Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation
Abstract
:1. Introduction
2. Encapsulation Techniques
2.1. Spray Drying
- Heating of the inlet air to the desired temperature (<220 °C).
- Two fluid nozzles operate the droplet formation, whereas an ultrasonic spray head operates the nano spray dryer.
- Drying chamber (heat exchange between drying gas and droplets).
- Particle collection using cyclones. Electrostatic particle collector for the nano spray dryer.
- Outlet filter for the collection of the finest particles.
- Drying gas is delivered by an aspirator. An aspirator or compressed air could be used by the nano spray dryer.
- Filtering of the drying gas.
2.2. Spray Cooling
2.3. Freeze Drying or Lyophilization
2.4. Fluidized Bed Coating
2.5. Extrusion
2.6. Ionic Gelation
2.7. Complex Coacervation
2.8. Electrospray and Electrospinning as Part of Nanoencapsulation
2.9. Emulsification
2.10. Encapsulation in Yeasts
- Autolysis: The permeability of yeast cells can be increased by this method or cells can be broken with the aim of obtaining intracellular products. When the cell’s own enzymes proceed to hydrolyze its macromolecules, this is called yeast autolysis. The cell wall structure is affected only in terms of permeability and retains its shape after the procedure is completed, as evidenced by electron microscopy. The main damage observed is located in the plasma membrane. In the food industry, autolysis takes place under conditions of temperatures of approx. 50 °C and pH = 5.5.
- Pulsed electric fields: The membrane of the yeast cells is exposed to an external high-voltage electric field, thus resulting in the formation of pores on the surface of the membrane, and this is called pulsed electric field treatment. Moderate electric field strengths of yeast cells do not cause cell disruption but lead to increased cell permeability at both the cell membrane and cell wall level. However, damage to the cell such as gaps in the cell wall and shrinkage of the cytoplasm is caused at very high electric field strengths (40 kV/cm). This technique has been studied in yeast cells with the aim of increasing the extraction of yeast extract, polysaccharides, proteins, and enzymes.
- High-pressure homogenization: It is a method to break up cells in large-scale processing. Intracellular products such as proteins from E. coli and S. cerevisiae can be obtained by this method. Being a non-thermal method, the pressure of the cell suspension is increased up to a pressure of 1000 bar, hence a specially designed valve system of the homogenizer releases the suspension. Cells receive a wide range of forces that can even cause their complete lysis and, by extension, an increase in cell permeability.
- Plasmolysis: plasmolysis involves cell incubation in the presence of NaCl or ethyl acetate, causing the cell membrane to rupture due to osmotic stress.
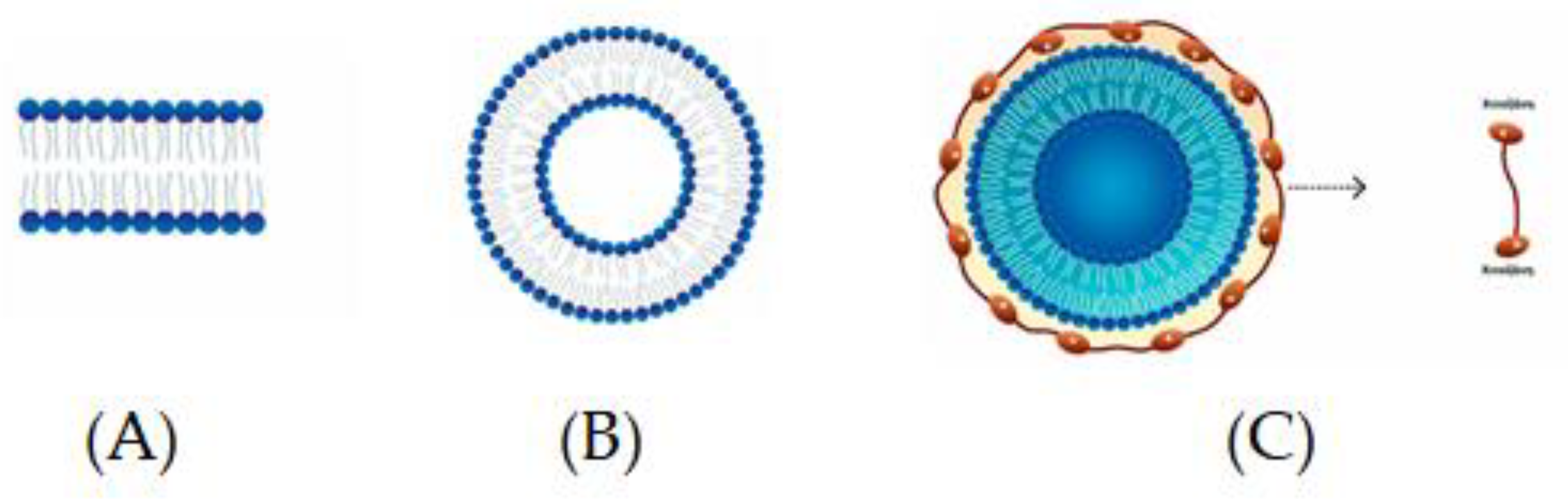
2.11. Liposomes
Chitosomes
2.12. Inclusion Complexation
2.13. Hydrogels
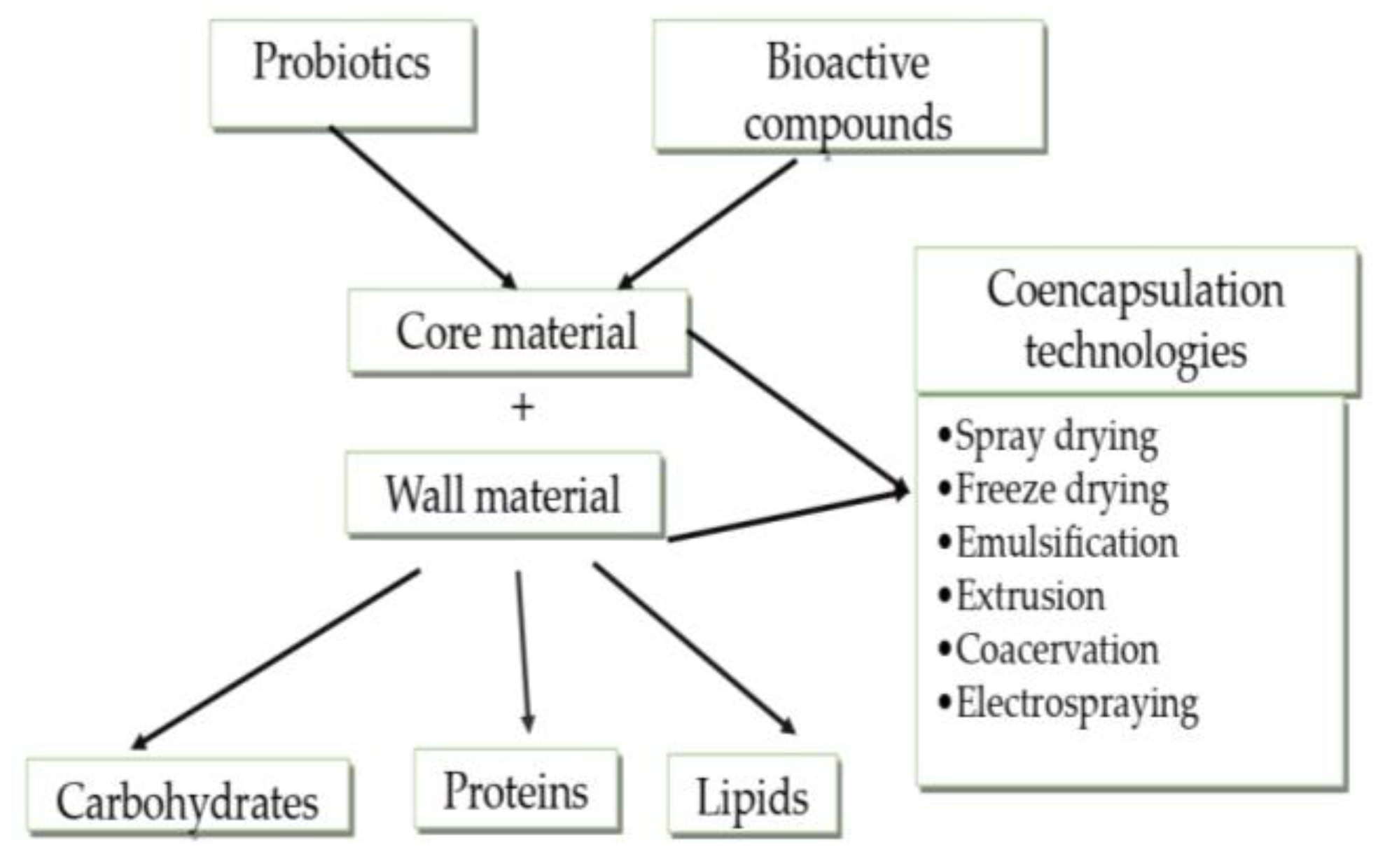
3. Co-Encapsulation of Probiotics
3.1. Co-Encapsulation of Probiotics with Bioactive Substances
3.1.1. Co-Encapsulation of Probiotics with Prebiotics
3.1.2. Co-Encapsulation of Multiple Probiotics
3.1.3. Co-Encapsulation of Probiotics and Omega-3 Fatty Acids
4. Controlled Release Mechanisms of Probiotics
5. Conclusions and Recommendations for Further Work
Author Contributions
Funding
Conflicts of Interest
References
- Sekhavatizadeh, S.S.; Pourakbar, N.; Ganje, M.; Shekarfroush, S.S.; Hosseinzadeh, S. Physicochemical and sensory properties of probiotic yogurt containing Lactobacillus plantarum ATCC 10241 microencapsulated with okra (Abelmoschus esculentus) mucilage and sodium alginate. Bioact. Carbohydr. Diet. Fibre 2023, 30, 100364. [Google Scholar] [CrossRef]
- Desobry, S.; Gaiani, C.; Jayaprakash, P.; Maudhuit, A. Encapsulation of bioactive compounds using competitive emerging techniques: Electrospraying, nano spray drying, and electrostatic spray drying. J. Food Eng. 2023, 339, 111260. [Google Scholar]
- Petsong, K.; Kaewthong, P.; Kingwascharapong, P.; Nilsuwan, K.; Karnjanapratum, S.; Tippayawat, P. Potential of jackfruit inner skin fibre for encapsulation of probiotics on their stability against adverse conditions. Sci. Rep. 2023, 13, 11158. [Google Scholar] [CrossRef]
- Alhamad, M.; Alrosan, M.; Aludatt, M.H.; Alzoubi, H.; Alzougl, R.; Gammoh, S.; Ghatasheh, S.; Ghozlan, K.; Rababah, T.; Tan, T.C.; et al. Encapsulation-based technologies for bioactive compounds and their application in the food industry: A roadmap for food-derived functional and healthy ingredients. Food Biosci. 2022, 50, 101971. [Google Scholar]
- Comunian, T.A.; Silva, M.P.; Souza, C.J.F. The use of food by-products as a novel for functional foods: Their use as ingredients and for the encapsulation process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Abdul-Mudalip, S.K.; Arshad, Z.I.; Che-Man, R.; Hashim, N.A.; Khatiman, M.N. A short review on encapsulation of bioactive compounds using different drying techniques. Mater. Today Proc. 2021, 42, 288–296. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications–A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef]
- Huang, K.T.; Lin, J.H.; Chang, T.X.; Lin, Y.L.; Lee, S.J.; Zheng, Y.Y.; Hsueh, Y.H. Incorporation of high molecular weight gamma-polyglutamic acid in maltodextrin-microencapsulated Bifidobacterium bifidum enhances resistance to simulated gastrointestinal fluids. Process Biochem. 2023, 133, 285–291. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Ismail, S.A.; Murad, H.A.; Khair, A.E.; Asmaa, G.; Azzaz, H.H. Characterization, encapsulation and evaluation of the newly isolated Enterococcus faecium as a probiotic for ruminants. Egypt. J. Chem. 2023, 66, 107–117. [Google Scholar]
- Yin, M.; Chen, M.; Yuan, Y.; Liu, F.; Zhong, F. Encapsulation of Lactobacillus rhamnosus GG in whey protein isolate-shortening oil and gum Arabic by complex coacervation: Enhanced the viability of probiotics during spray drying and storage. Food Hydrocoll. 2023, 146, 109252. [Google Scholar] [CrossRef]
- Akbari, A.; Gänzle, M.G.; Wu, J. Cruciferin improves stress resistance and simulated gastrointestinal survival of probiotic Limosilactobacillus reuteri in the model encapsulation system. Food Hydrocoll. Health 2023, 3, 100118. [Google Scholar] [CrossRef]
- Sun, Q.; Yin, S.; He, Y.; Cao, Y.; Jiang, C. Biomaterials and Encapsulation Techniques for Probiotics: Current Status and Future Prospects in Biomedical Applications. Nanomaterials 2023, 13, 2185. [Google Scholar] [CrossRef]
- Cakmak, H.; Ilyasoglu-Buyukkestelli, H.; Sogut, E.; Ozyurt, V.H.; Gumus-Bonacina, C.E.; Simsek, S. A review on recent advances of plant mucilages and their applications in food industry: Extraction, functional properties and health benefits. Food Hydrocoll. Health 2023, 100131. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, R.; Zhang, L.; Yan, L.; Jia, Y.; Yang, J.; Wang, X.; Lü, X. Enhanced viability of probiotics in composite hydrogel beads. J. Food Eng. 2023, 111621. [Google Scholar] [CrossRef]
- Wani, S.U.D.; Ali, M.; Mehdi, S.; Masoodi, M.H.; Zargar, M.I.; Shakeel, F. A review on chitosan and alginate-based microcapsules: Mechanism and applications in drug delivery systems. Int. J. Biol. Macromol. 2023, 248, 125875. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, B.; Prakash, S.; Binesh, A. Probiotic nanoparticles for food. In Recent Advances in Aquaculture Microbial Technology; Mathew, J., Radhakrishnan, E.K., Midhun, S.J., Kumar, A., Eds.; Academic Press: Boston, MA, USA, 2023; pp. 307–338. [Google Scholar]
- Brandelli, A.; Reque, P.M. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar]
- Bertkova, I.; Kvakova, M.; Savidge, T.C.; Stofilova, J. Co-encapsulated synbiotics and immobilized probiotics in human health and gut microbiota modulation. Nutraceuticals Funct. Foods 2021, 10, 1–28. [Google Scholar]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food development: A review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- Zhu, Q.; Tang, J.; Yao, S.; Feng, J.; Mi, B.; Zhu, W.; Chen, Q.; Liu, D.; Xu, E. Controllable structure of porous starch facilitates bioactive encapsulation by mild gelatinization. Food Hydrocoll. 2023, 145, 109135. [Google Scholar] [CrossRef]
- Li, Y.; Dong, L.; Liu, Y.; Chen, Q.; Wu, Z.; Liu, L.; Farag, M.A.; Liu, L. Ultrasound and enzyme assisted preparation of novel lactoferrin-cereal phenolic acid conjugates: Structural, physicochemical and functional properties. Food Chem. 2023, 435, 137572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, L.; Liu, L.; Wu, Z.; Pan, D.; Liu, L. Recent advances of stimuli-responsive polysaccharide hydrogels in delivery systems: A review. J. Agric. Food Chem. 2022, 70, 6300–6316. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, J.; Deng, Z. Editorial: Changes in food functional components during innovative processing technologies and delivery systems, digestion, and metabolism. Front. Nutr. 2023, 10, 1200010. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Álvarez, P.; González-Ávila, M.; Espinosa-Andrews, H. Recent Advances in Probiotic Encapsulation to Improve Viability under Storage and Gastrointestinal Conditions and Their Impact on Functional Food Formulation. Food Rev. Int. 2023, 39, 992–1013. [Google Scholar] [CrossRef]
- Rajam, R.; Subramanian, P. Encapsulation of probiotics: Past, present and future. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 46. [Google Scholar] [CrossRef]
- Alvarez-Salas, C.; Espinosa-Solis, V.; Leyva-Porras, C.; Piñón-Balderrama, C.; Saavedra-Leos, M.Z.; Terán-Figueroa, Y. Encapsulation of active ingredients in food industry by spray-drying and nano spray-drying technologies. Processes 2020, 8, 889. [Google Scholar]
- Akbari-Alavijeh, S.; Boostani, S.; Dima, C.; Falsafi, S.R.; Geranpour, M.; Hosseini, H.; Jafari, S.M.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Samborska, K.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar]
- Arepally, D.; Goswami, T.K. Effect of Inlet Air Temperature and Gum Arabic Concentration on Encapsulation of Probiotics by Spray Drying. LWT-Food Sci. Technol. 2019, 99, 583–593. [Google Scholar] [CrossRef]
- Gonzalez-Ferrero, C.; Irache, J.M.; Gonzalez-Navarro, C.J. Soybean Protein-Based Microparticles for Oral Delivery of Probiotics with Improved Stability during Storage and Gut Resistance. Food Chem. 2018, 239, 879–888. [Google Scholar] [CrossRef]
- Nunes, G.L.; Etchepare, M.D.A.; Cichoski, A.J.; Zepka, L.Q.; Jacob Lopes, E.; Barin, J.S.; Flores, E.M.D.M.; Da Silva, C.D.B.; De Menezes, C.R. Inulin, Hi-Maize, and Trehalose as Thermal Protectants for Increasing Viability of Lactobacillus acidophilus Encapsulated by Spray Drying. LWT-Food Sci. Technol. 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Rajam, R.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum (MTCC 5422) with Fructooligosaccharide as Wall Material by Spray Drying. LWT-Food Sci. Technol. 2015, 60, 773–780. [Google Scholar] [CrossRef]
- Liao, L.K.; Wei, X.Y.; Gong, X.; Li, J.H.; Huang, T.; Xiong, T. Microencapsulation of Lactobacillus casei LK-1 by Spray Drying Related to Its Stability and In Vitro Digestion. LWT-Food Sci. Technol. 2017, 82, 82–89. [Google Scholar] [CrossRef]
- Tantratian, S.; Wattanaprasert, S.; Suknaisilp, S. Effect of Partial Substitution of Milk-Non-Fat with Xanthan Gum on Encapsulation of a Probiotic Lactobacillus. J. Food Process. Preserv. 2018, 42, e13673. [Google Scholar] [CrossRef]
- Loyeau, P.A.; Spotti, M.J.; Vanden Braber, N.L.; Rossi, Y.E.; Montenegro, M.A.; Vinderola, G.; Carrara, C.R. Microencapsulation of Bifidobacterium Animalis Subsp. Lactis INL1 Using Whey Proteins and Dextrans Conjugates as Wall Materials. Food Hydrocoll. 2018, 85, 129–135. [Google Scholar]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.T. Effect of Drying Methods (Electrospraying, Freeze Drying and Spray Drying) on Survival and Viability of Microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods. 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Favaro-Trindade, C.S.; de Matos Junior, F.E.; Okuro, P.K.; Dias-Ferreira, J.; Cano, A.; Severino, P.; Zielińska, A.; Souto, E.B. Encapsulation of active pharmaceutical ingredients in lipid micro/nanoparticles for oral administration by spray-cooling. Pharmaceutics 2021, 13, 1186. [Google Scholar] [CrossRef] [PubMed]
- Duary, R.K.; Kumar, A.; Mahato, D.K.; Pandhi, S.; Premjit, Y.; Rai, D.C. Current trends in flavor encapsulation: A comprehensive review of emerging encapsulation techniques, flavor release and mathematical modelling. Food Res. Int. 2022, 151, 110879. [Google Scholar]
- Arslan-Tontul, S.; Erbas, M. Single and double layered microencapsulation of probiotics by spray drying and spray chilling. LWT-Food Sci. Technol. 2017, 81, 160–169. [Google Scholar] [CrossRef]
- Arslan-Tontul, S.; Erbas, M.; Gorgulu, A. The use of probiotic-loaded single-and double-layered microcapsules in cake production. Probiotics Antimicrob. Proteins 2019, 11, 840–849. [Google Scholar] [CrossRef]
- Bampi, G.B.; Backes, G.T.; Cansian, R.L.; de Matos, F.E.; Ansolin, I.M.A.; Poleto, B.C.; Corezzolla, L.R.; Favaro-Trindade, C.S. Spray chilling microencapsulation of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis and its use in the preparation of savory probiotic cereal bars. Food Bioproc. Technol. 2016, 9, 1422–1428. [Google Scholar]
- Dong, H.; Li, R.; Shen, J.; Wang, P.; Xu, X.; Yang, Z. Dual improvement in curcumin encapsulation efficiency and lyophilized complex dispersibility through ultrasound regulation of curcumin-protein assembly. Ultrason. Sonochem. 2022, 90, 106188. [Google Scholar] [CrossRef]
- Shu, G.; Wang, Z.; Chen, L.; Wan, H.; Chen, H. Characterization of Freeze-Dried Lactobacillus Acidophilus in Goat Milk Powder and Tablet: Optimization of the Composite Cryoprotectants and Evaluation of Storage Stability at Different Temperature. LWT-Food Sci. Technol. 2018, 90, 70–76. [Google Scholar] [CrossRef]
- Archacka, M.; Białas, W.; Dembczyński, R.; Olejnik, A.; Sip, A.; Szymanowska, D.; Celińska, E.; Jankowski, T.; Olejnik, A.; Rogodzińska, M. Method of Preservation and Type of Protective Agent Strongly Influence Probiotic Properties of Lactococcus lactis: A Complete Process of Probiotic Preparation Manufacture and Use. Food Chem. 2019, 274, 733–742. [Google Scholar] [CrossRef]
- Da Silva Guedes, J.; Pimentel, T.C.; Diniz-Silva, H.T.; Da Cruz Almeida, E.T.; Tavares, J.F.; Leite De Souza, E.; Garcia, E.F.; Magnani, M. Protective Effects of β-Glucan Extracted from Spent Brewer Yeast during Freeze-Drying, Storage and Exposure to Simulated Gastrointestinal Conditions of Probiotic Lactobacilli. LWT-Food Sci. Technol. 2019, 116, 108496. [Google Scholar] [CrossRef]
- Cloutier, S.; Nickerson, M.; Yan, C.; Zhang, W. Protection and masking of omega-3 and -6 oils via microencapsulation. In Microencapsulation in the Food Industry; Academic Press: Cambridge, MA, USA, 2014; pp. 485–500. [Google Scholar]
- Dewettinck, K.; Huyghebaert, A. Fluidized bed coating in food technology. Trends Food Sci. Technol. 1999, 10, 163–168. [Google Scholar] [CrossRef]
- Reddy, C.K.; Agarwal, R.K.; Shah, M.A.; Suriya, M. Encapsulation Techniques for Plant Xxtracts in Plant Extracts: Applications in Food Industry; Academic Press: Cambridge, MA, USA, 2022; pp. 75–88. [Google Scholar]
- Manojlović, V.; Nedović, V.A.; Kailasapathy, K.; Zuidam, N.J. Encapsulation of probiotics for use in food products. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer Science+Business Media: New York, NY, USA, 2010; pp. 269–302. [Google Scholar]
- Chávarri, M.; Maranon, I.; Villaran, M.C. Encapsulation technology to protect probiotic bacteria. In Probiotics; Rigobelo, E.C., Ed.; InTech: London, UK, 2012; pp. 501–540. [Google Scholar]
- Ozdal, T.; Yolci-Omeroglu, P.; Tamer, E.C. Role of encapsulation in functional beverages. In Biotechnological Progressand Beverage Consumption; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing, Elsevier: Cambridige, MA, USA, 2020; Volume 19, pp. 195–232. [Google Scholar]
- Strasser, S.; Neureiter, M.; Geppl, M.; Braun, R.; Danner, H. Influence of lyophilization, fluidized bed drying, addition of protectants, and storage on the viability of lactic acid bacteria. J. Appl. Microbiol. 2009, 107, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Albadran, H.A.; Chatzifragkou, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Stability of probiotic Lactobacillus plantarum in dry microcapsules under accelerated storage conditions. Food Res. Int. 2015, 74, 208–216. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Omura, M.H.; Cedran, M.F.; Dekker, R.F.; Barbosa-Dekker, A.M.; Garcia, S. Effect of natural polymers on the survival of Lactobacillus casei encapsulated in alginate microspheres. J. Microencapsul. 2017, 34, 431–439. [Google Scholar] [CrossRef]
- Giannou, V.; Frakolaki, G.; Topakas, E.; Tzia, C. Effect of various encapsulating agents on the beads morphology and the viability of cells during BB-12 encapsulation through extrusion. J. Food Eng. 2021, 294, 110423. [Google Scholar]
- Haghshenas, B.; Abdullah, N.; Nami, Y.; Radiah, D.; Rosli, R.; Yari Khosroushahi, A. Microencapsulation of probiotic bacteria Lactobacillus plantarum 15 HN using alginate-psyllium-fenugreek polymeric blends. J. Appl. Microbiol. 2015, 118, 1048–1057. [Google Scholar] [CrossRef]
- Etchepare, M.; Raddatz, G.C.; Flores, E.M.M.; Zepka, L.Q.; Jacob-Lopes, E.; Barin, J.S.; Raimundo, C.; Grosso, F.; de Menezes, C.R. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT-Food Sci. Technol. 2016, 65, 511–517. [Google Scholar] [CrossRef]
- Buensanteai, N.; Huy Hoang, N.; Kamkaew, A.; Le Thanh, T.; Papathoti, N.K.; Saengchan, C.; Sangpueak, R.; Thepbandit, W.; Treekoon, J. Chitosan nanoparticles-based ionic galation method: A promising candidate for plant disease management. Polymers 2022, 14, 662. [Google Scholar]
- Vega-Carranza, A.S.; Cervantes-Chávez, J.A.; Luna-Bárcenas, G.; Luna-González, A.; Diarte-Plata, G.; Nava-Mendoza, R.; Rodríguez-Morales, J.A.; Escamilla-Montes, R.; Pool, H. Alginate microcapsules as delivery and protective systems of Bacillus licheniformis in a simulated shrimp’s digestive tract. Aquaculture 2021, 540, 736675. [Google Scholar] [CrossRef]
- Ebrahimnejad, P.; Khavarpour, M.; Khalilid, S. Survival of Lactobacillus acidophilus as probiotic bacteria using chitosan nanoparticles. Int. J. Eng. 2017, 30, 456–463. [Google Scholar]
- Vaziri, A.S.; Alemzadeh, I.; Vossoughi, M. Improving Survivability of Lactobacillus plantarum in Alginate-Chitosan Beads Reinforced by Na-Tripolyphosphate Dual Cross-Linking. LWT-Food Sci. Technol. 2018, 97, 440–447. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Polk, D.B.; Tomasula, P.M.; Yan, F.; Liu, L.S. Preserving Viability of Lactobacillus rhamnosus GG in Vitro and in Vivo by a New Encapsulation System. J. Control. Release 2016, 230, 79–87. [Google Scholar] [CrossRef]
- Dafe, A.; Etemadi, H.; Dilmaghani, A.; Mahdavinia, G.R. Investigation of Pectin/Starch Hydrogel as a Carrier for Oral Delivery of Probiotic Bacteria. Int. J. Biol. Macromol. 2017, 97, 536–543. [Google Scholar] [CrossRef]
- Dafe, A.; Etemadi, H.; Zarredar, H.; Mahdavinia, G.R. Development of Novel Carboxymethyl Cellulose/k-Carrageenan Blends as an Enteric Delivery Vehicle for Probiotic Bacteria. Int. J. Biol. Macromol. 2017, 97, 299–307. [Google Scholar] [CrossRef]
- Liao, N.; Luo, B.; Gao, J.; Li, X.; Zhao, Z.; Zhang, Y.; Ni, Y.; Tian, F. Oligosaccharides as Co-Encapsulating Agents: Effect on Oral Lactobacillus fermentum Survival in a Simulated Gastrointestinal Tract. Biotechnol. Lett. 2019, 41, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Abdallah, N.A.; El-Shafei, K.; Tawfik, N.F.; El-Sayed, H.S. Survivability of Alginate-Microencapsulated Lactobacillus plantarum during Storage, Simulated Food Processing and Gastrointestinal Conditions. Heliyon 2020, 6, e03541. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, S.S.; Alemzadeh, I.; Vaziri, A.S.; Vossoughi, A. Optimization of Alginate-Whey Protein Isolate Microcapsules for Survivability and Release Behavior of Probiotic Bacteria. Appl. Biochem. Biotechnol. 2020, 190, 182–196. [Google Scholar] [CrossRef]
- Rather, S.A.; Akhter, R.; Masoodi, F.A.; Gani, A.; Wani, S.M. Effect of Double Alginate Microencapsulation on in vitro Digestibility and Thermal Tolerance of Lactobacillus plantarum NCDC201 and L. Casei NCDC297. LWT-Food Sci. Technol. 2017, 83, 50–58. [Google Scholar] [CrossRef]
- Safeer Abbas, M.; Afzaal, M.; Saeed, F.; Asghar, A.; Jianfeng, L.; Ahmad, A.; Ullah, Q.; Elahi, S.; Ateeq, H.; Shah, Y.A.; et al. Probiotic viability as affected by encapsulation materials: Recent updates and perspectives. Int. J. Food Prop. 2023, 26, 1324–1350. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Weinbreck, F.; de Vries, R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 2004, 9, 340–349. [Google Scholar] [CrossRef]
- Ghadermazi, R.; Asl, A.K.; Tamjidi, F. Optimization of whey protein isolate-quince seed mucilage complex coacervation. Int. J. Biol. Macromol. 2019, 131, 368–377. [Google Scholar] [CrossRef]
- Hernandez-Rodriguez, L.; Lobato-Calleros, C.; Pimentel-Gonzalez, D.J.; Vernon-Carter, E.J. Lactobacillus plantarum Protection by Entrapment in Whey Protein Isolate: κ-Carrageenan Complex Coacervates. Food Hydrocoll. 2014, 36, 181–188. [Google Scholar] [CrossRef]
- Eratte, D.; McKnight, S.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. Co-Encapsulation and Characterisation of Omega-3 Fatty Acids and Probiotic Bacteria in Whey Protein Isolate-Gum Arabic Complex Coacervates. J. Funct. Foods. 2015, 19, 882–892. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Moschakis, T.; Nigam, P.S.; Biliaderis, C.G. Growth Adaptation of Probiotics in Biopolymer-Based Coacervate Structures to Enhance Cell Viability. LWT-Food Sci. Technol. 2017, 77, 282–289. [Google Scholar] [CrossRef]
- Mao, L.; Pan, Q.; Yuan, F.; Gao, Y. Formation of Soy Protein Isolate-Carrageenan Complex Coacervates for Improved Viability of Bifidobacterium longum during Pasteurization and in vitro Digestion. Food Chem. 2019, 276, 307–314. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.M.; de Deus, C.; Fonseca, B.d.S.; Lopes, E.J.; Cichoski, A.J.; Esmerino, E.A.; Silva, C.d.B.d.; Muller, E.I.; Flores, E.M.M.; de Menezes, C.R. The Effect of Enzymatic Crosslinking on the Viability of Probiotic Bacteria (Lactobacillus acidophilus) Encapsulated by Complex Coacervation. Food Res. Int. 2019, 125, 108577. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, X.; Zhang, H.; Zhang, Y.; Ganzle, M.; Yang, N.; Nishinari, K.; Fang, Y. Probiotic Encapsulation in Water-in-Water Emulsion via Heteroprotein Complex Coacervation of Type-A Gelatin/Sodium Caseinate. Food Hydrocoll. 2020, 105, 105790. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Hubinger, M.D. Hydrophilic food compounds encapsulation by ionic gelation. Curr. Opin. Food Sci. 2017, 15, 50–55. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Zou, D.; Hui, Y.; Nigam, K.; Middelberg, A.P.; Zhao, C.X. Formulation of nanoparticles using mixing-induced nanoprecipitation for drug delivery. Ind. Eng. Chem. Res. 2019, 59, 4134–4149. [Google Scholar] [CrossRef]
- Modarres-Gheisari, S.M.M.; Gavagsaz-Ghoachani, R.; Malaki, M.; Safarpour, P.; Zandi, M. Ultrasonic nano-emulsification—A review. Ultrason. Sonochem. 2019, 52, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Walia, N.; Dasgupta, N.; Ranjan, S.; Ramalingam, C.; Gandhi, M. Methods for nanoemulsion and nanoencapsulation of food bioactives. Environ. Chem. Lett. 2019, 17, 1471–1483. [Google Scholar] [CrossRef]
- Han, S.Y.; Nguyen, D.T.; Kim, B.J.; Kim, N.; Kang, E.K.; Park, J.H.; Choi, I.S. Cytoprotection of probiotic Lactobacillus acidophilus with artificial nanoshells of nature-derived eggshell mem-brane hydrolysates and coffee melanoidins in single-cell nanoencapsulation. Polymers 2023, 15, 1104. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, S.; Maryam, A. Encapsulation of lactic acid bacteria and Bifidobacteria using starch-sodium alginate nanofibers to enhance viability in food model. J. Food Process. Preserv. 2021, 45, e16048. [Google Scholar] [CrossRef]
- Atraki, R.; Azizkhani, M. Survival of Probiotic Bacteria Nanoencapsulated within Biopolymers in a Simulated Gastrointestinal Model. Innov. Food Sci. Emerg. Technol. 2021, 72, 102750. [Google Scholar] [CrossRef]
- Li, S.; Fan, L.; Li, S.; Sun, X.; Di, Q.; Zhang, H.; Li, B.; Liu, X. Validation of layer-by-layer coating as a procedure to enhance Lactobacillus plantarum survival during in vitro digestion, storage, and fermentation. J. Agric. Food Chem. 2023, 71, 1701–1712. [Google Scholar] [CrossRef]
- Horuz, T.İ.; Belibağlı, K.B. Nanoencapsulation by electrospinning to improve stability and water solubility of carotenoids extracted from tomato peels. Food Chem. 2018, 268, 86–93. [Google Scholar] [CrossRef]
- Moncada, M.; Astete, C.; Sabliov, C.; Olson, D.; Boeneke, C.; Aryana, K.J. Nano spray-dried sodium chloride and its effects on the microbiological and sensory characteristics of surface-salted cheese crackers. J. Dairy Sci. 2015, 98, 5946–5954. [Google Scholar] [CrossRef]
- Vinitha, K.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Size-dependent enhancement in salt perception: Spraying approaches to reduce sodium content in foods. Powder Technol. 2021, 378, 237–245. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Farag, M.A.; Varzakas, T.; Lorenzo, J.M. Nanotechnology. In Sustainable Production Technology in Food; Lorenzo, J.M., Munecata, P.E.S., Barba, F.J., Eds.; Elsevier: Cambridge, MA, USA, 2021; pp. 179–202. [Google Scholar]
- Feng, K.; Huangfu, L.; Liu, C.; Bonfili, L.; Xiang, Q.; Wu, H.; Bai, Y. Electrospinning and Electrospraying: Emerging Techniques for Probiotic Stabilization and Application. Polymers 2023, 15, 2402. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.Q.; Xia, Y.N. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ban, Q.; Wang, W.; Hou, J.; Jiang, Z. Novel nano-encapsulated probiotic agents: Encapsulate materials, delivery, and encapsulation systems. J. Control. Release 2022, 349, 184–205. [Google Scholar] [CrossRef]
- Xu, C.; Ma, J.; Liu, Z.; Wang, W.; Liu, X.; Qian, S.; Chen, L.; Gu, L.; Sun, C.; Hou, J.; et al. Preparation of shell-core fiber-encapsulated Lactobacillus rhamnosus 1.0320 using coaxial electrospinning. Food Chem. 2023, 402, 134253. [Google Scholar] [CrossRef]
- Ma, J.; Li, T.; Wang, Q.; Xu, C.; Yu, W.; Yu, H.; Wang, W.; Feng, Z.; Chen, L.; Hou, J.; et al. Enhanced viability of probiotics encapsulated within synthetic/natural biopolymers by the addition of gum arabic via electrohydrodynamic processing. Food Chem. 2023, 413, 135680. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Katsimichas, A.; Oreopoulou, V.; Taoukis, P.; Tsimogiannis, D. Cell permeabilization processes for improved encapsulation of oregano essential oil in yeast cells. J. Food Eng. 2021, 294, 110408. [Google Scholar] [CrossRef]
- Errenst, C.; Kilzer, A.; Petermann, M. Encapsulation of limonene in yeast cells using the concentrated power from technology. J. Supercrit. Fluids 2021, 168, 105076. [Google Scholar] [CrossRef]
- Fumi, M.D.; Trioli, G.; Colombi, M.G.; Colagrande, O. Immobilization of Saccharomyces cerevisiae in calcium alginate gel and its application to bottle-fermented sparkling wine production. Am. J. Enol. Vitic. 1988, 39, 267–272. [Google Scholar] [CrossRef]
- Colagrande, O.; Silva, A.; Fumi, M.D. Recent applications of biotechnology in wine production. Biotechnol. Prog. 1994, 10, 2–18. [Google Scholar] [CrossRef]
- Moreno-García, J.; García-Martínez, T.; Mauricio, J.C.; Moreno, J. Yeast immobilization systems for alcoholic wine fermentations: Actual trends and future perspectives. Front. Microbiol. 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.J.; Maestre, O.; Mauricio, J.C. Use of a novel immobilization yeast system for winemaking. Biotechnol. Lett. 2005, 27, 1421–1424. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.J.; Villalba, J.M.; González-Reyes, J.A.; Ortega, J.M.; Mauricio, J.C. Yeast biocapsules: A new immobilization method and their applications. Enzyme Microb. Technol. 2006, 40, 79–84. [Google Scholar] [CrossRef]
- García-Martínez, T.; Puig-Pujol, A.; Peinado, R.A.; Moreno, J.; Mauricio, J.C. Potential use of wine yeasts immobilized on Penicillium chrysogenum for ethanol production. J. Chem. Technol. Biotechnol. 2012, 87, 51–359. [Google Scholar] [CrossRef]
- García-Martínez, T.; Moreno, J.; Mauricio, J.C.; Peinado, R. Natural sweet wine production by repeated use of yeast cells immobilized on Penicillium chrysogenum. LWT-Food Sci. Technol. 2015, 61, 503–509. [Google Scholar] [CrossRef]
- Puig-Pujol, A.; Bertran, E.; García-Martínez, T.; Capdevila, F.; Mínguez, S.; Mauricio, J.C. Application of a new organic yeast immobilization method for sparkling wine production. Am. J. Enol. Vitic. 2013, 64, 386–394. [Google Scholar] [CrossRef]
- López de Lerma, N.; Peinado, R.A.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Influence of two yeast strains in free, bioimmobilized or immobilized with alginate forms on the aromatic profile of long aged sparkling wines. Food Chem. 2018, 250, 22–29. [Google Scholar] [CrossRef]
- Ogawa, M.; Carmona-Jiménez, P.; García-Martínez, T.; Jorrín-Novo, J.V.; Moreno, J.; Rey, M.D.; Moreno-García, J. Use of yeast biocapsules as a fungal-based immobilized cell technology for Indian Pale Ale-type beer brewing. Appl. Microbiol. Biotechnol. 2022, 106, 7615–7625. [Google Scholar] [CrossRef]
- Liu, M.; Qin, X.; Wu, X. Study on the technology of brewing red raspberry wine by using new immobilized yeast technology. Sci. Rep. 2022, 12, 21344. [Google Scholar] [CrossRef]
- García-Martínez, T.; Peinado, R.A.; Moreno, J.; García-García, I.; Mauricio, J.C. Co-culture of Penicillium chrysogenum and Saccharomyces cerevisiae leading to the immobilization of yeast. J. Chem. Technol. Biotechnol. 2011, 86, 812–817. [Google Scholar] [CrossRef]
- López-Menchero, J.R.; Ogawa, M.; Mauricio, J.C.; Moreno, J. Effect of calcium alginate coating on the cell retention and fermentation of a fungus-yeast immobilization system. LWT-Food Sci. Technol. 2021, 144, 111250. [Google Scholar] [CrossRef]
- Luquez-Caravaca, L.; Ogawa, M.; Rai, R.; Nitin, N.; Moreno, J.; Garcia-Martinez, T.; Mauricio, J.C.; Jimenez-Uceda, J.C.; Moreno-Garcia, J. Yeast cell vacuum infusion into fungal pellets as a novel cell encapsulation methodology. Appl. Microbiol. Biotechnol. 2023, 107, 5715–5726. [Google Scholar] [CrossRef] [PubMed]
- Esposto, B.S.; Jauregi, P.; Martelli-Tosi, M.; Tapia-Blacido, D.R. Liposomes vs. chitosomes: Encapsulating food bioactives. Trends Food Sci. Technol. 2021, 108, 40–48. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Hamishehkar, H.; Mohammadi, M.; Nobari-Azar, F.A.; Pezeshki, A. Nanostructured lipid carriers: Promising delivery systems for encapsulation of food ingredients. J. Agric. Food Res. 2020, 2, 100084. [Google Scholar]
- Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Encapsulation of food bioactives and nutraceuticals by various chitosan-based nanocarriers. Food Hydrocoll. 2020, 105, 105774. [Google Scholar] [CrossRef]
- Adeel, M.; Afzaal, M.; Saeed, F.; Ahmed, A.; Mahmood, K.; Abbas shah, Y.; Ateeq, H.; Sibat, A.; Khan, M.R.; Busquets, R. Encapsulation of probiotic bacteria using polyelectrolytes stabilized nanoliposomes for improved viability under hostile conditions. J. Food Sci. 2023, 88, 3839–3848. [Google Scholar] [CrossRef]
- Feng, B.; Tan, C.; Xia, S.; Xia, W.; Zhang, X. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar]
- Farias, T.G.S.; Ladislau, H.F.L.; Stamford, T.C.M.; Medeiros, J.A.C.; Soares, B.L.M.; Arnaud, T.M.S.; Stamford, T.L.M. Viabilities of Lactobacillus rhamnosus ASCC 290 and Lactobacillus casei ATCC 334 (in free form or encapsulated with calcium alginate-chitosan) in yellow mombin ice cream. LWT-Food Sci. Technol. 2019, 100, 391–396. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, L.; Li, Y.; Chen, Q.; Wang, L.; Farag, M.A.; Liu, L.; Zhan, S.; Wu, Z.; Liu, L. Soy protein isolate-citrus pectin composite hydrogels induced by TGase and ultrasonic treatment: Potential targeted delivery system for probiotics. Food Hydrocoll. 2023, 143, 108901. [Google Scholar] [CrossRef]
- Ghandi, A.; Adhikari, B.; Powell, I.B. 24—Powders containing microorganisms and enzymes. In Handbook of Food Powders; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 593–624. [Google Scholar]
- Naja, G.M.; Volesky, B. Biosorption for industrial applications. In Comprehensive Biotechnology, 2nd ed.; Pergamon Press: Oxford, UK, 2011; pp. 685–700. [Google Scholar]
- Chan, E.S.; Choo, W.S.; Pushpamalar, J.; Sultana, M. Advances in extrusion-dripping encapsulation of probiotics and omega-3 rich oils. Trends Food Sci. Technol. 2022, 123, 69–86. [Google Scholar]
- Télessy, I.G. Nutraceuticals. In The Role of Functional Food Security in Global Health; Singh, R.B., Watson, R.R., Takahashi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 409–421. ISBN 978-0-12-813148-0. [Google Scholar]
- Aguilar, C.N.; Al-Sahlany, S.T.G.; Ibrahim, S.A.; Niamah, A.K.; Patel, A.R.; Singh, S.; Thakur, M.; Utama, G.L.; Verma, D.K. Electro-hydrodynamic processing for encapsulation of probiotics: A review on recent trends, technological development, challenges and future prospect. Food Biosci. 2021, 44, 101458. [Google Scholar]
- Gu, Q.; Liu, F.; Liu, X.; McClements, D.J.; Yan, X.; Yin, Y. Encapsulation of multiple probiotics, synbiotics or nutrabiotics for improved health effects: A review. Adv. Colloid Interface Sci. 2022, 309, 102781. [Google Scholar] [CrossRef]
- Babazadeh, A.; Bahrami, A.; Jafari, S.M.; Rashidinejad, A.; Rehman, A.; Rezaei, A.; Singh, H. Co-encapsulation of probiotics with prebiotics and their application in functional/symbiotic dairy products. Food Sci. Nutr. 2022, 62, 2470–2494. [Google Scholar]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R. Double layer co-encapsulation of probiotics and prebiotics by electro-hydrodynamic atomization. LWT-Food Sci. Technol. 2019, 110, 102–109. [Google Scholar]
- PPoletto, G.; Raddatz, G.C.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; Wagner, R.; de Menezes, C.R. Study of viability and storage stability of Lactobacillus acidophillus when encapsulated with the prebiotics rice bran, inulin and hi-maize. Food Hydrocoll. 2019, 95, 238–244. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, G. Synbiotic encapsulation of probiotic Latobacillus plantarum by alginate -arabinoxylan composite microspheres. LWT-Food Sci. Technol. 2018, 93, 135–141. [Google Scholar] [CrossRef]
- Atia, A.; Gomma, A.I.; Fliss, I.; Beyssac, E.; Garrait, G.; Subirade, M. Molecular and biopharmaceutical investigation of alginate–inulin synbiotic coencapsulation of probiotic to target the colon. J. Microencapsul. 2017, 34, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, T.; Song, Y.; Shu, G.; Chen, H. Effect of xanthan-chitosan-xanthan double layer encapsulation on survival of Bifidobacterium BB01 in simulated gastrointestinal conditions, bile salt solution and yogurt. LWT-Food Sci. Technol. 2017, 81, 274–280. [Google Scholar] [CrossRef]
- Zhou, T.; Kang, W.; Han, Y.; Li, Y.; Shan, H.; Ma, W.; Yin, B.; Yang, R.; Liu, X.; Li, S.; et al. Combinedly increased viability of Lactiplantibacillus plantarum grx16 by co-encapsulation of cryoprotectants and porous starch within calcium alginate capsules. Int. J. Food Sci. Technol. 2023, 58, 5291–5298. [Google Scholar] [CrossRef]
- Sultana, M.; Chan, E.S.; Janarthanan, P.; Choo, W.S. Functional orange juice with Lactobacillus casei and tocotrienol-enriched flaxseed oil co-encapsulation: Physicochemical properties, probiotic viability, oxidative stability, and sensorial acceptability. LWT-Food Sci. Technol. 2023, 188, 115388. [Google Scholar] [CrossRef]
- Vaziri, A.S.; Alemzadeh, I.; Vossoughi, M.; Khorasani, A.C. Co-microencapsulation of Lactobacillus plantarum and DHA fatty acid in alginate-pectin-gelatin biocomposites. Carbohydr. Polym. 2018, 199, 266–275. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, S.G.; Michel, S.G.F.; Corral, R.I.A.; García-Galaz, A.; Moreno-Rivas, S.C.; Vázquez-Moreno, L.; Montfort, G.R.C. Caracterizacion del co-encapsulamiento de Lactobacillus plantarum y ácidos grasos omega-3 en una matriz de alginato-pectina. Biotecnia 2019, 21, 38–46. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Ribeiro, A.M.; Rocha, F. Improvement of vitamin E microencapsulation and release using different biopolymers as encapsulating agents. Food Bioprod. Process. 2021, 130, 23–33. [Google Scholar]
- Jafari, S.M.; McClements, D.J. Nanotechnology approaches for increasing nutrient bioavailability. In Advances in Food and Nutrition Research, 1st ed.; Toldrá, F., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 1–30. [Google Scholar]
- Flores, F.; Kong, F. In vitro release kinetics of microencapsulated materials and the effect of the food matrix. Ann. Rev. Food Sci. Technol. 2017, 8, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Perrie, Y.; Rades, T. Pharmaceutics—Drug Delivery and Targeting, 2nd ed.; Pharmaceutical Press: London, UK, 2009; pp. 1–240. [Google Scholar]
- Boostani, S.; Jafari, S.M. A comprehensive review on the controlled release of encapsulated food ingredients: Fundamental concepts to design and applications. Trends Food Sci. Technol. 2021, 109, 303–321. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Xue, C.; Wei, Z. Latest developments in food-grade delivery systems for probiotics: A systematic review. Crit. Rev. Food Sci. Nutr. 2023, 63, 4371–4388. [Google Scholar] [CrossRef]
- Boostani, S.; Jafari, S.M. Controlled release of nanoencapsulated food ingredients. In Release and Bioavailability of Nanoencapsulated Food Ingredients; Jafari, S.M., Ed.; Academic Press: Boston, MA, USA, 2020; pp. 27–78. [Google Scholar]
- McClements, D.J. Nanoparticle- and Microparticle-Based Delivery Systems: Encapsulation, Protection and Release of Active Components; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Sultana, A.; Tanaka, Y.; Fushimi, Y.; Yoshii, H. Stability and release behavior of encapsulated flavor from spray-dried Saccharomyces cerevisiae and maltodextrin powder. Food Res. Int. 2018, 106, 809–816. [Google Scholar] [CrossRef]
- Pothakamury, U.R.; Barbosa-Canovas, G.V. Fundamental aspects of controlled release in foods. Trends Food Sci. Technol. 1995, 6, 397–406. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly (lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Jafari, S.M.; Katouzian, I.; Rajabi, H.; Ganje, M. Bioavailability and release of bioactive components from nanocapsules. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Jafari, S.M., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 494–523. [Google Scholar]
- Hoseyni, S.Z.; Jafari, S.M.; Tabarestani, H.S.; Ghorbani, M.; Assadpour, E.; Sabaghi, M. Production and characterization of catechin-loaded electrospun nanofibers from Azivash gum-polyvinyl alcohol. Carbohydr. Polym. 2020, 235, 115979. [Google Scholar] [CrossRef]
- Mokhtari, S.; Khomeiri, M.; Jafari, S.M.; Maghsoudlou, Y.; Ghorbani, M. Descriptive analysis of bacterial profile, physicochemical and sensory characteristics of grape juice containing Saccharomyces cerevisiae cell wall-coated probiotic microcapsules during storage. Int. J. Food Sci. Technol. 2017, 52, 1042–1048. [Google Scholar] [CrossRef]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M.; Maghsoudlou, Y.; Ghorbani, M. The cell wall compound of Saccharomyces cerevisiae as a novel wall material for encapsulation of probiotics. Food Res. Int. 2017, 96, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Rostami, M.; Yousefi, M.; Khezerlou, A.; Mohammadi, M.A.; Jafari, S.M. Application of different biopolymers for nanoencapsulation of antioxidants via electrohydrodynamic processes. Food Hydrocoll. 2019, 97, 105170. [Google Scholar] [CrossRef]
- Basu, S.; Banerjee, D.; Chowdhury, R.; Bhattacharya, P. Controlled release of microencapsulated probiotics in food matrix. J. Food Eng. 2018, 238, 61–69. [Google Scholar] [CrossRef]
- Banerjee, D.; Chowdhury, R.; Bhattacharya, P. In-vitro evaluation of targeted release of probiotic Lactobacillus casei (2651 1951 RPK) from synbiotic microcapsules in the gastrointestinal (GI) system: Experiments and modeling. LWT-Food Sci. Technol. 2017, 83, 243–253. [Google Scholar] [CrossRef]
- Luo, Y.; De Souza, C.; Ramachandran, M.; Wang, S.; Yi, H.; Ma, Z.; Zhang, L.; Lin, K. Precise oral delivery systems for probiotics: A review. J. Control. Release 2022, 352, 371–384. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Dalbhagat, C.G.; Mishra, H.N. Emerging Technologies and Coating Materials for Improved Probiotication in Food Products: A Review. Food Bioprocess Technol. 2022, 15, 998–1039. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, T.; Li, C.; Zheng, S.; Li, H.; Yu, J. Development of a microencapsulated synbiotic product and its application in yoghurt. LWT-Food Sci. Technol. 2020, 122, 109033. [Google Scholar] [CrossRef]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Crit. Rev. Food Sci. Nutr. 2021, 61, 1515–1536. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Elvan, M.; Ozer, M.; Harsa, S. Effect of different microencapsulating materials on the viability of S. thermophiles CCM4757 incorporated into dark and milk chocolates. Food Biosci. 2021, 44, 101413. [Google Scholar] [CrossRef]
| Encapsulation Process | Probiotics | Wall Material(s) | Effect | Reference |
|---|---|---|---|---|
| Νanocoating | Lactobacillus acidophilus | Artificial nanoshells | Enhanced viability of nanocoating L. acidophilus in simulated gastric fluid (SGF) by 49% compared with free probiotics. | [82] |
| Electrospinning | Probiotic strains of LAB and Bifidobacteria | Corn starch (CS) and sodium alginate (SA) nanofiber | After a 20-day storage of yogurt, the count of nanoencapsulated LAB and bifidobacteria declined by 0.19 (97.9% survival) and 0.28 (96.9% survival) log CFU, respectively. | [83] |
| Electrospinning | Probiotic strains | Starch/sodium alginate | Enhanced viability rate of lactobacilli and bifidobacteria strains in the acidic environment and simulated gastrointestinal conditions. | [84] |
| Layer-By-Layer Coating | Lactobacillus plantarum 90 (LP90) | Whey protein isolate fibrils (WPIFs), sodium alginate (ALG), carboxymethyl cellulose (CMC), and xanthan gum (XG) | Enhanced survival of Lactobacillus plantarum 90 (LP90). | [85] |
| Advantages | Disadvantages | References |
|---|---|---|
| Spray drying | ||
| Short drying time; Low cost; Flexibility; High productivity process; Large-scale production; High speed. | Decreased number of viable probiotic organisms; Wall material must be soluble in water; Particles with irregular geometry and porous surface; Process losses of heat-sensitive substances have not been fully addressed. | [2,24,25,28,36,59,88] |
| Spray cooling | ||
| Low-cost, fast process; Dense, spherical, and smooth particle surface; It can be applied on an industrial scale. | Low performance; Not suitable for heat-sensitive bioactives; Possibility of loss of bioactives. | [4,36] |
| Freeze drying or lyophilization | ||
| Mild conditions; Suitable for heat-sensitive compounds; Almost unchanged nutritional value; Rapid comprehensive dehydration. | Decreased number of viable probiotic organisms; High cost of installation and operation of the equipment; Unsuccessful on foods that are difficult to dehydrate; Risk of cell damage. | [41,88] |
| Fluidized bed coating | ||
| Suitable for thermally sensitive components; Mass production of biospheres. | Complicated process; Direct exposure to high temperature can cause molecules to degrade; High cost. | [24,25,45] |
| Extrusion | ||
| Simple, low-cost technology; Mild conditions; Ideal for thermosensitive bioactives. | Reduced viability after the extrusion process; Formation of large particles; Slow production rate; Not applicable on an industrial scale; The use of hydrocolloid solution is necessary. | [25,26,36] |
| Ionic gelation | ||
| Simple and economical method; No special equipment or organic solvents are required; Mild conditions. | It is not easy to produce particles of uniform size; | [4,7,58,60] |
| Emulsification | ||
| Easy to scale up; Simple and flexible technique; High sustainability of bioactives, e.g., probiotics. | Not suitable for mass production; Non-uniform particle shape and size. | [16,25,59,88] |
| Encapsulation in yeasts | ||
| Simple and low-cost process; High encapsulation efficiency; Good mechanical strength and easy cultivation on an industrial scale; No additional materials are used. | Mainly used for the encapsulation of lipophilic molecules. | [37] |
| Liposomes | ||
| Encapsulation of hydrophilic and hydrophobic molecules; Production of uniform pellets on an industrial scale; No use of organic solvents. | Thermodynamically unstable method; The structure of the liposomes can be disrupted and the encapsulated substances can be released. | [111,112] |
| Inclusion complexation | ||
| Economical to produce it on a large scale (β-cyclodextrin); Also suitable for volatile compounds; It allows the controlled release of bioactives. | High doses of cyclodextrin may be harmful. | [4,37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agriopoulou, S.; Tarapoulouzi, M.; Varzakas, T.; Jafari, S.M. Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation. Microorganisms 2023, 11, 2896. https://doi.org/10.3390/microorganisms11122896
Agriopoulou S, Tarapoulouzi M, Varzakas T, Jafari SM. Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation. Microorganisms. 2023; 11(12):2896. https://doi.org/10.3390/microorganisms11122896
Chicago/Turabian StyleAgriopoulou, Sofia, Maria Tarapoulouzi, Theodoros Varzakas, and Seid Mahdi Jafari. 2023. "Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation" Microorganisms 11, no. 12: 2896. https://doi.org/10.3390/microorganisms11122896
APA StyleAgriopoulou, S., Tarapoulouzi, M., Varzakas, T., & Jafari, S. M. (2023). Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation. Microorganisms, 11(12), 2896. https://doi.org/10.3390/microorganisms11122896









