Synergistic Antibacterial Efficacy of Melittin in Combination with Oxacillin against Methicillin-Resistant Staphylococcus aureus (MRSA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates and Chemicals
2.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.3. Bacterial Growth Curve Assay and Time-Kill Assay
2.4. Effects on Biofilm Formation
2.5. Leakage of Proteins and Nucleic Acids
2.6. Efflux of Potassium and Phosphate Ions
2.7. Hemolytic Activity
2.8. Cytotoxicity in Keratinocytes by Flow Cytometry
2.9. Statistical Analysis
2.10. Protein Extraction and Quantification
2.11. Enzymatic Digestion of Proteins
2.12. Mass Spectrometry
2.13. Analysis of Mass Spectrometry Data
3. Results
3.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
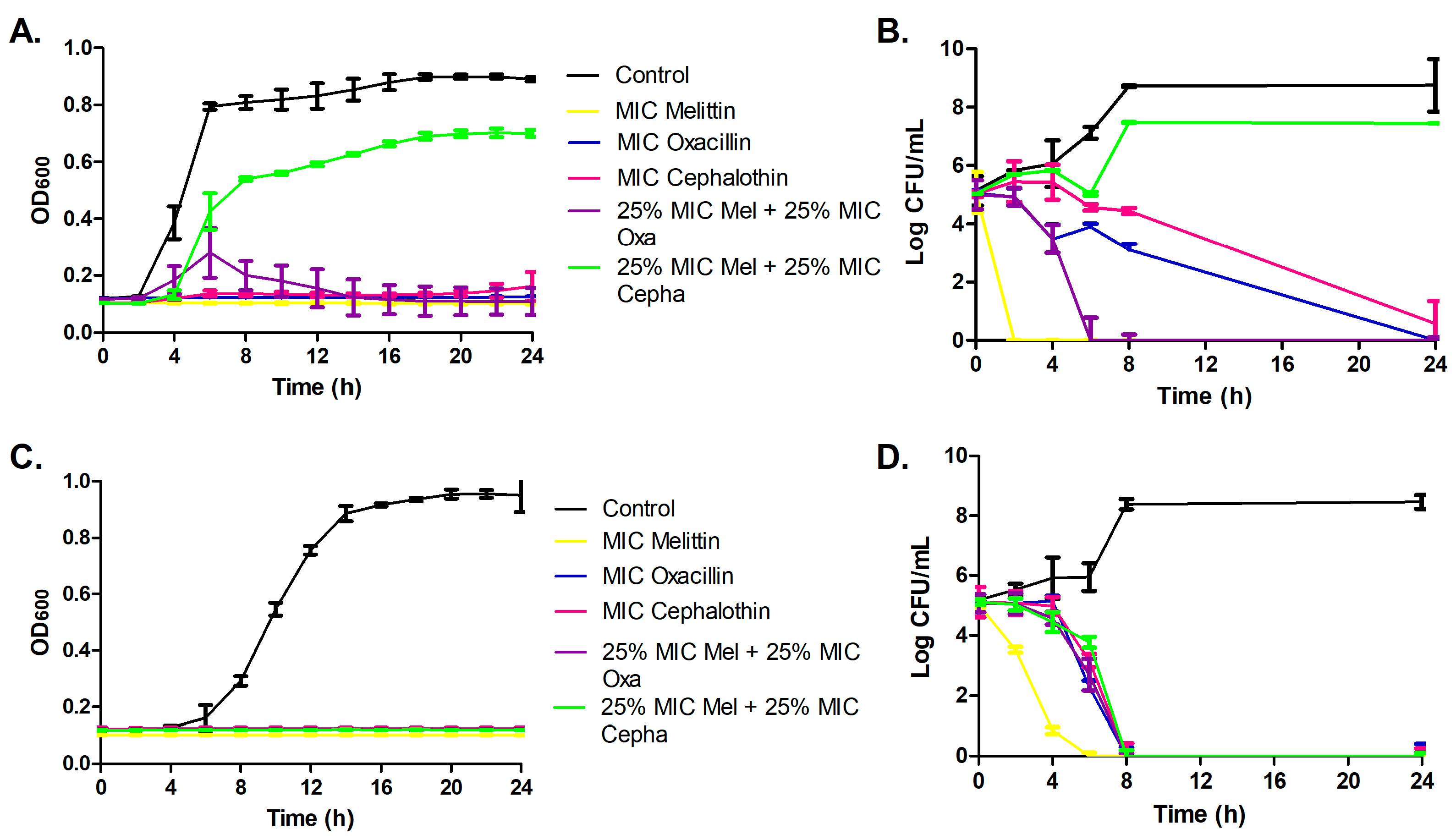
3.2. Bacterial Growth Curve Assay and Time-Kill Assay
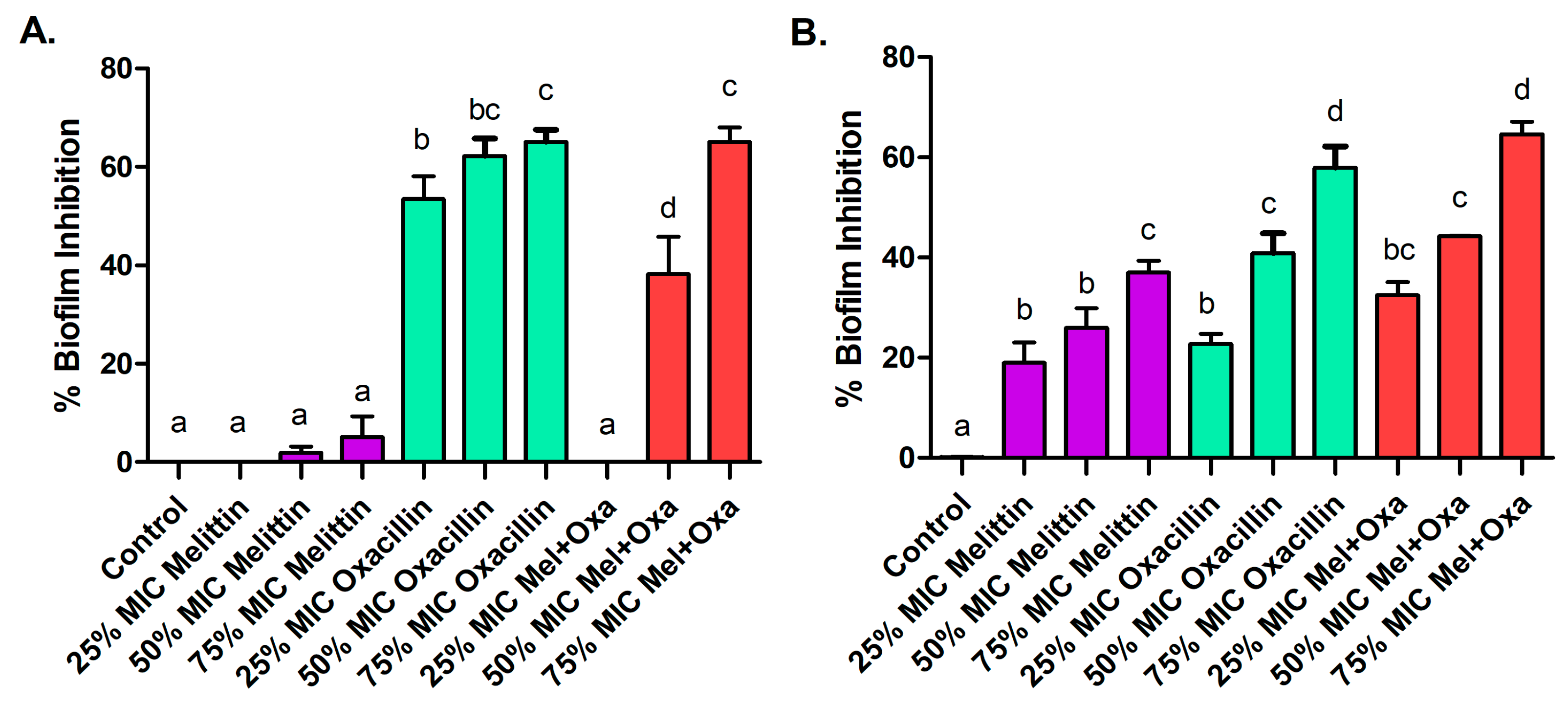
3.3. Effects on Biofilm Formation
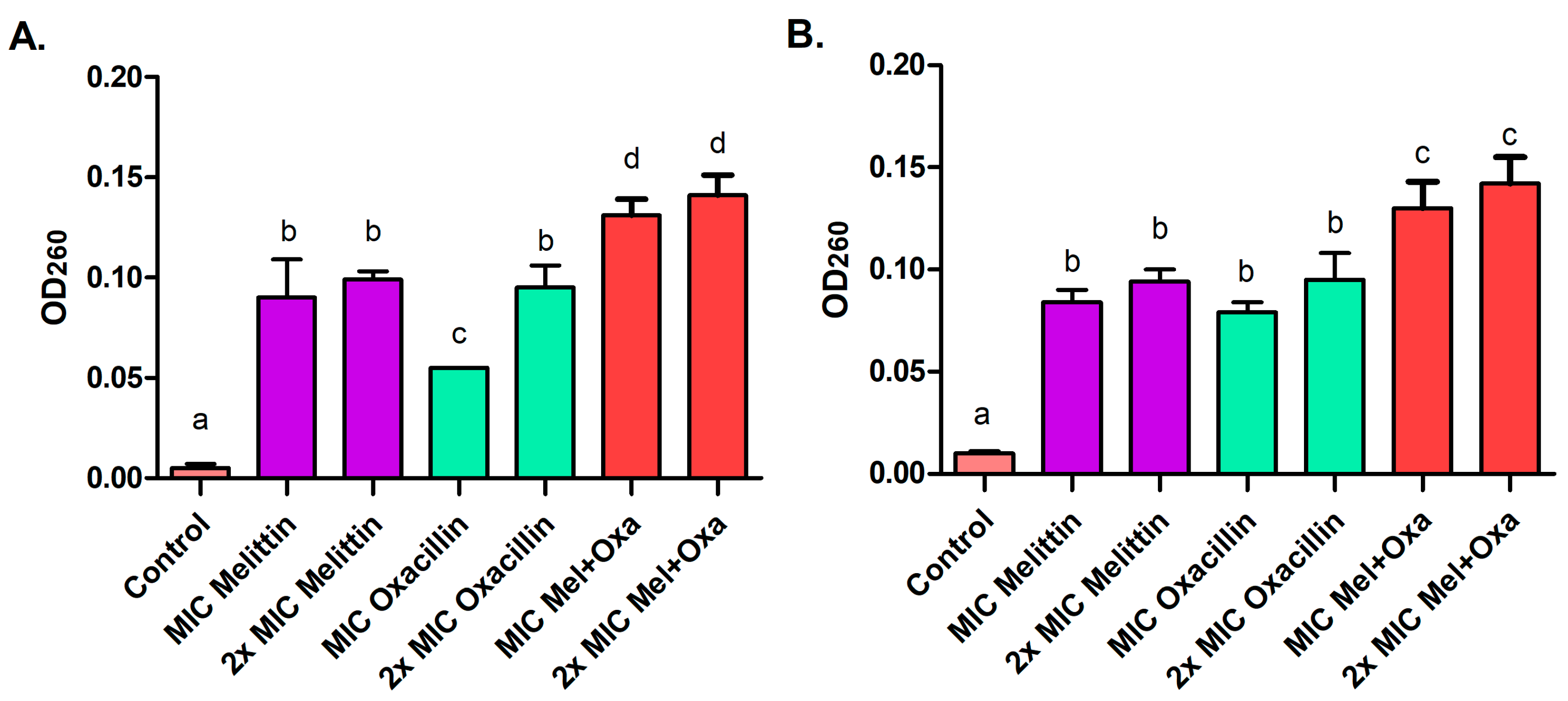
3.4. Leakage of Proteins and Nucleic Acids
3.5. Efflux of Potassium and Phosphate Ions
3.6. Hemolytic Activity
3.7. Cytotoxicity in Keratinocytes by Flow Cytometry
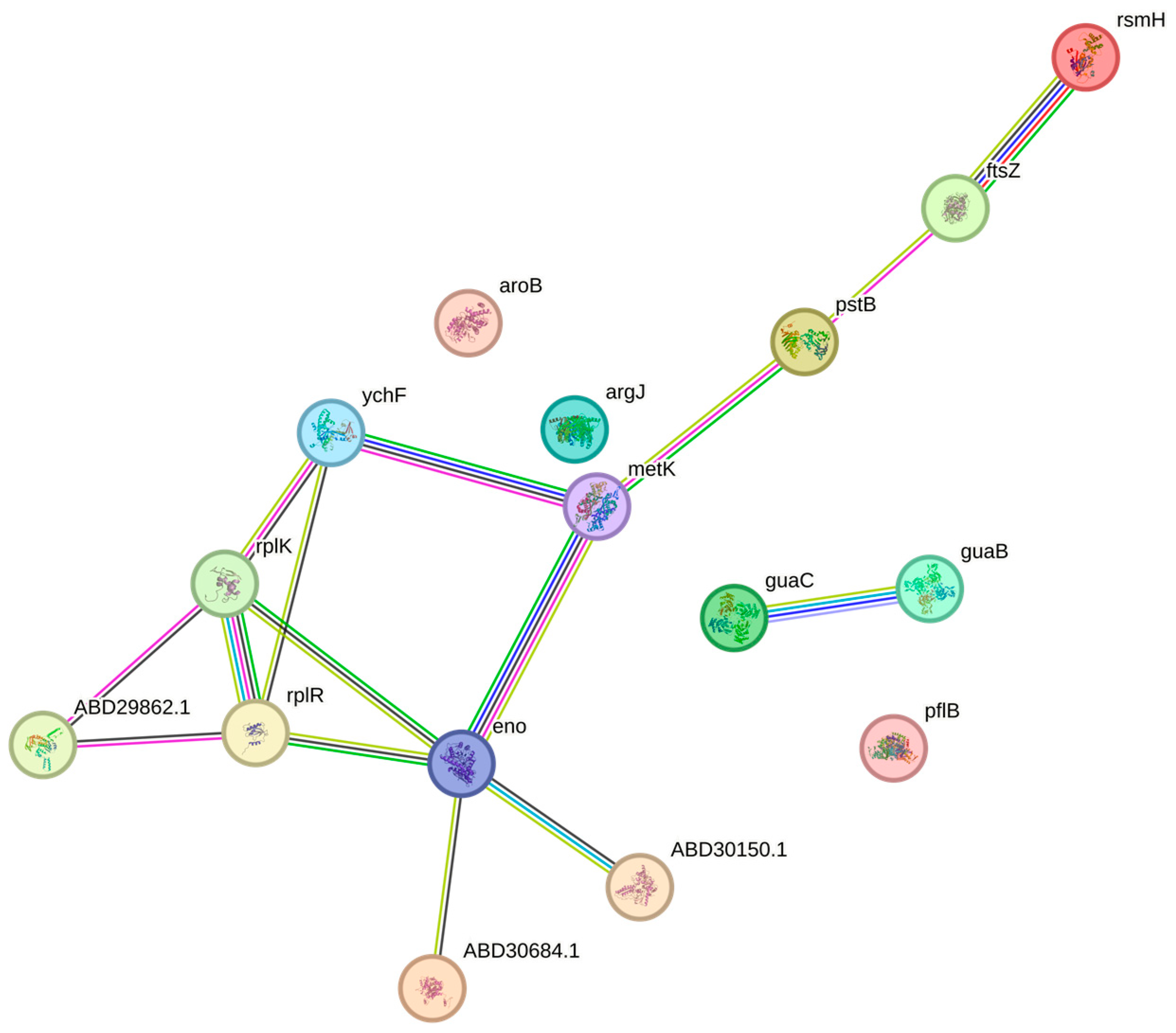
3.8. Proteomic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pecoraro, C.; Carbone, D.; Parrino, B.; Cascioferro, S.; Diana, P. Recent Developments in the Inhibition of Bacterial Adhesion as Promising Anti-Virulence Strategy. Int. J. Mol. Sci. 2023, 24, 4872. [Google Scholar] [CrossRef] [PubMed]
- Mestrovic, T.; Aguilar, G.R.; Swetschinski, L.R.; Ikuta, K.S.; Gray, A.P.; Weaver, N.D.; Han, C.; Wool, E.E.; Hayoon, A.G.; Hay, S.I.; et al. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: A cross-country systematic analysis. Lancet Public Health 2022, 7, e897–e913. [Google Scholar] [CrossRef] [PubMed]
- Parastan, R.; Kargar, M.; Solhjoo, K.; Kafilzadeh, F. Staphylococcus aureus biofilms: Structures, antibiotic resistance, inhibition, and vacines. Gene Rep. 2020, 20, 100739. [Google Scholar] [CrossRef]
- Lai, C.K.C.; Ng, R.W.Y.; Leung, S.S.Y.; Hui, M.; Ip, M. Overcoming the rising incidence and evolving mechanisms of antibiotic resistance by novel drug delivery approaches—An overview. Adv. Drug Deliv. Rev. 2022, 181, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, C.; Carbone, D.; Deng, D.; Cascioferro, S.M.; Diana, P.; Giovannetti, E. Biofilm Formation as Valuable Target to Fight against Severe Chronic Infections. Curr. Med. Chem. 2022, 25, 4307–4310. [Google Scholar] [CrossRef] [PubMed]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef]
- Paray, B.A.; Ahmad, A.; Khan, J.M.; Taufiq, F.; Pathan, A.; Malik, A.; Ahmed, M.Z. The role of the multifunctional antimicrobial peptide melittin in gene delivery. Drug Discov. Today 2021, 26, 1053–1059. [Google Scholar] [CrossRef]
- Lima, W.G.; Batista Filho, F.L.; Lima, I.P.; Simião, D.C.; Brito, J.C.M.; da Cruz Nizer, W.S.; Cardoso, V.N.; Fernandes, S.O.A. Antibacterial, anti-biofilm, and anti-adhesive activities of melittin, a honeybee venom-derived peptide, against quinolone-resistant uropathogenic Escherichia coli (UPEC). Nat. Prod. Res. 2022, 24, 6381–6388. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J.; Hassan, K.T.S. Cucurbitacin B interacts synergistically with antibiotics against Staphylococcus aureus clinical isolates and exhibits antiviral activity against HSV-1. S. Afr. J. Bot. 2017, 108, 90–94. [Google Scholar] [CrossRef]
- Mirzaei, R.; Gouvarchin, E.; Ghaleh, H.; Ranjbar, R. Antibiofilm effect of melittin alone and in combination with conventional antibiotics toward strong biofilm of MDR-MRSA and -Pseudomonas aeruginosa. Front. Microbiol. 2023, 14, 1030401. [Google Scholar] [CrossRef]
- Li, W.; O’Brien-Simpson, N.M.; Holden, J.A.; Otvos, L.; Reynolds, E.C.; Separovic, F.; Hossain, M.A.; Wade, J.D. Covalent conjugation of cationic antimicrobial peptides with a beta-lactam antibiotic core. Pept. Sci. 2018, 110, e24059. [Google Scholar] [CrossRef]
- Desgranges, S.; Ruddle, C.C.; Burke, L.P.; McFadden, T.M.; Doherty, T.; O’Brien, J.E.; Fitzgerald-Hughes, D.; Humphreys, H.; Smythe, T.P.; Devocelle, M. β-Lactam-host defence peptide conjugates as antibioticprodrug candidates targeting resistant bacteria. RSC Adv. 2012, 2, 2480–2492. [Google Scholar] [CrossRef]
- Pereira, A.F.M.; Albano, M.; Alves, F.C.B.; Andrade, B.F.M.T.; Furlanetto, A.; Rall, V.L.M.; dos Santos, L.D.; Orsi, R.O.; Fernandes Júnior, A. Influence of apitoxin and melittin from Apis mellifera bee on Staphylococcus aureus strains. Microb. Pathog. 2020, 141, 104011. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, F.; Bevalian, P.; Akbari, R.; Bagheri, K.P. Single dose eradication of extensively drug resistant Acinetobacter spp. in a mouse model of burn infection by melittin antimicrobial peptide. Microb. Pathog. 2019, 127, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. J. Pathog. Microbiol. Immunol. 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Han, J.-H.; Rather, I.A.; Park, C.; Lim, J.; Paek, W.K.; Lee, J.S.; Yoo, J.-I.; Park, Y.-H. Characterization and antibacterial potential of lactic acid bacterium Pediococcus pentosaceus 4I1 isolated from freshwater fish Zacco koreanus. Front. Microbiol. 2016, 7, 2037. [Google Scholar] [CrossRef]
- Zarrinnahad, H.; Mahmoodzadeh, A.; Hamidi, M.P.; Mahdavi, M.; Moradi, A.; Bagheri, K.P.; Shahbazzadeh, D. Apoptotic effect of melittin purified from iranian honey bee venom on human cervical cancer HeLa cell line. Int. J. Pept. Res. Ther. 2017, 23, 563–570. [Google Scholar] [CrossRef]
- Cavalcante, J.S.; Almeida, C.A.S.; Clasen, M.A.; Silva, E.L.; Barros, L.C.; Marinho, A.D.; Rossini, B.C.; Marino, C.L.; Carvalho, P.C.; Jorge, R.J.B.; et al. A fingerprint of plasma proteome alteration after local tissue damage induced by Bothrops Leucurus snake venom in mice. J. Proteom. 2022, 253, 104464. [Google Scholar] [CrossRef]
- Calzetta, L.; Koziol-White, C. Pharmacological interactions: Synergism, or not synergism, that is the question. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100046. [Google Scholar] [CrossRef]
- Bevalian, P.; Pashaie, F.; Akbari, R.; Bagheri, K.P. Eradication of vancomycin-resistant Staphylococcus aureus on a mouse model of third-degree burn infection by melittin: An antimicrobial peptide from bee venom. Toxicon 2021, 199, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Akbari, R.; Hakemi-Vala, M.; Pashaie, F.; Bevalian, P.; Hashemi, A.; Bagheri, K. Highly synergistic effects of melittin with conventional antibiotics against multidrug-resistant isolates of Acinetobacter baumannii and Pseudomonas aeruginosa. Microb. Drug Resist. 2019, 25, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Maisetta, G.; Esin, S.; Batoni, G. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front. Microbiol. 2017, 8, 2409. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Alikhani, M.Y.; Arciola, C.R.; Sedighi, I.; Yousefimashouf, R.; Bagheri, K.P. Prevention, inhibition, and degradation effects of melittin alone and in combination with vancomycin and rifampin against strong biofilm producer strains of methicillin-resistant Staphylococcus epidermidis. Biomed. Pharmacother. 2022, 142, 112670. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Ghimire, J.; Wu, E.; Wimley, W.C. Mechanistic landscape of membrane-permeabilizing peptides. Chem. Rev. 2019, 119, 6040–6085. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Alikhani, M.Y.; Arciola, C.R.; Sedighi, I.; Irajian, G.; Jamasbi, E.; Yousefimashouf, R.; Bagheri, K.P. Highly synergistic effects of melittin with vancomycin and rifampin against vancomycin and rifampin resistant Staphylococcus epidermidis. Front. Microbiol. 2022, 13, 869650. [Google Scholar] [CrossRef]
- Wilson, D.N.; Hauryliuk, V.; Atkinson, G.C.; O’Neill, A.J. Target protection as a key antibiotic resistance mechanism. Nat. Rev. Microbiol. 2020, 18, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Bennison, D.J.; Nakamoto, J.A.; Craggs, T.D.; Milón, P.; Rafferty, J.B.; Corrigan, R.M. The Stringent Response Inhibits 70S Ribosome Formation in Staphylococcus aureus by Impeding GTPase-Ribosome Interactions. mBio 2021, 12, e0267921. [Google Scholar] [CrossRef]
- Siddiqui, Z.I. Ribosome biogenesis in prokaryotes. In Emerging Concepts in Ribosome Structure, Biogenesis, and Function; Academic Press: Cambridge, MA, USA, 2021; pp. 151–181. [Google Scholar] [CrossRef]
- Riganti, C.; Gazzano, E.; Polimeni, M.; Aldieri, E.; Ghigo, D. The pentose phosphate pathway: An antioxidant defense and a crossroad in tumor cell fate. Free Radic. Biol. Med. 2012, 53, 421–436. [Google Scholar] [CrossRef]
- Vanlis, R.; Popek, M.; Couté, Y.; Kosta, A.; Drapier, D.; Nitschke, W.; Atteia, A. Concerted up-regulation of aldehyde/alcohol dehydrogenase (adhe) and starch in Chlamydomonas reinhardtii increases survival under dark anoxia. J. Biol. Chem. 2017, 292, 2395–2410. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.; Lin, S.; Wu, M.; Han, L.; Tian, C.; Zhang, X.; Zang, J. Octameric structure of Staphylococcus aureus enolase in complex with phosphoenolpyruvate. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 2457–2470. [Google Scholar] [CrossRef] [PubMed]
- Balemans, W.; Vranckx, L.; Lounis, N.; Pop, O.; Guillemont, J.; Vergauwen, K.; Mol, S.; Gilissen, R.; Motte, M.; Lançois, D.; et al. Novel antibiotics targeting respiratory ATP synthesis in gram-positive pathogenic bacteria. Antimicrob. Agents Chemother. 2012, 56, 4131–4139. [Google Scholar] [CrossRef] [PubMed]
- Vasu, D.; Sunitha, M.M.; Sreekanth, L.; Swarupa, V.; Prasad, U.V.; Sireesha, K.; Yeswanth, S.; Kumar, P.S.; Venkatesh, K.; Chaudhary, A.; et al. In Staphylococcus aureus the regulation of pyruvate kinase activity by serine/threonine protein kinase favors biofilm formation. 3 Biotech 2015, 5, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, H.; Nakanishi, Y.; Kuno, S.; Ota, T.; Mochidome, K.; Saito, Y.; Sugihara, F.; Takakusagi, Y.; Aoki, I.; Nagatoishi, S.; et al. Design strategy for serine hydroxymethyltransferase probes based on retro-aldol-type reaction. Nat. Commun. 2019, 10, 876. [Google Scholar] [CrossRef] [PubMed]
- Fourie, K.R.; Wilson, H.L. Understanding GroEL and DnaK stress response proteins as antigens for bacterial diseases. Vaccines 2020, 8, 773. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Margolin, W.; Beuria, T.K. Targeting the achilles heel of FtsZ: The interdomain cleft. Front. Microbiol. 2021, 12, 647955. [Google Scholar] [CrossRef]
- Ferrer-González, E.; Kaul, M.; Parhi, A.K.; Lavoie, E.J.; Pilch, D.S. β-Lactam antibiotics with a high affinity for PBP2 act synergistically with the FtsZ-targeting agent TXA707 against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2017, 61, e00863-17. [Google Scholar] [CrossRef]
- Leibig, M.; Liebeke, M.; Mader, D.; Lalk, M.; Peschel, A.; Götz, F. Pyruvate formate lyase acts as a formate supplier for metabolic processes during anaerobiosis in Staphylococcus aureus. J. Bacteriol. 2011, 193, 952–962. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon metabolism in health and disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Landwehr, V.; Milanov, M.; Hong, J.; Koch, H.G. The Role of the Universally conserved ATPase YchF/Ola1 in translation regulation during cellular stress. Microorganisms 2021, 10, 14. [Google Scholar] [CrossRef]
- Swinehart, W.E.; Jackman, J.E. Diversity in mechanism and function of tRNA methyltransferases. RNA Biol. 2015, 12, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G.; Aharoni, A. Shikimate pathway and aromatic amino acid biosynthesis. eLS 2012, 8, 32. [Google Scholar] [CrossRef]
- Wu, H.; Gong, Y.; Ji, P.; Xie, Y.; Jiang, Y.; Liu, G.-Y. Targeting nucleotide metabolism: A promising approach to enhance cancer immunotherapy. J. Hematol. Oncol. 2022, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.L.; Dassa, E.; Orelle, C.; Chen, J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 2008, 72, 317–364. [Google Scholar] [CrossRef] [PubMed]
- Eitinger, T.; Rodionov, D.A.; Grote, M.; Schneider, E. Canonical and ECF-Type ATP-Binding cassette importers in prokaryotes: Diversity in modular organization and cellular functions. FEMS Microbiol. Rev. 2011, 35, 3–67. [Google Scholar] [CrossRef]
- Alves, F.C.B.; Albano, M.; Andrade, B.F.M.T.; Chechi, J.L.; Pereira, A.F.M.; Furlanetto, A.; Rall, V.L.M.; Fernandes, A.A.H.; dos Santos, L.D.; Barbosa, L.N.; et al. Comparative proteomics of methicillin-resistant Staphylococcus aureus subjected to synergistic effects of the lantibiotic nisin and oxacillin. Microb. Drug Resist. 2020, 26, 179–189. [Google Scholar] [CrossRef]




| MRSA | Melittin | Oxacillin | Cephalothin | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| ATCC | 5.3 | 8.0 | 16.0 | 16.0 | 16.0 | 32.0 |
| Isolate | 4.0 | 8.0 | 8.0 | 16.0 | 2.0 | 2.0 |
| Potassium Ions | Phosphate Ions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0h | T2h | T4h | T0h | T2h | T4h | |||||||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| MIC | 1× | 2× | 1× | 2× | 1× | 2× | 1× | 2× | 1× | 2× | 1× | 2× |
| Melittin | 0 | 0 | 0/250 * | 250 | 250 | 250 | 0 | 0 | 10 | 10 | 10 | 10 |
| Oxacillin | 0 | 0 | 0 | 250 | 250 | 250 | 0 | 0 | 0 | 10 | 10 | 10 |
| Mel+Oxa | 0 | 0 | 0 | 250 | 250 | 250 | 0 | 0 | 0 | 10 | 10 | 10 |
| 4× MIC | 2× MIC | MIC | 50% MIC | 25% MIC | |
|---|---|---|---|---|---|
| Melittin | 100 ± 0.9 aA | 4.2 ± 7.2 bA | 0.2 ± 0.2 cA | 0.2 ± 0.4 cA | 0.2 ± 0.5 cA |
| Oxacillin | 0 ± 0.8 aB | 0 ± 0.5 aB | 0 ± 0.2 aA | 0 ± 0.0 aA | 0 ± 0.0 aA |
| Mel+Oxa | 100 ± 3.8 aA | 0 ± 0.2 bB | 0 ± 0.5 bA | 0.0 ± 0.1 aA | 0.0 ± 0.0 aA |
| Treatments | UL (Late Apoptotis/Necrosis) % | UR (Late Apoptosis/ Necrosis) % | LL (Live Cells) % | LR (Early Apoptosis) % |
|---|---|---|---|---|
| Basal negative control (autofluorescence) | 0.00 | 0.01 | 99.97 | 0.02 |
| Basal negative control | 0.50 | 5.40 | 87.99 | 6.11 |
| MIC Melittin | 0.77 | 1.64 | 96.52 | 1.06 |
| 2× MIC Melittin | 0.78 | 3.94 | 93.62 | 1.66 |
| MIC Oxacillin | 0.89 | 3.95 | 90.88 | 4.29 |
| 2× MIC Oxacillin | 0.55 | 5.94 | 89.42 | 4.10 |
| MIC Mel+Oxa | 0.21 | 3.53 | 92.22 | 4.04 |
| 2× MIC Mel+Oxa | 0.36 | 2.54 | 95.65 | 1.44 |
| Proteins | Molecular Function | Biological Process | Cellular Component | Protein Expression | |||
|---|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | ||||
| 30S ribosomal protein S2 * | Structural component; Binding—nucleic acid | Translation | Ribosome | ✓ | down | ||
| 30S ribosomal protein S3 * | Structural component; Binding—nucleic acid | Translation | Ribosome | ✓ | down | ||
| 30S ribosomal protein S19 * | Structural component; Binding—nucleic acid | Translation | Ribosome | ✓ | up | up | |
| 50S ribosomal protein L9 * | Structural component; Binding—nucleic acid | Translation | Ribosome | down | down | ||
| 50S ribosomal protein L10 | Structural component; Binding—nucleic acid | Translation | Ribosome | up | |||
| 50S ribosomal protein L11 * | Structural component; Binding—nucleic acid | Translation | Ribosome | ✓ | down | down | |
| 50S ribosomal protein L18 | Structural component; Binding—nucleic acid | Translation | Cytoplasm; Ribosome | up | |||
| 6-phospho-beta-galactosidase * | Glycosidase; Hydrolase | Lactose catabolic process via | – | ✓ | down | ||
| 6-phosphogluconate dehydrogenase, decarboxylating * | Binding; Oxidoreductase | Gluconate utilization; Pentose phosphate pathway | – | ✓ | up | up | |
| ABC transporter, ATP-binding protein | Binding—ATP; Hydrolase; Transport | Carbohydrate transport | – | up | |||
| Aldehyde-alcohol dehydrogenase * | Binding; Oxidoreductase | Metabolic process of alcohol; Carbon utilization | – | ✓ | up | up | |
| Aspartate–tRNA ligase | Binding—nucleic acid and ATP; Ligase activity | Protein biosynthesis | Cytoplasm | ✓ | up | ||
| ATP synthase subunit beta | ATP-dependent; Ligase activity; Transport | ATP synthesis; Transport | Cell membrane | ✓ | down | ||
| Cell division protein FtsZ | Binding; Hydrolase | Cell division | Cytoplasm; Cell division site | down | |||
| Chaperone protein DnaK | ATP-dependent; Binding; Chaperone; Hydrolase | Stress response | – | ✓ | down | ||
| Cold shock protein CspA | Structural component; Binding—nucleic acid | Stress response | Cytoplasm | up | |||
| DAHP synthetase-chorismate mutase | Lyase activity | Amino acid biosynthesis aromatics; Chorismate metabolic process | – | up | |||
| Elongation factor G | Translation factor; Hydrolase | Protein biosynthesis | Cytoplasm | ✓ | down | ||
| Enolase * | Binding; Lyase activity | Glycolysis | Extracellular region; Cell surface | up | up | up | |
| Extracellular matrix-binding protein ebh | Structural component | – | Cell membrane | ✓ | down | ||
| Formate acetyltransferase | Transferase activity | Glucose metabolic process | Cytoplasm | up | |||
| Glyceraldehyde-3-phosphate dehydrogenase | Binding; Oxidoreductase | Glucose metabolic process | – | ✓ | up | ||
| GMP reductase | Oxidoreductase | Nucleotide metabolic process | Macromolecular complex | up | |||
| Inosine-5’-monophosphate dehydrogenase *# | Binding; Oxidoreductase | GMP biosynthesis | – | ✓ | down | ||
| N-acetyltransferase * | Transferase activity | – | – | ✓ | down | down | down |
| Phosphoenolpyruvate-protein phosphotransferase | Transferase activity | Transport | Cytoplasm | ✓ | down | ||
| PstB | ATP-dependent; Binding—ATP | Phosphate transport | Cell membrane | down | |||
| Piruvate kinase | Binding—ATP; Transferase activity | Glycolysis | – | down | |||
| Ribosomal RNA small subunit methyltransferase H | Binding; Transferase activity | rRNA processing | – | down | |||
| Ribosome-binding ATPase YchF | ATP-dependent; Binding—ATP and ions; Hydrolase | – | – | up | |||
| S-adenosylmethionine synthase | Binding—ATP; Transferase activity | One-carbon metabolism | Cytoplasm | ✓ | down | up | |
| Serine hydroxymethyltransferase * | Binding; Transferase activity | One-carbon metabolism; Amino acid biosynthesis | Cytoplasm | ✓ | down | down | |
| Thymidylate synthase * | Transferase activity | Nucleotide biosynthesis | Cytoplasm | up | |||
| Uncharacterized protein | – | – | – | up | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.F.M.; Sani, A.A.; Zapata, T.B.; Sousa, D.S.M.d.; Rossini, B.C.; Santos, L.D.d.; Rall, V.L.M.; Riccardi, C.d.S.; Fernandes Júnior, A. Synergistic Antibacterial Efficacy of Melittin in Combination with Oxacillin against Methicillin-Resistant Staphylococcus aureus (MRSA). Microorganisms 2023, 11, 2868. https://doi.org/10.3390/microorganisms11122868
Pereira AFM, Sani AA, Zapata TB, Sousa DSMd, Rossini BC, Santos LDd, Rall VLM, Riccardi CdS, Fernandes Júnior A. Synergistic Antibacterial Efficacy of Melittin in Combination with Oxacillin against Methicillin-Resistant Staphylococcus aureus (MRSA). Microorganisms. 2023; 11(12):2868. https://doi.org/10.3390/microorganisms11122868
Chicago/Turabian StylePereira, Ana Flávia Marques, Alessandra Aguirra Sani, Tatiane Baptista Zapata, Débora Silva Marques de Sousa, Bruno César Rossini, Lucilene Delazari dos Santos, Vera Lúcia Mores Rall, Carla dos Santos Riccardi, and Ary Fernandes Júnior. 2023. "Synergistic Antibacterial Efficacy of Melittin in Combination with Oxacillin against Methicillin-Resistant Staphylococcus aureus (MRSA)" Microorganisms 11, no. 12: 2868. https://doi.org/10.3390/microorganisms11122868
APA StylePereira, A. F. M., Sani, A. A., Zapata, T. B., Sousa, D. S. M. d., Rossini, B. C., Santos, L. D. d., Rall, V. L. M., Riccardi, C. d. S., & Fernandes Júnior, A. (2023). Synergistic Antibacterial Efficacy of Melittin in Combination with Oxacillin against Methicillin-Resistant Staphylococcus aureus (MRSA). Microorganisms, 11(12), 2868. https://doi.org/10.3390/microorganisms11122868






