Distinct Effects of Moxifloxacin and Bedaquiline on Growing and ‘Non-Culturable’ Mycobacterium abscessus
Abstract
:1. Introduction
2. Materials and Methods
3. Results
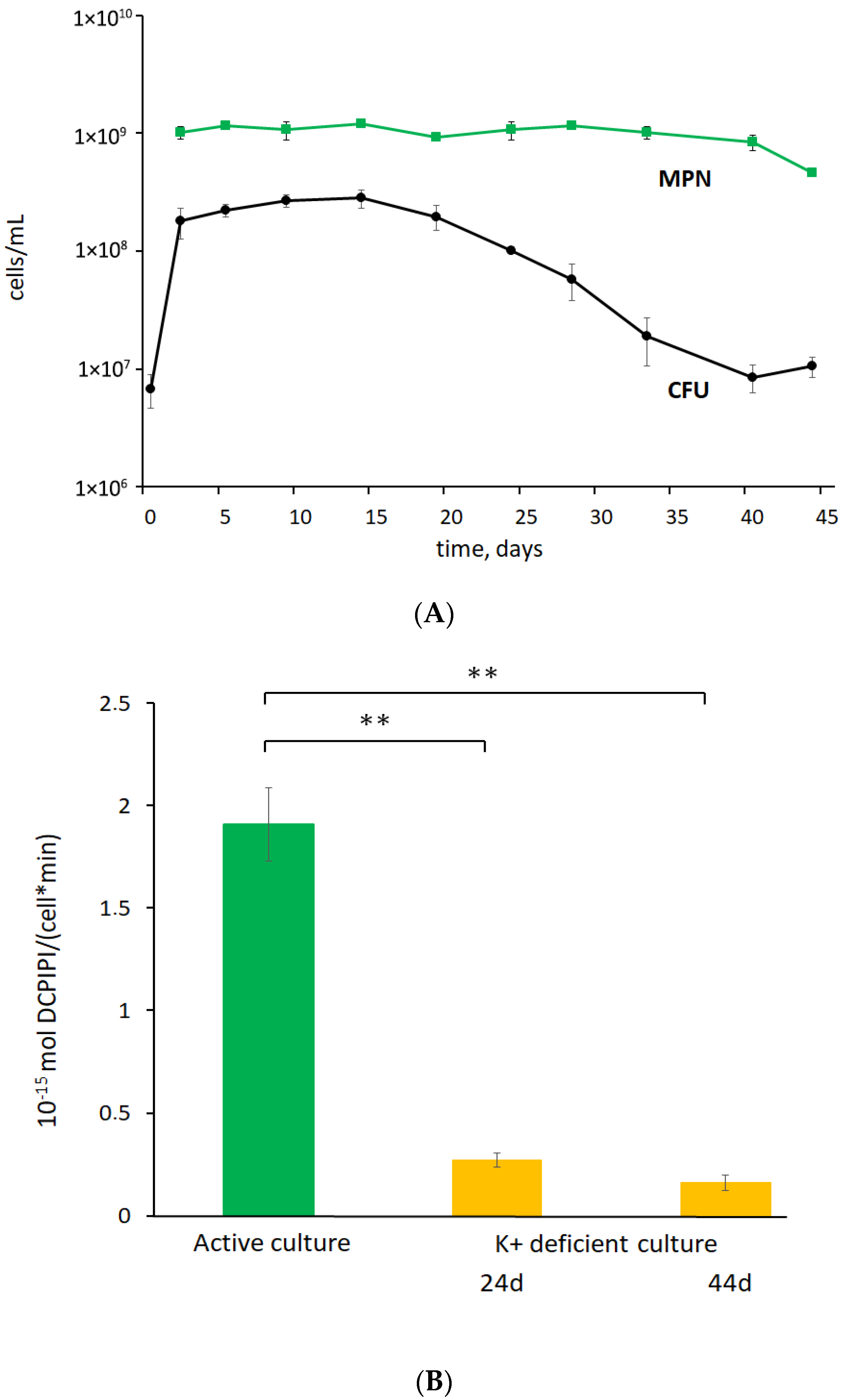
3.1. K+ Sequestration Causes M. abscessus to Enter a Metabolically Dormant State with a Loss of Colony-Forming Ability
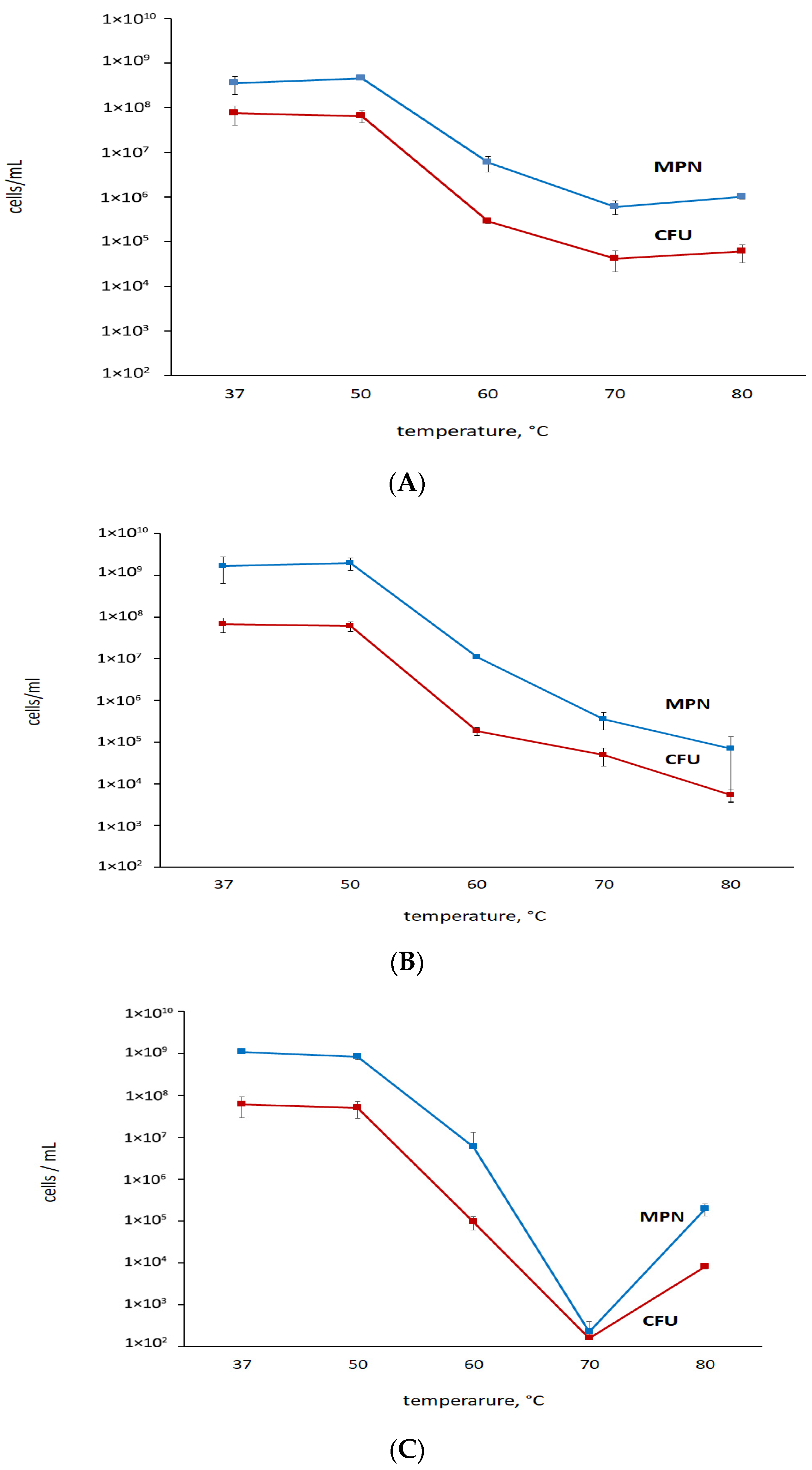
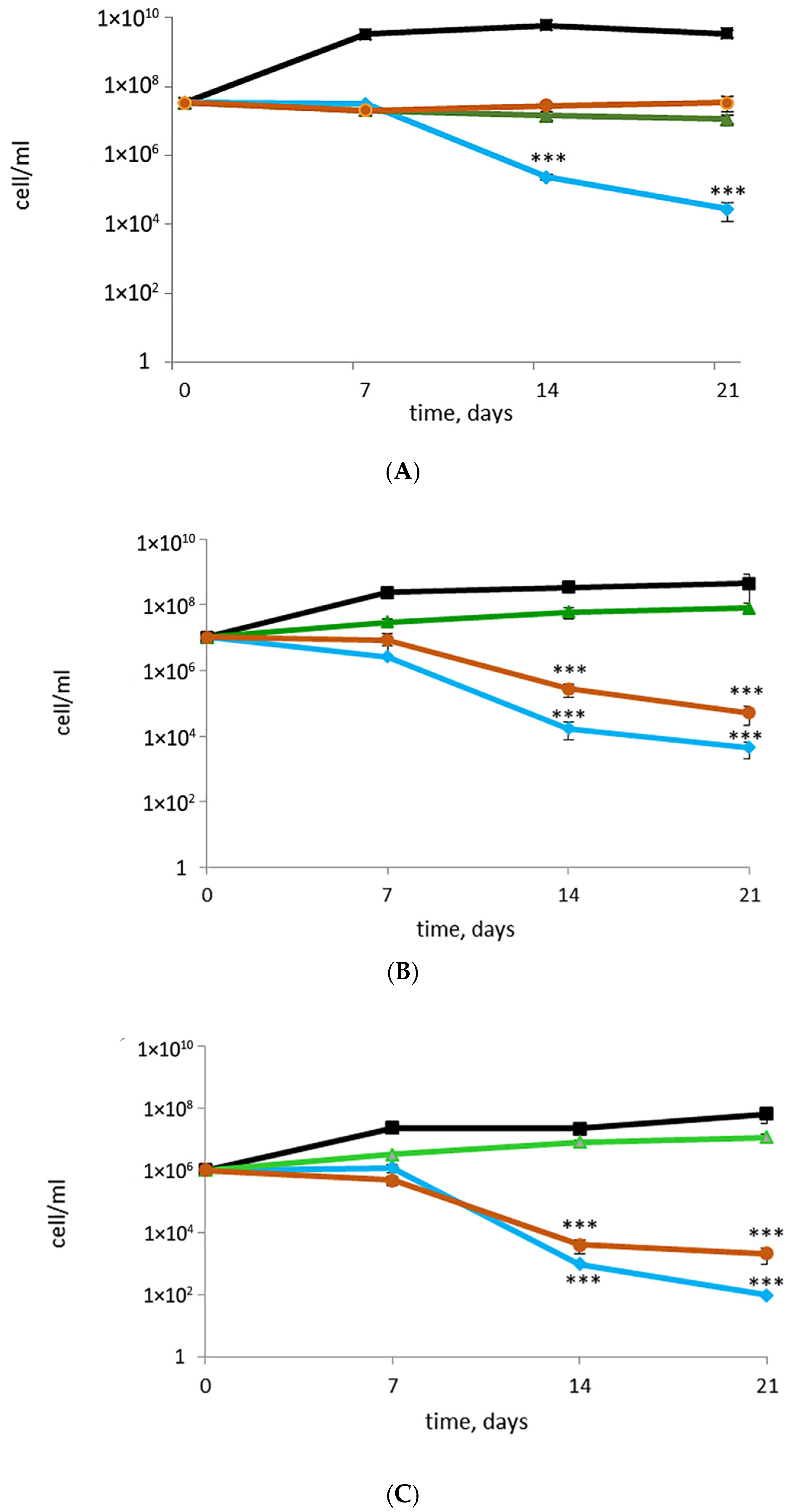
3.2. Rifampicin, Moxifloxacin, and Bedaquiline Exert Different Effects on Growing and Metabolically Inert M. abscessus Cells: Results from Commonly Applied CFU Tests
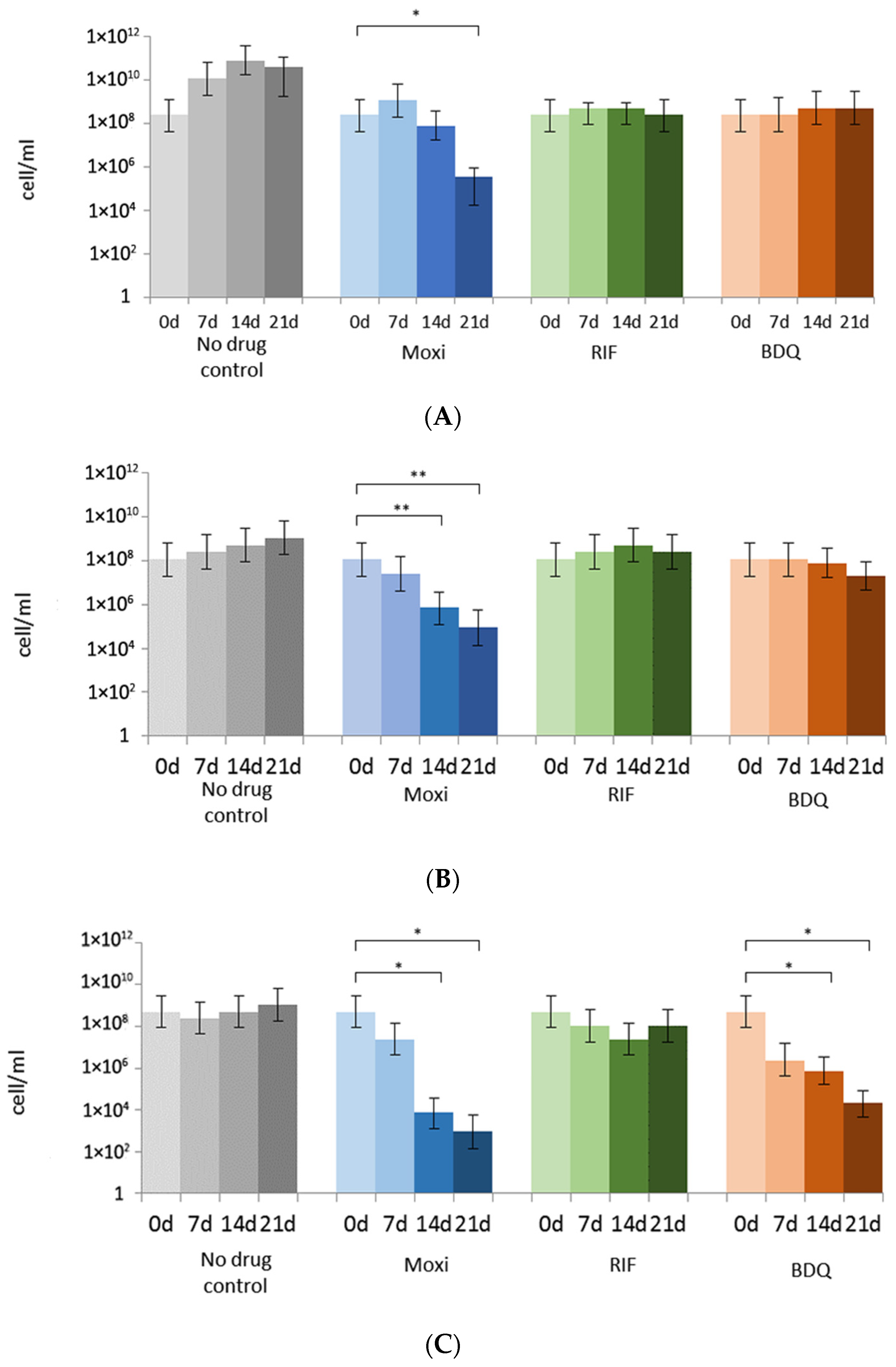
3.3. Putative or Confirmed Bactericidal Activity against M. abscessus: Findings from MPN Assays and Comparisons with CFU
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johansen, M.D.; Herrmann, J.-L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef] [PubMed]
- López-Roa, P.; Esteban, J.; Muñoz-Egea, M.-C. Updated Review on the Mechanisms of Pathogenicity in Mycobacterium abscessus, a Rapidly Growing Emerging Pathogen. Microorganisms 2022, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Tortoli, E.; Fedrizzi, T.; Meehan, C.J.; Trovato, A.; Grottola, A.; Giacobazzi, E.; Serpini, G.F.; Tagliazucchi, S.; Fabio, A.; Bettua, C.; et al. The new phylogeny of the genus Mycobacterium: The old and the news. Infect. Genet. Evol. 2017, 56, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Degiacomi, G.; Sammartino, J.C.; Chiarelli, L.R.; Riabova, O.; Makarov, V.; Pasca, M.R. An emerging and worrisome pathogen among cystic fibrosis patients. Int. J. Mol. Sci. 2019, 20, 5868. [Google Scholar] [CrossRef]
- Catherinot, E.; Roux, A.-L.; Vibet, M.-A.; Bellis, G.; Ravilly, S.; Lemonnier, L.; Le Roux, E.; Bernède-Bauduin, C.; Le Bourgeois, M.; Herrmann, J.-L.; et al. Mycobacterium avium and Mycobacterium abscessus complex target distinct cystic fibrosis patient subpopulations. J. Cyst. Fibros. 2013, 12, 74–80. [Google Scholar] [CrossRef]
- Luthra, S.; Rominski, A.; Sander, P. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus Drug Resistance. Front. Microbiol. 2018, 9, 2179. [Google Scholar] [CrossRef]
- Trias, J.; Jarlier, V.; Benz, R. Porins in the cell wall of mycobacteria. Science 1992, 258, 1479–1481. [Google Scholar] [CrossRef]
- Nessar, R.; Cambau, E.; Reyrat, J.M.; Murray, A.; Gicquel, B. Mycobacterium abscessus: A new antibiotic nightmare. J. Antimicrob. Chemother. 2012, 67, 810–818. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, L.; Mao, Y.; Ye, M.; Guo, Q.; Zhang, Y.; Xu, L.; Zhang, Z.; Li, B.; Chu, H. Clinical efficacy and adverse effects of antibiotics used to treat. Front. Microbiol. 2019, 10, 1977. [Google Scholar] [CrossRef]
- Recchia, D.; Stelitano, G.; Stamilla, A.; Gutierrez, D.L.; Degiacomi, G.; Chiarelli, L.R.; Pasca, M.R. Mycobacterium abscessus infections in cystic fibrosis individuals: A review on therapeutic options. Int. J. Mol. Sci. 2023, 24, 4635. [Google Scholar] [CrossRef]
- Lagune, M.; Kremer, L.; Herrmann, J.-L. Mycobacterium abscessus, a complex of three fast-growing subspecies sharing virulence traits with slow-growing mycobacteria. Clin. Microbiol. Infect. 2023, S1198-743X(23)00485-8. [Google Scholar] [CrossRef] [PubMed]
- Floto, R.A.; Olivier, K.N.; Saiman, L.; Daley, C.L.; Herrmann, J.-L.; Nick, J.A.; Noone, P.G.; Bilton, D.; Corris, P.; Gibson, R.L.; et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 2016, 71 (Suppl. S1), i1–i22. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020, 56, 2000535. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.M.; Gomes, M.S.; Silva, T. Looking beyond Typical Treatments for Atypical Mycobacteria. Antibiotics 2020, 9, 18. [Google Scholar] [CrossRef]
- Quang, N.T.; Jang, J. Current molecular therapeutic agents and drug candidates for Mycobacterium abscessus. Front. Pharmacol. 2021, 12, 724725. [Google Scholar] [CrossRef]
- Berube, B.J.; Castro, L.; Russell, D.; Ovechkina, Y.; Parish, T. Novel screen to assess bactericidal activity of compounds against non-replicating Mycobacterium abscessus. Front. Microbiol. 2018, 9, 2417. [Google Scholar] [CrossRef]
- Yam, Y.-K.; Alvarez, N.; Go, M.-L.; Dick, T. Extreme drug tolerance of Mycobacterium abscessus “persisters”. Front. Microbiol. 2020, 11, 359. [Google Scholar] [CrossRef]
- Bald, D.; Koul, A. Advances and strategies in discovery of new antibacterials for combating metabolically resting bacteria. Drug Discov. Today 2013, 18, 250–255. [Google Scholar] [CrossRef]
- Pieterman, E.D.; Sarink, M.J.; Sala, C.; Cole, S.T.; de Steenwinkel, J.E.M.; Bax, H.I. Advanced quantification methods to improve the 18b dormancy model for assessing the activity of tuberculosis drugs. Antimicrob. Agents Chemother. 2020, 64, e00280-20. [Google Scholar] [CrossRef]
- Salina, E.G.; Ryabova, O.; Vocat, A.; Nikonenko, B.; Cole, S.T.; Makarov, V. New 1-hydroxy-2-thiopyridine derivatives active against both replicating and dormant Mycobacterium tuberculosis. J. Infect. Chemother. 2017, 23, 794–797. [Google Scholar] [CrossRef]
- Makarov, V.; Manina, G.; Mikusova, K.; Möllmann, U.; Ryabova, O.; Saint-Joanis, B.; Dhar, N.; Pasca, M.R.; Buroni, S.; Lucarelli, A.P.; et al. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science 2009, 324, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.; Lechartier, B.; Zhang, M.; Neres, J.; van der Sar, A.M.; Raadsen, S.A.; Hartkoorn, R.C.; Ryabova, O.B.; Vocat, A.; Decosterd, L.A.; et al. Towards a new combination therapy for tuberculosis with next generation benzothiazinones. EMBO Mol. Med. 2014, 6, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Manjunatha, U.; Boshoff, H.I.M.; Ha, Y.H.; Niyomrattanakit, P.; Ledwidge, R.; Dowd, C.S.; Lee, I.Y.; Kim, P.; Zhang, L.; et al. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 2008, 322, 1392–1395. [Google Scholar] [CrossRef]
- Stancil, S.L.; Mirzayev, F.; Abdel-Rahman, S.M. Profiling pretomanid as a therapeutic option for TB infection: Evidence to date. Drug Des. Devel Ther. 2021, 15, 2815–2830. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, U.; Boshoff, H.I.; Barry, C.E. The mechanism of action of PA-824: Novel insights from transcriptional profiling. Commun. Integr. Biol. 2009, 2, 215–218. [Google Scholar] [CrossRef]
- Koul, A.; Vranckx, L.; Dendouga, N.; Balemans, W.; Van den Wyngaert, I.; Vergauwen, K.; Göhlmann, H.W.; Willebrords, R.; Poncelet, A.; Guillemont, J.; et al. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J. Biol. Chem. 2008, 283, 25273–25280. [Google Scholar] [CrossRef]
- Lanni, A.; Borroni, E.; Iacobino, A.; Russo, C.; Gentile, L.; Fattorini, L.; Giannoni, F. Activity of drug combinations against Mycobacterium abscessus grown in aerobic and hypoxic conditions. Microorganisms 2022, 10, 1421. [Google Scholar] [CrossRef]
- Shleeva, M.; Mukamolova, G.V.; Young, M.; Williams, H.D.; Kaprelyants, A.S. Formation of ‘non-culturable’ cells of Mycobacterium smegmatis in stationary phase in response to growth under suboptimal conditions and their Rpf-mediated resuscitation. Microbiology 2004, 150 Pt 6, 1687–1697. [Google Scholar] [CrossRef]
- Salina, E.; Ryabova, O.; Kaprelyants, A.; Makarov, V. New 2-thiopyridines as potential candidates for killing both actively growing and dormant Mycobacterium tuberculosis cells. Antimicrob. Agents Chemother. 2014, 58, 55–60. [Google Scholar] [CrossRef]
- Salina, E.G.; Waddell, S.J.; Hoffmann, N.; Rosenkrands, I.; Butcher, P.D.; Kaprelyants, A.S. Potassium availability triggers Mycobacterium tuberculosis transition to, and resuscitation from, non-culturable (dormant) states. Open Biol. 2014, 4, 140106. [Google Scholar] [CrossRef]
- Connell, N. Mycobacterium: Isolation, maintenance, transformation, and mutant selection. Methods Cell Biol. 1995, 45, 107–125. [Google Scholar] [CrossRef]
- de Man, J.C. The probability of most probable numbers. J. Appl. Microbiol. 1975, 1, 67–78. [Google Scholar]
- Myers, A. Use of DCPIP in a colorimetric method to investigate electron transport in crude heart mitochondrial extracts. J. Biol. Educ. 1990, 24, 123–127. [Google Scholar] [CrossRef]
- Palomino, J.-C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Mulyukin, A.L.; Kudykina, Y.K.; Shleeva, M.O.; Anuchin, A.M.; Suzina, N.E.; Danilevich, V.N.; Duda, V.I.; Kaprelyants, A.S.; El’-Registan, G.I. Intraspecies diversity of dormant forms of Mycobacterium smegmatis. Microbiology 2010, 79, 461–471. [Google Scholar] [CrossRef]
- Verma, A.; Ghoshal, A.; Dwivedi, V.P.; Bhaskar, A. Tuberculosis: The success tale of less explored dormant Mycobacterium tuberculosis. Front. Cell Infect. Microbiol. 2022, 12, 1079569. [Google Scholar] [CrossRef]
- Ayrapetyan, M.; Williams, T.; Oliver, J.D. Relationship between the viable but nonculturable state and antibiotic persister cells. J. Bacteriol. 2018, 200, e00249-18. [Google Scholar] [CrossRef]
- Ayrapetyan, M.; Williams, T.C.; Oliver, J.D. Bridging the gap between viable but non-culturable and antibiotic persistent bacteria. Trends Microbiol. 2015, 23, 7–13. [Google Scholar] [CrossRef]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef]
- Oliver, J.D. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol. Rev. 2010, 34, 415–425. [Google Scholar] [CrossRef]
- Bowness, R.; Boeree, M.J.; Aarnoutse, R.; Dawson, R.; Diacon, A.; Mangu, C.; Heinrich, N.; Ntinginya, N.E.; Kohlenberg, A.; Mtafya, B.; et al. The relationship between Mycobacterium tuberculosis MGIT time to positivity and cfu in sputum samples demonstrates changing bacterial phenotypes potentially reflecting the impact of chemotherapy on critical sub-populations. J. Antimicrob. Chemother. 2015, 70, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Maurer, F.P.; Bruderer, V.L.; Ritter, C.; Castelberg, C.; Bloemberg, G.V.; Böttger, E.C. Lack of antimicrobial bactericidal activity in Mycobacterium abscessus. Antimicrob. Agents Chemother. 2014, 58, 3828–3836. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayana, S.B.; Huat, T.B.; Ho, P.C.; Manjunatha, U.H.; Dartois, V.; Dick, T.; Rao, S.P.S. Comprehensive physicochemical, pharmacokinetic and activity profiling of anti-TB agents. J. Antimicrob. Chemother. 2015, 70, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Hurst-Hess, K.R.; Saxena, A.; Rudra, P.; Yang, Y.; Ghosh, P. Mycobacterium abscessus HelR interacts with RNA polymerase to confer intrinsic rifamycin resistance. Mol. Cell 2022, 82, 3166–3177.e5. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Park, E.M.; Kim, H.; Kwon, O.J.; Chang, C.L.; Lew, W.J.; Kil Park, Y.; Koh, W.-J. In vitro antimicrobial susceptibility of Mycobacterium abscessus in Korea. J. Korean Med. Sci. 2008, 23, 49–52. [Google Scholar] [CrossRef]
- Nie, W.; Duan, H.; Huang, H.; Lu, Y.; Bi, D.; Chu, N. Species identification of Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii using rpoB and hsp65, and susceptibility testing to eight antibiotics. Int. J. Infect. Dis. 2014, 25, 170–174. [Google Scholar] [CrossRef]
- Nie, W.J.; Xie, Z.Y.; Gao, S.; Teng, T.L.; Zhou, W.Q.; Shang, Y.Y.; Jing, W.; Shi, W.H.; Wang, Q.F.; Huang, X.R.; et al. Efficacy of Moxifloxacin against Mycobacterium abscessus in Zebrafish Model in vivo. Biomed. Environ. Sci. 2020, 33, 350–358. [Google Scholar] [CrossRef]
- Vesenbeckh, S.; Schönfeld, N.; Roth, A.; Bettermann, G.; Krieger, D.; Bauer, T.T.; Rüssmann, H.; Mauch, H. Bedaquiline as a potential agent in the treatment of Mycobacterium abscessus infections. Eur. Respir. J. 2017, 49, 1700083. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Wallace, R.J., Jr. Susceptibility testing of bedaquiline against Mycobacterium abscessus complex. Antimicrob. Agents Chemother. 2019, 63, e01919-18. [Google Scholar] [CrossRef]
- Gil, E.; Sweeney, N.; Barrett, V.; Morris-Jones, S.; Miller, R.F.; Johnston, V.J.; Brown, M. Bedaquiline as treatment for disseminated nontuberculous Mycobacteria infection in 2 patients co-infected with HIV. Emerg. Infect. Dis. 2021, 27, 944–948. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulyukin, A.L.; Recchia, D.; Kostrikina, N.A.; Artyukhina, M.V.; Martini, B.A.; Stamilla, A.; Degiacomi, G.; Salina, E.G. Distinct Effects of Moxifloxacin and Bedaquiline on Growing and ‘Non-Culturable’ Mycobacterium abscessus. Microorganisms 2023, 11, 2690. https://doi.org/10.3390/microorganisms11112690
Mulyukin AL, Recchia D, Kostrikina NA, Artyukhina MV, Martini BA, Stamilla A, Degiacomi G, Salina EG. Distinct Effects of Moxifloxacin and Bedaquiline on Growing and ‘Non-Culturable’ Mycobacterium abscessus. Microorganisms. 2023; 11(11):2690. https://doi.org/10.3390/microorganisms11112690
Chicago/Turabian StyleMulyukin, Andrey L., Deborah Recchia, Nadezhda A. Kostrikina, Maria V. Artyukhina, Billy A. Martini, Alessandro Stamilla, Giulia Degiacomi, and Elena G. Salina. 2023. "Distinct Effects of Moxifloxacin and Bedaquiline on Growing and ‘Non-Culturable’ Mycobacterium abscessus" Microorganisms 11, no. 11: 2690. https://doi.org/10.3390/microorganisms11112690






