.; Friedler, A.; Hust, M.; Egelman, E.H.; et al. Bacterial flagellar capping proteins adopt diverse oligomeric states. Elife 2016, 5, e18857. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, N.; Sassi, A.; Berg, H.C.; Tu, Y. A multi-state dynamic process confers mechano-adaptation to a biological nanomachine. Nat. Commun. 2022, 13, 5327. [Google Scholar] [CrossRef] [PubMed]
- Mondino, S.; San Martin, F.; Buschiazzo, A. 3D cryo-EM imaging of bacterial flagella: Novel structural mechanistic insights into cell motility. J. Biol. Chem. 2022, 298, 102105. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, H.; Barker, C.S.; Yoon, Y.-H.; Wolf, M.; Samatey, F.A. Complete structure of the bacterial flagellar hook reveals extensive set of stabilizing interactions. Nat. Commun. 2016, 7, 13425. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Kinoshita, M.; Namba, K. Insight into Distinct Functional Roles of the Flagellar ATPase Complex for Flagellar Assembly in Salmonella. Front. Microbiol. 2022, 13, 864178. [Google Scholar] [CrossRef]
- Cairns, L.S.; Marlow, V.L.; Kiley, T.B.; Birchall, C.; Ostrowski, A.; Aldridge, P.D.; Stanley-Wall, N.R. FlgN is required for flagellum-based motility by Bacillus subtilis. J. Bacteriol. 2014, 196, 2216–2226. [Google Scholar] [CrossRef]
- Kinoshita, M.; Hara, N.; Imada, K.; Namba, K.; Minamino, T. Interactions of bacterial flagellar chaperone-substrate complexes with FlhA contribute to co-ordinating assembly of the flagellar filament. Mol. Microbiol. 2013, 90, 1249–1261. [Google Scholar] [CrossRef]
- Minamino, T.; Kinoshita, M.; Morimoto, Y.V.; Namba, K. The FlgN chaperone activates the Na+-driven engine of the Salmonella flagellar protein export apparatus. Commun. Biol. 2021, 4, 335. [Google Scholar] [CrossRef]
- Minamino, T.; Kinoshita, M.; Hara, N.; Takeuchi, S.; Hida, A.; Koya, S.; Glenwright, H.; Imada, K.; Aldridge, P.D.; Namba, K. Interaction of a bacterial flagellar chaperone FlgN with FlhA is required for efficient export of its cognate substrates. Mol. Microbiol. 2012, 83, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Kutsukake, K.; Okada, T.; Yokoseki, T.; Iino, T. Sequence analysis of the flgA gene and its adjacent region in Salmonella typhimurium, and identification of another flagellar gene, flgN. Gene 1994, 143, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Imada, K. The bacterial flagellar motor and its structural diversity. Trends Microbiol. 2015, 23, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Kearns, D.B. The structure and regulation of flagella in Bacillus subtilis. Annu. Rev. Genet. 2014, 48, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, S.; Martínez-Granero, F.M.; Sánchez-Contreras, M.; Rivilla, R.; Martín, M. Analysis of Pseudomonas fluorescens F113 genes implicated in flagellar filament synthesis and their role in competitive root colonization. Microbiology 2004, 150 Pt 11, 3889–3897. [Google Scholar] [CrossRef] [PubMed]
- Kalmokoff, M.; Lanthier, P.; Tremblay, T.L.; Foss, M.; Lau, P.C.; Sanders, G.; Austin, J.; Kelly, J.; Szymanski, C.M. Proteomic analysis of Campylobacter jejuni 11168 biofilms reveals a role for the motility complex in biofilm formation. J. Bacteriol. 2006, 188, 4312–4320. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Gryllos, I.; Tomás, J.M.; Shaw, J.G. Motility and the polar flagellum are required for Aeromonas caviae adherence to HEp-2 cells. Infect. Immun. 2001, 69, 4257–4267. [Google Scholar] [CrossRef]
- Tsai, J.-Y.; Yeh, Y.-H.; Lin, L.-D.; Sun, Y.-J.; Hsiao, C.-D. Crystal structure of the flagellin protein FlaG from Helicobacter pylori. J. Chin. Chem. Soc. 2019, 66, 1178–1185. [Google Scholar] [CrossRef]
- Kurniyati, K.; Liu, J.; Zhang, J.-R.; Min, Y.; Li, C. A pleiotropic role of FlaG in regulating the cell morphogenesis and flagellar homeostasis at the cell poles of Treponema denticola. Cell. Microbiol. 2019, 21, e12886. [Google Scholar] [CrossRef]
- Inoue, T.; Barker, C.S.; Matsunami, H.; Aizawa, S.I.; Samatey, F.A. The FlaG regulator is involved in length control of the polar flagella of Campylobacter jejuni. Microbiology 2018, 164, 740–750. [Google Scholar] [CrossRef]
- Kajikawa, A.; Suzuki, S.; Igimi, S. The impact of motility on the localization of Lactobacillus agilis in the murine gastrointestinal tract. BMC Microbiol. 2018, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Bron, P.A.; Marco, M.L.; Van Pijkeren, J.P.; O’Connell Motherway, M.; Hill, C.; Pot, B.; Roos, S.; Klaenhammer, T. Identification of probiotic effector molecules: Present state and future perspectives. Curr. Opin. Biotechnol. 2018, 49, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, R.A.; Mazzeo, M.F. Molecular mechanisms of probiotic action: A proteomic perspective. Curr. Opin. Microbiol. 2012, 15, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Young, C.; Neu, J. Molecular modulation of intestinal epithelial barrier: Contribution of microbiota. J. Biomed. Biotechnol. 2010, 2010, 305879. [Google Scholar] [CrossRef]
- Troge, A.; Scheppach, W.; Schroeder, B.O.; Rund, S.A.; Heuner, K.; Wehkamp, J.; Stange, E.F.; Oelschlaeger, T.A. More than a marine propeller—The flagellum of the probiotic Escherichia coli strain Nissle 1917 is the major adhesin mediating binding to human mucus. Int. J. Med. Microbiol. 2012, 302, 304–314. [Google Scholar] [CrossRef]

| Product | P. beninensis LMG 25373T | P. fabalis LMG 26217T | P. fabaria LMG 24289T | P. ghanensis DSM 19935T |
|---|---|---|---|---|
| flagellar motor protein MotB | WP_205144093.1 KAK10_03145 | WP_168721826.1 KAR41_09605 | WP_230096995.1 KAR50_04000 | WP_230096995.1 KAR53_05550 |
| MotA/TolQ/ExbB proton channel family protein | WP_205144094.1 KAK10_03150 | WP_168721825.1 KAR41_09610 | WP_230096996.1 KAR50_04005 | WP_230097992.1 KAR53_05545 |
| flagellar basal body rod protein FlgB | WP_205144095.1 KAK10_03155 | WP_168721824.1 KAR41_09615 | WP_230096997.1 KAR50_04010 | WP_230096997.1 KAR53_05540 |
| flagellar basal body rod protein FlgC | WP_205144096.1 KAK10_03160 | WP_168721823.1 KAR41_09620 | WP_230096998.1 KAR50_04015 | WP_230097993.1 KAR53_05535 |
| flagellar hook–basal body complex protein FliE | WP_205144097.1 KAK10_03165 | WP_210726990.1 KAR41_09625 | WP_230096999.1 KAR50_04020 | WP_230097994.1 KAR53_05530 |
| flagellar M-ring protein FliF | WP_205144098.1 KAK10_03170 | WP_168721822.1 KAR41_09630 | WP_230097000.1 KAR50_04025 | WP_230097995.1 KAR53_05525 |
| flagellar motor switch protein FliG | WP_205144099.1 KAK10_03175 | WP_168721821.1 KAR41_09635 | WP_230097001.1 KAR50_04030 | WP_230097996.1 KAR53_05520 |
| flagellar assembly protein FliH | WP_205144100.1 KAK10_03180 | WP_168721820.1 KAR41_09640 b | WP_230097002.1 KAR50_04035 b | WP_230097997.1 KAR53_05515 b |
| flagellar protein export ATPase FliI | WP_205144101.1 KAK10_03185 | WP_168721819.1 KAR41_09645 | WP_230097003.1 KAR50_04040 | WP_230097998.1 KAR53_05510 |
| flagellar export protein FliJ | WP_205144102.1 KAK10_03190 | WP_168721818.1 KAR41_09650 b | WP_230096704.1 KAR50_04045 b | WP_230097999.1 KAR53_05505 b |
| flagellar hook–length control protein FliK | WP_205144103.1 KAK10_03195 | WP_168721817.1 KAR41_09655 | WP_230097005.1 KAR50_04050 | WP_230098000.1 KAR53_05500 |
| flagellar basal body rod modification protein FlgD | WP_205144104.1 KAK10_03200 | WP_210726989.1 KAR41_09660 | WP_230097006.1 KAR50_04055 | WP_230097006.1 KAR53_05495 |
| flagellar hook–basal body complex protein FlgE | WP_205144105.1 KAK10_03205 | WP_168721816.1 KAR41_09665 | WP_230097007.1 KAR50_04060 | WP_230098001.1 KAR53_05490 |
| flagellar FlbD family protein | WP_205144106.1 KAK10_03210 | WP_168721815.1 KAR41_09670 | WP_230097008.1 KAR50_04065 | WP_230098002.1 KAR53_05485 |
| flagellar basal body-associated FliL family protein | WP_205144107.1 KAK10_03215 | WP_168721814.1 KAR41_09675 | WP_230097009.1 KAR50_04070 | WP_230098003.1 KAR53_05480 |
| flagellar biosynthetic protein FliO | WP_205144108.1 KAK10_03220 | WP_168721813.1 KAR41_09680 | WP_230097010.1 KAR50_04075 | WP_230098004.1 KAR53_05475 |
| flagellar type III secretion system pore protein FliP | WP_205144109.1 KAK10_03225 | WP_168721812.1 KAR41_09685 | WP_230097011.1 KAR50_04080 | WP_230097011.1 KAR53_05470 |
| flagellar biosynthesis protein FliQ | WP_205144110.1 KAK10_03230 | WP_168721811.1 KAR41_09690 | WP_230097012.1 KAR50_04085 | WP_230097012.1 KAR53_05465 |
| flagellar biosynthetic protein FliR | WP_205144111.1 KAK10_03235 | WP_168721810.1 KAR41_09695 | WP_230097013.1 KAR50_04090 | WP_230098005.1 KAR53_05460 |
| flagellar biosynthesis protein FlhB | WP_205144112.1 KAK10_03240 | WP_168721809.1 KAR41_09700 | WP_230096714.1 KAR50_04095 | WP_230098006.1 KAR53_05455 |
| flagellar biosynthesis protein FlhA | WP_205144113.1 KAK10_03245 | WP_168721808.1 KAR41_09705 | WP_230097015.1 KAR50_04100 | WP_230098007.1 KAR53_05450 |
| FliA/WhiG family RNA polymerase sigma factor | WP_205144114.1 KAK10_03250 | WP_168721807.1 KAR41_09710 | WP_230097016.1 KAR50_04105 | WP_230098008.1 KAR53_05445 |
| flagellar hook–basal body complex protein FlgEFG | WP_205144115.1 KAK10_03255 | WP_168721806.1 KAR41_09715 | WP_230097017.1 KAR50_04110 | WP_230098009.1 KAR53_05440 |
| flagellar hook–basal body protein FlgEFG | WP_205144116.1 KAK10_03260 | WP_168721805.1 KAR41_09720 | WP_230097018.1 KAR50_04115 | WP_230098010.1 KAR53_05435 |
| methyl-accepting chemotaxis protein | WP_205144117.1 KAK10_03265 | WP_168721804.1 KAR41_09725 | WP_230097019.1 KAR50_04120 | WP_230098011.1 KAR53_05430 |
| chemotaxis protein CheW | WP_205144118.1 KAK10_03270 | WP_168721803.1 KAR41_09730 | WP_230097020.1 KAR50_04125 | WP_230097020.1 KAR53_05425 |
| chemotaxis protein CheD | WP_205144119.1 KAK10_03275 | WP_168721802.1 KAR41_09735 | WP_230097021.1 KAR50_04130 | WP_230098012.1 KAR53_05420 |
| chemotaxis-specific protein-glutamate methyltransferase CheB | WP_205144120.1 KAK10_03280 | WP_168721801.1 KAR41_09740 | WP_230097022.1 KAR50_04135 | WP_230098013.1 KAR53_05415 |
| protein-glutamate O-methyltransferase CheR | WP_205144121.1 KAK10_03285 | WP_168721800.1 KAR41_09745 | WP_230097057.1 KAR50_04140 | WP_230098035.1 KAR53_05410 |
| chemotaxis protein CheA | WP_205144122.1 KAK10_03290 | WP_168721799.1 KAR41_09750 | WP_230097023.1 KAR50_04145 | WP_230098014.1 KAR53_05405 |
| chemotaxis protein CheC | WP_205144123.1 KAK10_03295 | WP_168721798.1 KAR41_09755 | WP_230097024.1 KAR50_04150 | WP_230098015.1 KAR53_05400 |
| response regulator | WP_205144124.1 KAK10_03300 | WP_168721797.1 KAR41_09760 | WP_230097025.1 KAR50_04155 | WP_230097025.1 KAR53_05395 |
| chemotaxis protein CheW | WP_205144125.1 KAK10_03305 | WP_168721796.1 KAR41_09765 | WP_230097026.1 KAR50_04160 | WP_230097026.1 KAR53_05390 |
| flagellar motor switch protein FliM | WP_205144126.1 KAK10_03310 | WP_168721795.1 KAR41_09770 | WP_230097027.1 KAR50_04165 | WP_230098016.1 KAR53_05385 |
| flagellar motor switch phosphatase FliY | WP_205144127.1 KAK10_03315 | WP_168721794.1 KAR41_09775 | WP_230097028.1 KAR50_04170 | WP_230098017.1 KAR53_05380 |
| methyl-accepting chemotaxis protein | WP_205144169.1 KAK10_03445 | WP_168721793.1 KAR41_09780 | WP_230097029.1 KAR50_04175 | WP_230098018.1 KAR53_05375 |
| methyl-accepting chemotaxis protein | WP_239517065.1 KAK10_03450 | WP_168721792.1 KAR41_09785 | WP_230097030.1 KAR50_04180 | WP_230098019.1 KAR53_05370 |
| methyl-accepting chemotaxis protein | WP_239517064.1 KAK10_03455 | na | na | na |
| flagellar biosynthesis anti-sigma factor FlgM | WP_205144168.1 KAK10_03460 | WP_168721790.1 KAR41_09795 | WP_230097032.1 KAR50_04190 | WP_230098021.1 KAR53_05360 |
| Flagella synthesis protein FlgN | WP_205144167.1 b KAK10_03465 | WP_168721789.1 b KAR41_09800 | WP_230097033.1 b KAR50_04195 | WP_230098022.1 b KAR53_05355 |
| flagellar hook-associated protein FlgK | WP_205144166.1 KAK10_03470 | WP_168721788.1 KAR41_09805 | WP_230097034.1 KAR50_04200 | WP_230098023.1 KAR53_05350 |
| flagellar hook-associated protein FlgL | WP_205144165.1 KAK10_03475 | WP_168721787.1 KAR41_09810 | WP_230097035.1 KAR50_04205 | WP_230098024.1 KAR53_05345 |
| Flagellin FliC1 | WP_205144164.1 KAK10_03485 | WP_168721786.1 KAR41_01540 | WP_230097036.1 KAR50_04210 | WP_230098025.1 KAR53_05340 |
| FliC2 | na | WP_168721785.1 KAR41_01545 | na | na |
| flagellar protein FlaG | WP_205144163.1 KAK10_03490 | na | na | na |
| flagellar filament-capping protein FliD | WP_205144162.1 KAK10_03495 | WP_168721784.1 KAR41_06490 | WP_230097037.1 KAR50_04215 | WP_230098026.1 KAR53_05335 |
| flagellar export chaperone FliS | WP_205144160.1 KAK10_03505 | WP_168721782.1 KAR41_01555 | WP_230097038.1 KAR50_04225 | WP_230098028.1 KAR53_05325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanelli, F.; Montemurro, M.; Chieffi, D.; Cho, G.-S.; Low, H.-Z.; Hille, F.; Franz, C.M.A.P.; Fusco, V. Motility in Periweissella Species: Genomic and Phenotypic Characterization and Update on Motility in Lactobacillaceae. Microorganisms 2023, 11, 2923. https://doi.org/10.3390/microorganisms11122923
Fanelli F, Montemurro M, Chieffi D, Cho G-S, Low H-Z, Hille F, Franz CMAP, Fusco V. Motility in Periweissella Species: Genomic and Phenotypic Characterization and Update on Motility in Lactobacillaceae. Microorganisms. 2023; 11(12):2923. https://doi.org/10.3390/microorganisms11122923
Chicago/Turabian StyleFanelli, Francesca, Marco Montemurro, Daniele Chieffi, Gyu-Sung Cho, Hui-Zhi Low, Frank Hille, Charles M. A. P. Franz, and Vincenzina Fusco. 2023. "Motility in Periweissella Species: Genomic and Phenotypic Characterization and Update on Motility in Lactobacillaceae" Microorganisms 11, no. 12: 2923. https://doi.org/10.3390/microorganisms11122923
APA StyleFanelli, F., Montemurro, M., Chieffi, D., Cho, G.-S., Low, H.-Z., Hille, F., Franz, C. M. A. P., & Fusco, V. (2023). Motility in Periweissella Species: Genomic and Phenotypic Characterization and Update on Motility in Lactobacillaceae. Microorganisms, 11(12), 2923. https://doi.org/10.3390/microorganisms11122923








