Rhizospheric Bacillus spp. Exhibit Miticidal Efficacy against Oligonychus coffeae (Acari: Tetranychidae) of Tea
Abstract
:1. Introduction
2. Material and Methods
2.1. Bacterial Strains
2.2. Preparation of Bacterial Cell Suspension
2.3. Collection and Rearing of Red Spider Mite
2.4. Screening of Miticidal (Acaricidal and Ovicidal) Potential of Bacillus spp. against O. coffeae
2.5. Setup for Adulticidal Activity
2.6. Setup for Ovicidal Activity
2.7. Virulence of the Three Bacillus Isolates against Adults and Eggs of O. Coffeae
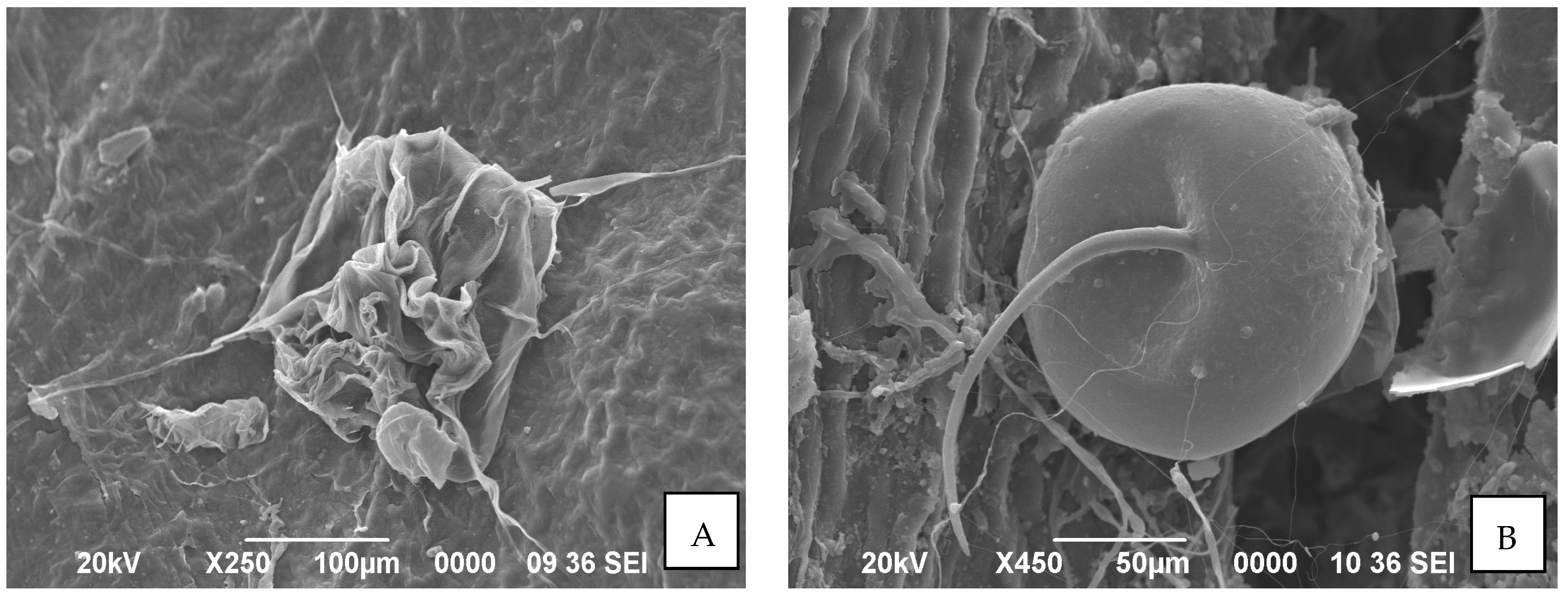
2.8. Electron Microscopy Study
2.9. Characterization of Pesticidal Metabolites through LC-MS Profiling
2.10. Statistical Analysis
3. Results
3.1. In Vitro Adulticidal Activity of Bacillus spp. against O. Coffeae
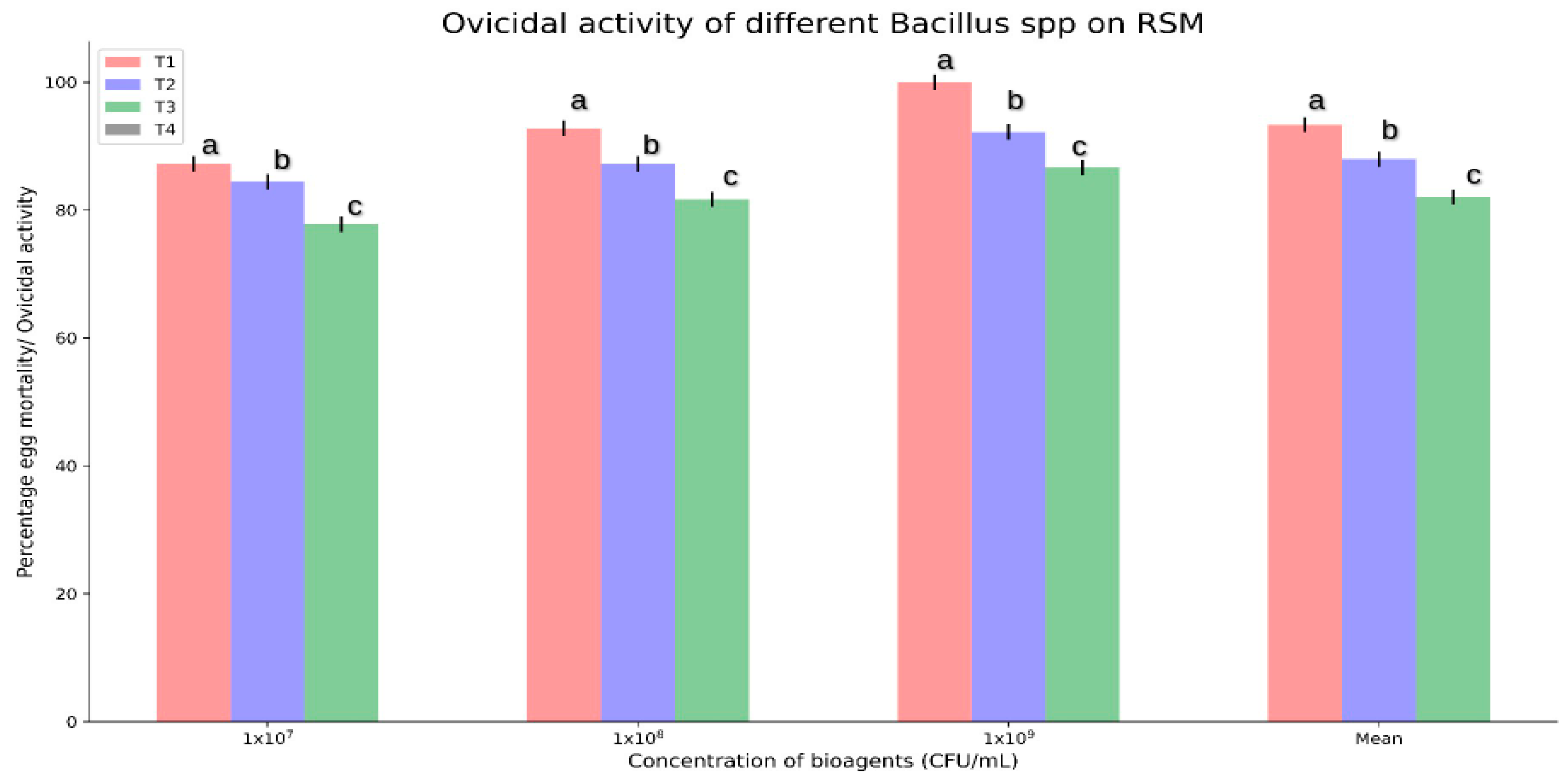
3.2. In Vitro Ovicidal Activity of Bacillus spp. against O. coffeae
3.3. Virulence of the Three Bacillus Isolates against Adults and Eggs of O. Coffeae
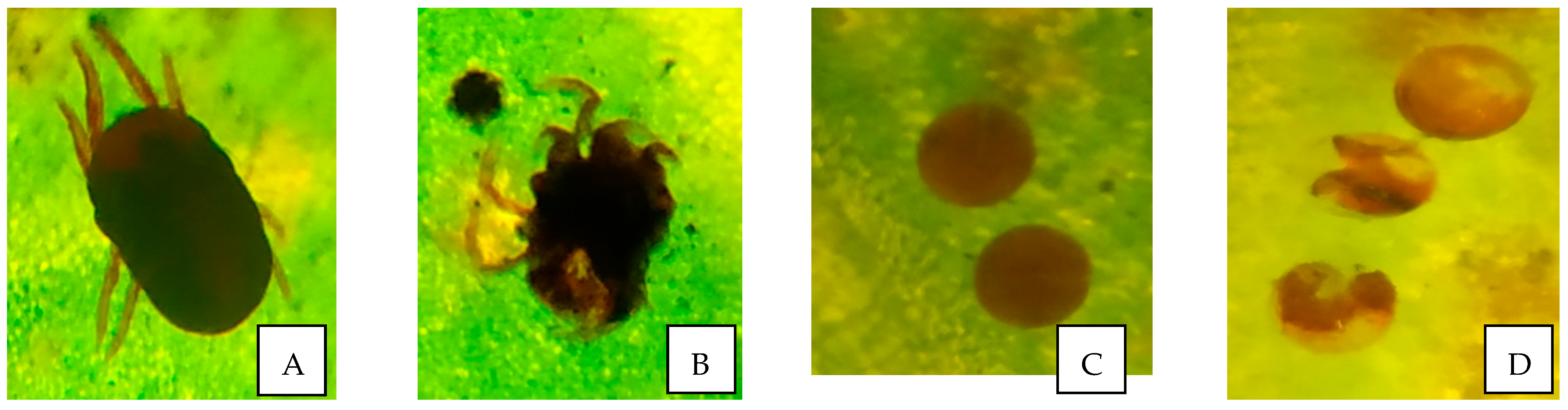
3.4. Morphological Changes in O. coffeae in Response to Bacillus Treatment
3.5. Profiling and Identification of Pesticidal Metabolites of Bacillus spp.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bora, P.; Bora, L.C. Microbial antagonists and botanicals mediated disease management in tea, Camellia sinensis (L.) O. Kuntze: An overview. Crop Prot. 2021, 148, 105711. [Google Scholar] [CrossRef]
- Bora, P.; Bora, L.C.; Bhuyan, R.P.; Hashem, A.; Abd-Allah, E.F. Bioagent consortia assisted suppression in grey blight disease with enhanced leaf nutrients and biochemical properties of tea (Camellia sinensis). Biol. Control 2022, 170, 104907. [Google Scholar] [CrossRef]
- Roy, S. Detection and biochemical characterization of acaricides resistance in field population of tea red spider mite, Oligonychus coffeae (Acari: Tetranychidae) in Assam tea plantation of India. Int. J. Acarol. 2019, 45, 47–476. [Google Scholar] [CrossRef]
- Hazarika, L.K.; Bhuyan, M.; Hazarika, B.N. Insect pests of tea and their management. Annu. Rev. Entomol. 2009, 54, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, M.S.; Haque, M.M.; Ahmad, M.; Yasin, M. Physiological and biochemical changes in tea leaves and made tea due to red spider mite infestation. Asian J. Pl. Sci. 2016, 15, 16–25. [Google Scholar]
- Meyer, M. Tea red mite, Oligonychus coffeae (Nitener). In Tropical Fruit Pests and Pollinators [Electronic Resource]: Biology, Economic Importance, Natural Enemies, and Control; CABI Publishing: New York, NY, USA, 2001. [Google Scholar]
- Gurusubramanian, G.; Rahman, A.; Sarmah, M.; Ray, S.; Bora, S. Pesticides usage pattern in tea ecosystem, their retrospects and alternative measures. J. Environ. Biol. 2008, 29, 813–826. [Google Scholar]
- Roy, S.; Handique, G.; Dutta, R.; Bora, A.; Gogoi, H.; Bhattacharjee, A.; Rahman Sarmah, M.; Babu, A. Insecticide resistance amongst field population of Hyposidra talaca Walker (Geometriae: Lepidoptera) in tea plantation of Assam, India: Detection through biochemical approach. Phytoparasitica 2021, 49, 433–442. [Google Scholar] [CrossRef]
- Bora, P.; Bora, L.C. Disease management in horticultural crops through microbial interventions: An overview. Indian J. Agric. Sci. 2020, 90, 1389–1396. [Google Scholar] [CrossRef]
- Mochizuki, M. Effectiveness and pesticide susceptibility of the pyrethroid-resistant predatory mite Amblyseius womersleyi in the integrated pest management of tea pests. Bio. Control 2003, 48, 207–221. [Google Scholar]
- Kunimi, Y. Current status and prospects on microbial control in Japan. J. Invertebr. Pathol. 2007, 95, 181–186. [Google Scholar] [CrossRef]
- Kavya, D.; Somnath, R.; Akanksha, N.; Roy, S.M.; Flood, J.; Prasad, A.K.; Ravinder, K.; Neave, S.; Muraleedharan, N. Pest management through Bacillus thuringiensis (Bt) in a tea-silkworm ecosystem: Status and potential prospects. Appl. Microbiol. Biotechnol. 2017, 101, 1795–1803. [Google Scholar]
- Banik, A.; Chattopadhyay, A.; Ganguly, S.; Mukhopadhyay, S. Characterization of a tea pest specific Bacillus thuringiensis and identification of its toxin by MALDI-TOF mass spectrometry. Ind. Crops Prod. 2019, 137, 549–556. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmad, I.; Ali, M. Biocontrol studies on rhizospheric microorganisms against black rot disease of tea caused by Corticium theae Bernard. Bangladesh J. Bot. 2018, 47, 985–991. [Google Scholar] [CrossRef]
- Kim, G.H.; Lim, M.T.; Hur, J.S.; Yum, K.J.; Koh, Y.J. Biological control of tea anthracnose using an antagonistic bacterium of Bacillus subtilis isolated from tea leaves. Plant Pathol. J. 2009, 25, 99–102. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, Z.; Lv, F.; Zhao, H.; Wang, Y.; Bie, X. Antagonistic action of Bacillus subtilis strain fmbj on the postharvest pathogen Rhizopus stolonifer. J. Food Sci. 2011, 76, 581. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Mukhopadhyay, A.; Gurusubramanian, G. Relative susceptibility of tea mosquito bug, Helopeltis theivora Waterhouse and red spider mite, Oligonychus coffeae Nietner eggs to commonly used pesticides. J. Plant Prot. Res. 2010, 50, 244–249. [Google Scholar] [CrossRef]
- Ebeling, W.; Pence, R.J. Pesticides formulation, influence of formulation on effectiveness. J. Agric. Food Chem. 1953, 1, 386–397. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Roy, S.; Rahman, A.; Phukan, A.K.; Muraleedharan, N.N. Terminalia chebula Retz. (Combretaceae): Source of a botanical acaricide against Oligonychus coffeae Nietner (Acarina: Tetranychidae). Int. J. Acarol. 2014, 40, 138–144. [Google Scholar] [CrossRef]
- Orion, D.; Wergin, W.; Chitwood, D.; Erbe, E. Low-temperature scanning electron microscope observations of the Meloidogyne incognita egg mass: The gelatinous matrix and embryo development. J. Nematol. 1994, 26, 402. [Google Scholar]
- Willcott, M.R. MestRe Nova; ACS Publications: Washington, DC, USA, 2009. [Google Scholar]
- IBM SPSS. IBM SPSS Statistics for Windows, version 20.0; IBM Corp.: New York, NY, USA, 2011; Volume 440, p. 394. [Google Scholar]
- Kumhar, K.C.; Babu, A.; Arulmarianathan, J.P. Role of beneficial fungi in managing diseases and insect pests of tea plantation. Egypt J. Biol. Pest. Control 2020, 30, 78. [Google Scholar] [CrossRef]
- Roobakkumar, A.; Rahman, V.J.; VasanthaKumar, D.; Babu, A.; Subramaniam, R.M.S. Utilization of the bacterium, Pseudomonas putida as a potential biocontrol agent against red spider mite, Oligonychus coffeae (Acari: Tetranychidae) infesting tea. J. Plant. Crops 2011, 39, 236–238. [Google Scholar]
- Wu, S.; Xie, H.; Li, M.; Xu, X.; Lei, Z. Highly virulent Beauveria bassiana strains against the two-spotted spider mite, Tetranychus urticae, show no pathogenicity against five phytoseiid mite species. Exp. Appl. Acarol. 2016, 70, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Ajuna, H.B.; Won, S.J.; Choub, V.; Kim, C.-W.; Moon, J.H.; Ahn, Y.S. The insecticidal potential of Bacillus velezensis CE 100 against Dasineura jujubifolia Jiao & Bu (Diptera: Cecidomyiidae) larvae infestation and its role in the enhancement of yield and fruit quality of jujube (Zizyphus jujuba Miller var. inermis Rehder). Crop Prot. 2023, 163, 106098. [Google Scholar]
- Liang, L.; Fu, Y.; Deng, S.; Wu, Y.; Gao, M. Genomic, antimicrobial, and aphicidal traits of Bacillus velezensis ATR2, and its biocontrol potential against ginger rhizome rot disease caused by Bacillus pumilus. Microorganisms 2021, 10, 63. [Google Scholar] [CrossRef]
- Pappas, M.L.; Samaras, K.; Koufakis, I.; Broufas, G.D. Beneficial soil microbes negatively affect spider mites and aphids in pepper. Agronomy 2021, 11, 1831. [Google Scholar] [CrossRef]
- Lopez, I.G.; Alvarez, A.E.; Petroselli, G.; Erra, B.R.; Audisio, M.C. Aphicidal activity of Bacillus amyloliquefaciens strains in the peach-potato aphid (Myzus persicae). Microbiol. Res. 2019, 226, 41–47. [Google Scholar] [CrossRef]
- Akpor, O.B.; Akinwusi, O.D.; Ogunnusi, T.A. Production, characterization and pesticidal potential of Bacillus species metabolites against sugar ant (Camponotus consobrinus). Heliyon 2021, 7, e08447. [Google Scholar] [CrossRef]
- Al-Azzazy, M.M.; Abdullah, S.; Alsohim, C.E.Y. Biological effects of three bacterial species on Tetranychus urticae (Acari: Tetrenychidae) infesting eggplant under laboratory and greenhouse conditions. Acarologia 2020, 60, 587–594. [Google Scholar] [CrossRef]
- Majeed, M.Z.; Riaz, M.A.; Khan, M.A.; Ma, C.S.; Ahmad, S. Pathogenicity of indigenous soil isolate of Bacillus thuringiensis to Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2018, 28, 38. [Google Scholar] [CrossRef]
- Patterson, R.R.M.; Simmonds, M.S.J.; Blaney, W.M. Mycopesticidal effects of characterized extracts of Penicillium isolates and purified metabolites (including mycotoxins) on Drosphila melanogasier and Spodopiera litoralis. J. Invertebr. Pathol. 1987, 50, 124–133. [Google Scholar] [CrossRef]
- McInerney, B.V.; Gregson, R.P.; Lacey, M.J.; Akhurst, R.J.; Lyons, G.R.; Rhodes, S.H.; Smith, D.R.; Engelhardt, L.M.; White, A.H. Biologically active metabolites from Xenorhabdus spp., part 1. Dithiolopyrrolone derivatives with antibiotic activity. J. Nat. Prod. 1991, 54, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Rolinson, G.N. Microbial metabolites with insecticidal properties. Appl. Microbiol. 1972, 24, 660–662. [Google Scholar] [CrossRef]
- Mishima, H. Milbemycin: A family of macrolide antibiotics with insecticidal activity. J. Nat. Prod. 1983, 129–134. [Google Scholar] [CrossRef]
- Rodriguez, J.; Potts, M.; Rodriguez, L. Mycotoxin toxicity to Tyrophagus putrescentiae. J. Econ. Entomol. 1980, 73, 282–284. [Google Scholar] [CrossRef]
- He, H.; Suh, L.A.H.; Handelsman, J.; Clardy, J. Zwittermycin A, an antifungal and plant protection agent from Bacillus cereus. Tetrahedron Lett. 1994, 35, 2499–2502. [Google Scholar] [CrossRef]
- Stabb, E.V.; Jacobson, L.M.; Handelsman, J. Zwittermicin A-producing strains of Bacillus cereus from diverse soils. Appl. Environ. Microbiol. 1994, 60, 4404–4412. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Choi, Y.L.; Sun, M.; Yu, Z. Novel roles of Bacillus thuringiensis to control plant diseases. Appl. Microbiol. Biotechnol. 2008, 80, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Goodman, R.M.; Raffa, K.F.; Handelsman, J. Synergy between zwittermicin A and Bacillus thuringiensis subsp. kurstaki against gypsy moth (Lepidoptera: Lymantriidae). Environ. Entomol. 2000, 29, 101–107. [Google Scholar] [CrossRef]
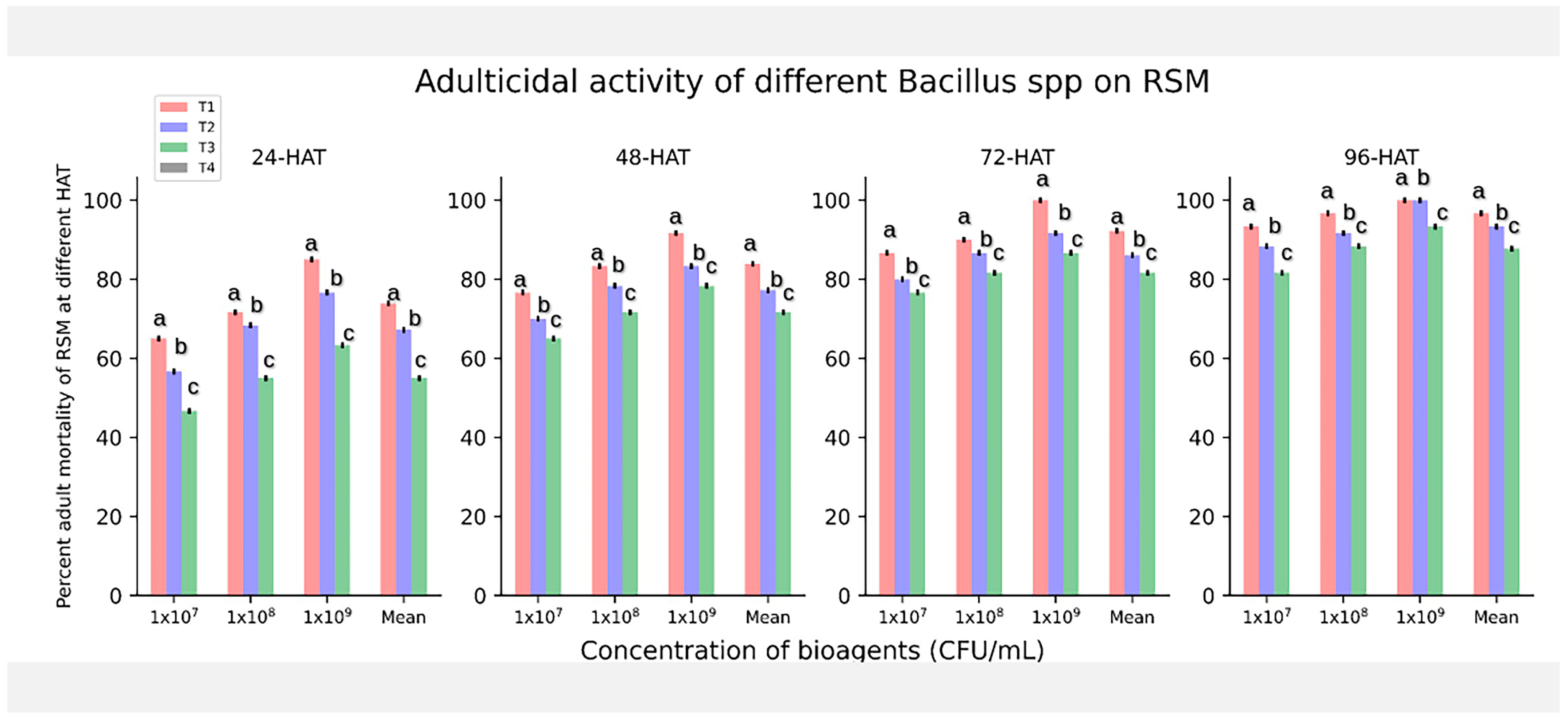

| Treatment | Conc. (CFU/mL) | Adult Mortality (%) after 96 h | Log of Conc. | Probit Mortality | Regression Statistics (a = Slope, b = Intercept) | Regression Equation Y = aX + b | LC50 (Y = 5) LC50 = antilogX | LC50 Value |
|---|---|---|---|---|---|---|---|---|
| T1: Bacillus velezensis AB22 | 1 × 105 | 70.00 (56.79) | 5 | 5.18 | a = 0.262; b =3.832 | Y = 0.262x + 3.832 | 28,708.79737 | 0.28 × 105 |
| 1 × 106 | 81.67 (64.60) | 6 | 5.39 | |||||
| 1 × 107 | 93.33 (75.00) | 7 | 5.67 | |||||
| 1 × 108 | 96.67 (79.37) | 8 | 5.81 | |||||
| 1 × 109 | 100.00 (90.00) | 9 | 6.28 | |||||
| T2: B. amyloliquefaciens BAC1 | 1 × 105 | 63.33 (52.71) | 5 | 5.08 | a = 0.273; b = 3.643 | Y = 0.273x + 3.643 | 106,151.4425 | 1.06 × 105 |
| 1 × 106 | 76.67 (61.07) | 6 | 5.28 | |||||
| 1 × 107 | 88.33 (70.00) | 7 | 5.52 | |||||
| 1 × 108 | 91.67 (73.15) | 8 | 5.61 | |||||
| 1 × 109 | 100.00 (90.00) | 9 | 6.28 | |||||
| T3: B. subtilis LB22 | 1 × 105 | 61.67 (51.71) | 5 | 5.05 | a = 0.161; b = 4.229 | Y = 0.161x + 4.229 | 51,286,138.4 | 5.12 × 107 |
| 1 × 106 | 68.33 (55.73) | 6 | 5.15 | |||||
| 1 × 107 | 81.67 (64.60) | 7 | 5.39 | |||||
| 1 × 108 | 88.33 (70.00) | 8 | 5.52 | |||||
| 1 × 109 | 93.33 (75.00) | 9 | 5.67 |
| Treatment | Conc. (CFU/mL) | Ovicidal Activity (%) after 14 Days | Log of Conc. | Probit Mortality | Regression Statistics (a = Slope, b = Intercept) | Regression Equation Y = aX + b | LC50 (Y = 5) LC50 = antilogX | LC50 Value |
|---|---|---|---|---|---|---|---|---|
| T1: Bacillus velezensis AB22 | 1 × 105 | 73.33 (58.89) | 5 | 5.61 | a = 0.238; b =3.936 | Y= 0.238x + 3.936 | 29,552.09 | 0.29 × 105 |
| 1 × 106 | 81.11 (64.23) | 6 | 5.88 | |||||
| 1 × 107 | 87.22 (69.06) | 7 | 6.13 | |||||
| 1 × 108 | 92.78 (74.41) | 8 | 6.48 | |||||
| 1 × 109 | 100.00 (90.00) | 9 | 8.95 | |||||
| T2: B. amyloliquefaciens BAC1 | 1 × 105 | 63.89 (53.01) | 5 | 5.36 | a = 0.130; b = 4.400 | Y = 0.130x + 4.40 | 41,246.26 | 0.41 × 105 |
| 1 × 106 | 76.67 (61.07) | 6 | 5.74 | |||||
| 1 × 107 | 84.44 (66.77) | 7 | 5.99 | |||||
| 1 × 108 | 87.22 (69.06) | 8 | 6.13 | |||||
| 1 × 109 | 92.22 (73.81) | 9 | 6.41 | |||||
| T3: B. subtilis LB22 | 1 × 105 | 61.11 (51.41) | 5 | 5.28 | a = 0.110; b = 4.490 | Y = 0.110x + 4.49 | 43,287.61 | 0.43 × 105 |
| 1 × 106 | 71.67 (57.80) | 6 | 5.58 | |||||
| 1 × 107 | 77.78 (61.87) | 7 | 5.77 | |||||
| 1 × 108 | 81.67 (64.65) | 8 | 5.92 | |||||
| 1 × 109 | 86.67 (68.58) | 9 | 6.13 |
| Sl. No. | Compound Name | Formula | Match Score | Retention Time (RT) | Predicted m/z | Matched m/z |
|---|---|---|---|---|---|---|
| 1. | Brevianamide A | C21H23N3O3 | 0.961 | 27.27 | 404.1371 | 403.8991 |
| 2. | Citromycin | C13H10O5 | 0.909 | 10.18 | 285.0160 | 284.8307 |
| 3. | Emodin | C15H10O5 | 0.912 | 28.51 | 303.0863 | 302.8130 |
| 4. | Heptadecanoic acid | C17H34O2 | 0.953 | 28.36 | 303.2894 | 302.9980 |
| 5. | Paxillines | C27H33NO4 | 0.872 | 12.46 | 458.2302 | 458.0682 |
| 6. | Peramine | C12H17N5O | 0.905 | 10.18 | 285.1202 | 284.8307 |
| 7. | Thiolutin | C8H8N2O2S2 | 0.927 | 9.71 | 246.0365 | 245.7579 |
| 8. | Versimide | C9H11NO4 | 0.926 | 18.91 | 220.0580 | 219.8944 |
| 9. | Zwittermicin A | C13H28N6O8 | 0.856 | 31.86 | 414.2307 | 413.9633 |
| Sl. No. | Compound Name | Formula | Match Score | Retention Time (RT) | Predicted m/z | Matched m/z |
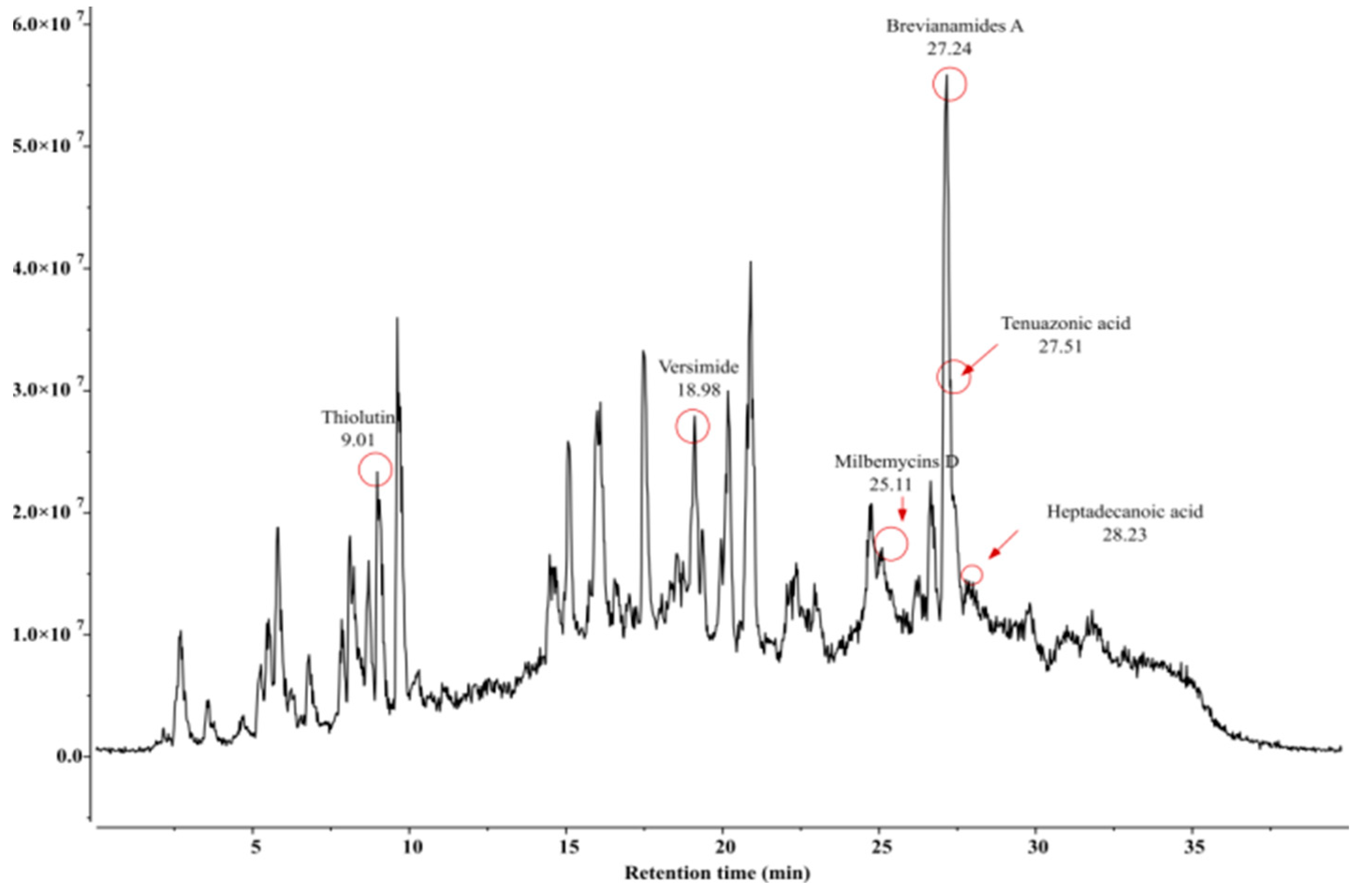
|---|---|---|---|---|---|---|
| 1. | Brevianamides A | C21H23N3O3 | 0.933 | 27.24 | 404.1371 | 404.0471 |
| 2. | Heptadecanoic acid | C17H34O2 | 0.904 | 28.23 | 303.2894 | 302.9980 |
| 3. | Milbemycins D | C33H48O7 | 0.888 | 25.11 | 574.3738 | 574.1025 |
| 4. | Tenuazonic acid | C8H16O2 | 0.956 | 27.51 | 485.9953 | 486.2257 |
| 5. | Thiolutin | C8H8N2O2S2 | 0.970 | 9.01 | 246.0365 | 245.7579 |
| 6. | Versimide | C9H11NO4 | 0.923 | 18.98 | 220.0580 | 219.7834 |
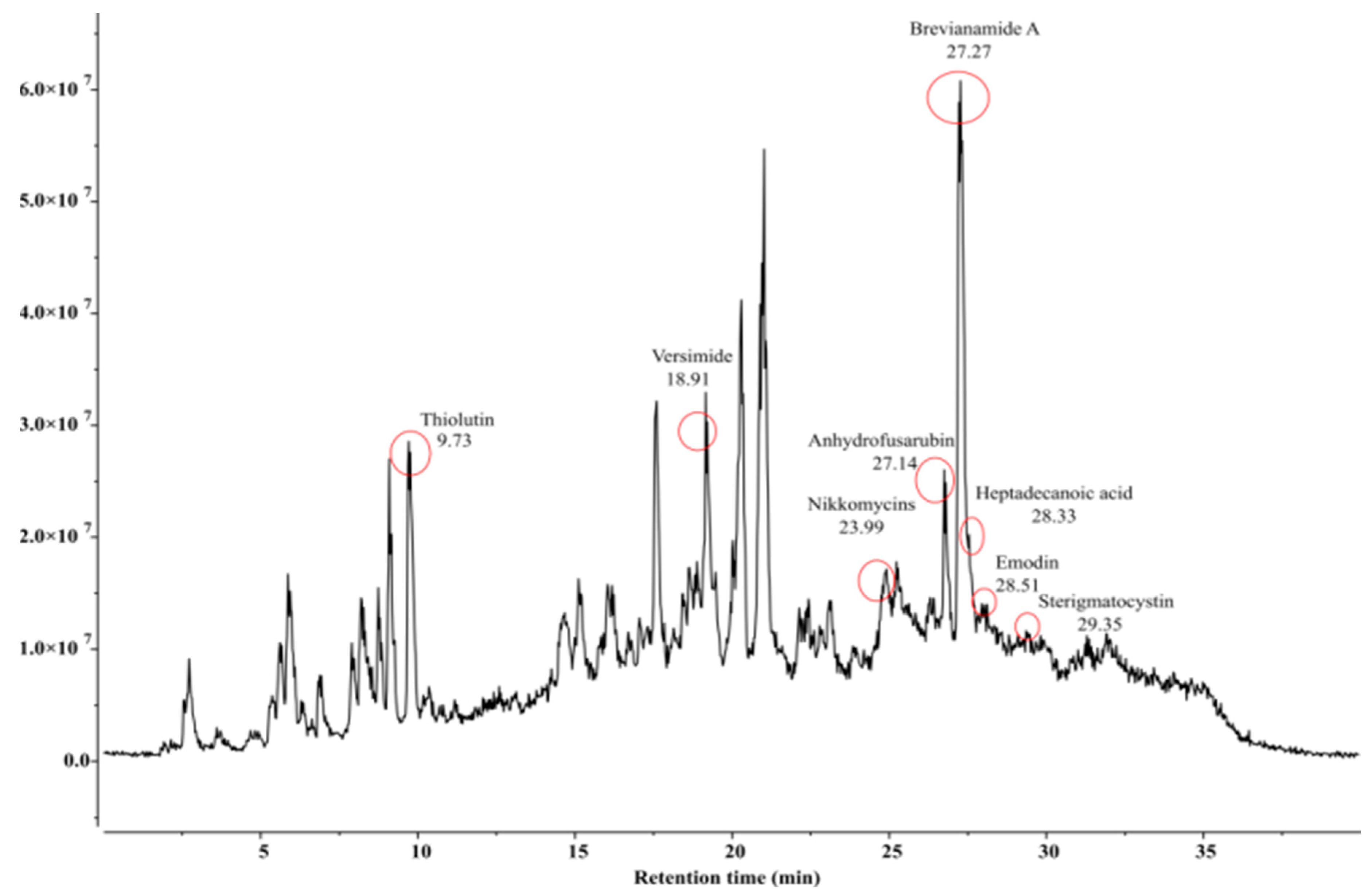
| Sl. No. | Compound Name | Formula | Match Score | Retention Time (RT) | Predicted m/z | Matched m/z |
|---|---|---|---|---|---|---|
| 1. | Anhydrofusarubin | C15H12O6 | 0.955 | 27.14 | 306.0972 | 305.9951 |
| 2. | Brevianamide A | C21H23N3O3 | 0.961 | 27.27 | 404.1371 | 403.8991 |
| 3. | Emodin | C15H10O5 | 0.912 | 28.51 | 303.0863 | 302.8130 |
| 4. | Heptadecanoic acid | C17H34O2 | 0.906 | 28.33 | 303.2894 | 303.0721 |
| 5. | Nikkomycins | C20H25N5O10 | 0.925 | 23.99 | 518.1494 | 518.3794 |
| 6. | Sterigmatocystin | C18H12O6 | 0.878 | 29.35 | 342.0972 | 341.8858 |
| 7. | Thiolutin | C8H8N2O2S2 | 0.982 | 9.73 | 246.0365 | 245.7949 |
| 8. | Versimide | C9H11NO4 | 0.926 | 18.91 | 220.0580 | 219.8944 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bora, P.; Gogoi, S.; Deshpande, M.V.; Garg, P.; Bhuyan, R.P.; Altaf, N.; Saha, N.; Borah, S.M.; Phukon, M.; Tanti, N.; et al. Rhizospheric Bacillus spp. Exhibit Miticidal Efficacy against Oligonychus coffeae (Acari: Tetranychidae) of Tea. Microorganisms 2023, 11, 2691. https://doi.org/10.3390/microorganisms11112691
Bora P, Gogoi S, Deshpande MV, Garg P, Bhuyan RP, Altaf N, Saha N, Borah SM, Phukon M, Tanti N, et al. Rhizospheric Bacillus spp. Exhibit Miticidal Efficacy against Oligonychus coffeae (Acari: Tetranychidae) of Tea. Microorganisms. 2023; 11(11):2691. https://doi.org/10.3390/microorganisms11112691
Chicago/Turabian StyleBora, Popy, Sukanya Gogoi, Mukund Vinayak Deshpande, Pankaj Garg, Rana P. Bhuyan, Nilofar Altaf, Nikita Saha, Sapna Mayuri Borah, Mousumi Phukon, Nabajit Tanti, and et al. 2023. "Rhizospheric Bacillus spp. Exhibit Miticidal Efficacy against Oligonychus coffeae (Acari: Tetranychidae) of Tea" Microorganisms 11, no. 11: 2691. https://doi.org/10.3390/microorganisms11112691
APA StyleBora, P., Gogoi, S., Deshpande, M. V., Garg, P., Bhuyan, R. P., Altaf, N., Saha, N., Borah, S. M., Phukon, M., Tanti, N., Saikia, B., Ahmed, S. S., Borah, S. R., Dutta, A., & Sarmah, B. K. (2023). Rhizospheric Bacillus spp. Exhibit Miticidal Efficacy against Oligonychus coffeae (Acari: Tetranychidae) of Tea. Microorganisms, 11(11), 2691. https://doi.org/10.3390/microorganisms11112691




