Taxifolin as a Metallo-β-Lactamase Inhibitor in Combination with Augmentin against Verona Imipenemase 2 Expressing Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Materials and Methods
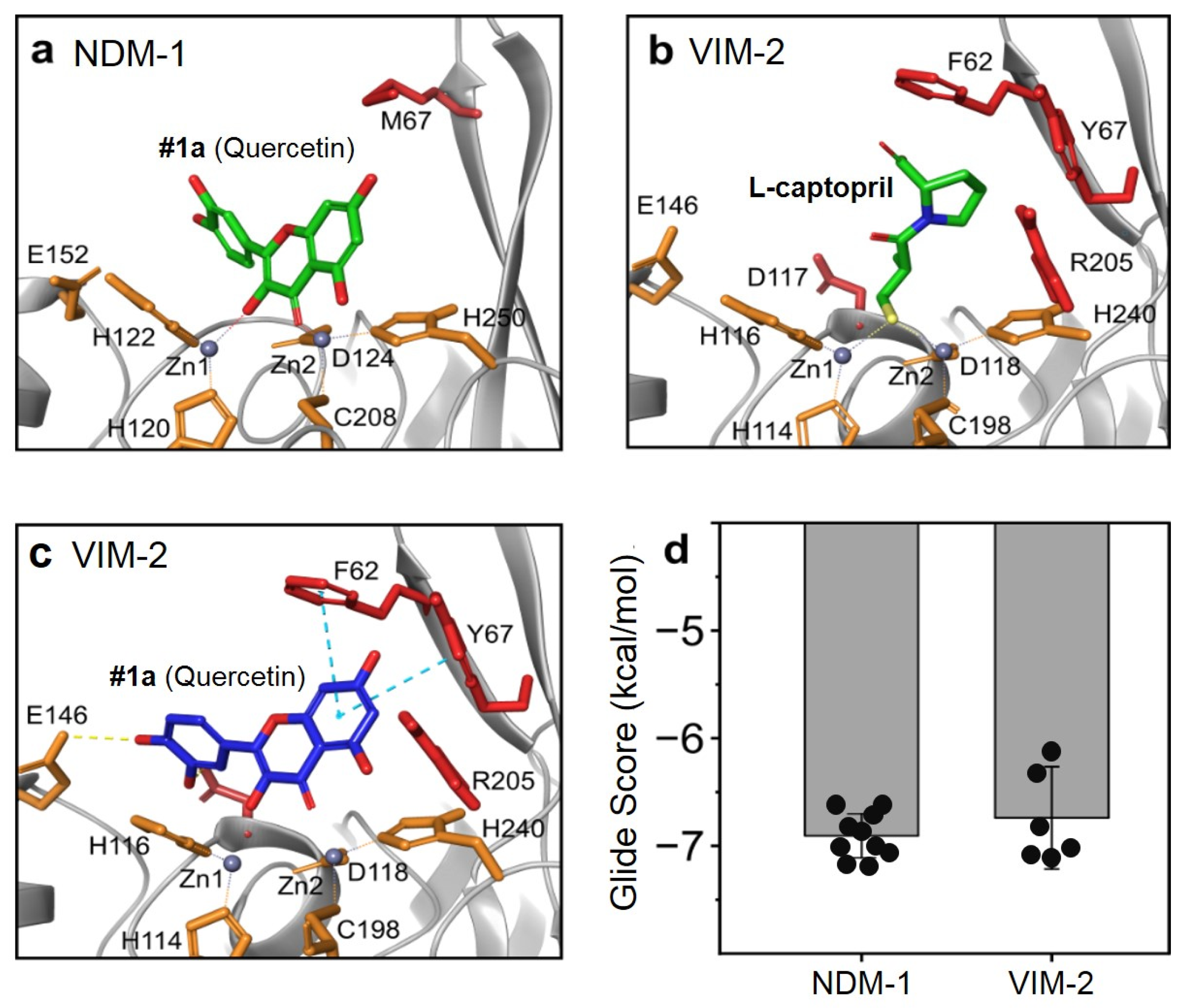
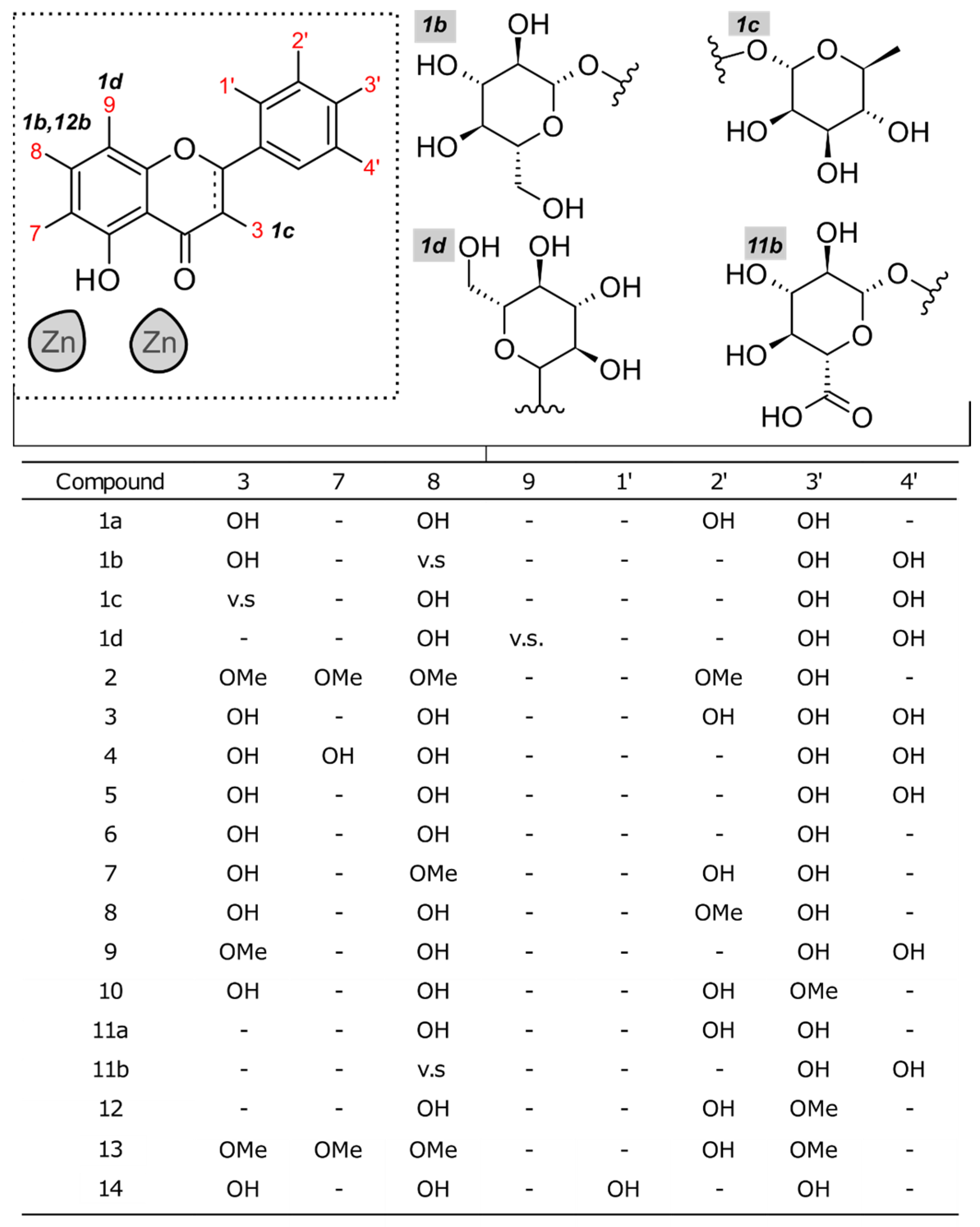
2.1. Docking Studies
2.2. Biochemical Assays
2.3. Bacteria Transformation
2.4. Disc Diffusion Assay
2.5. Single-Dose Inhibition Assay
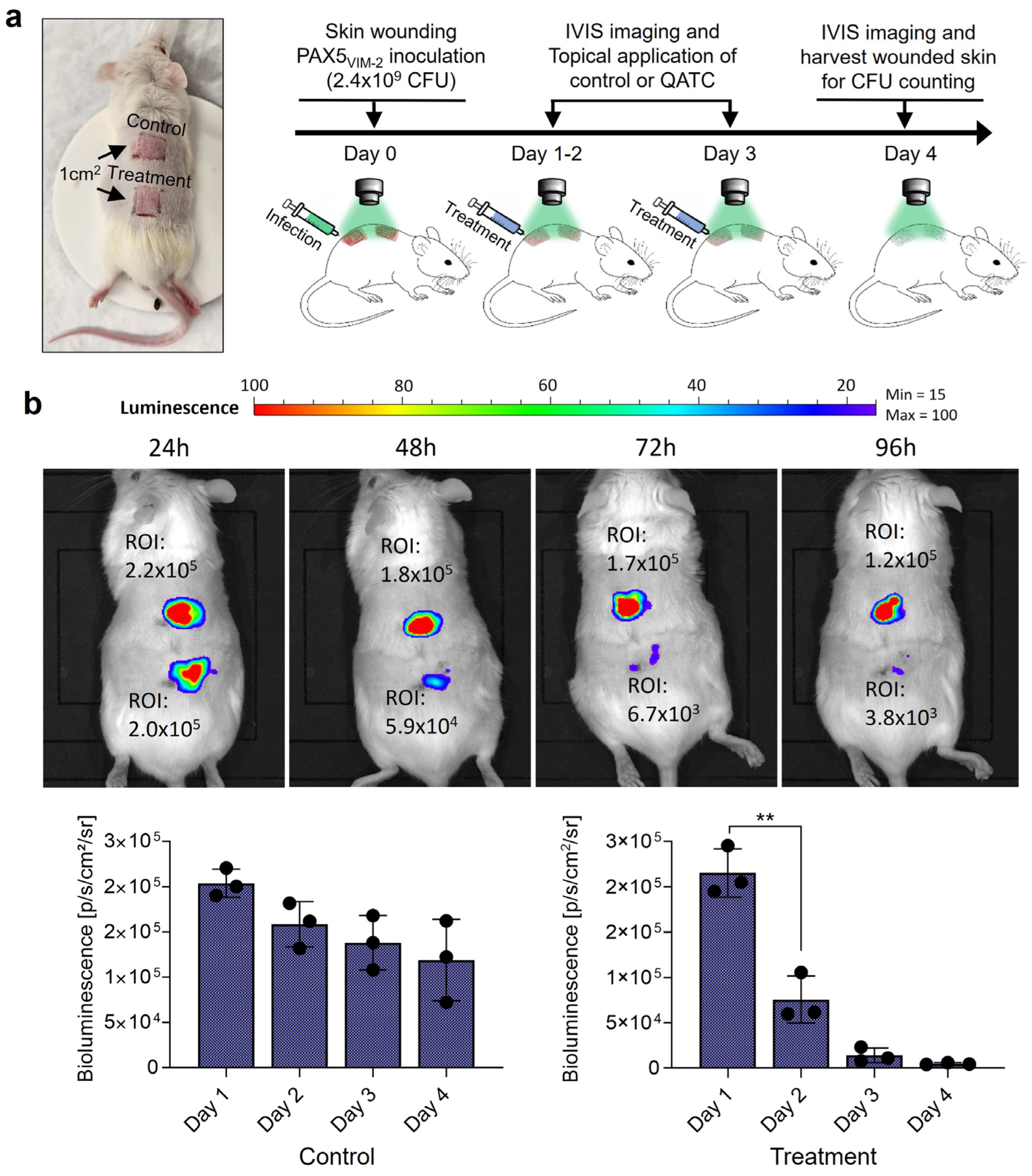
2.6. Luminescence Imaging
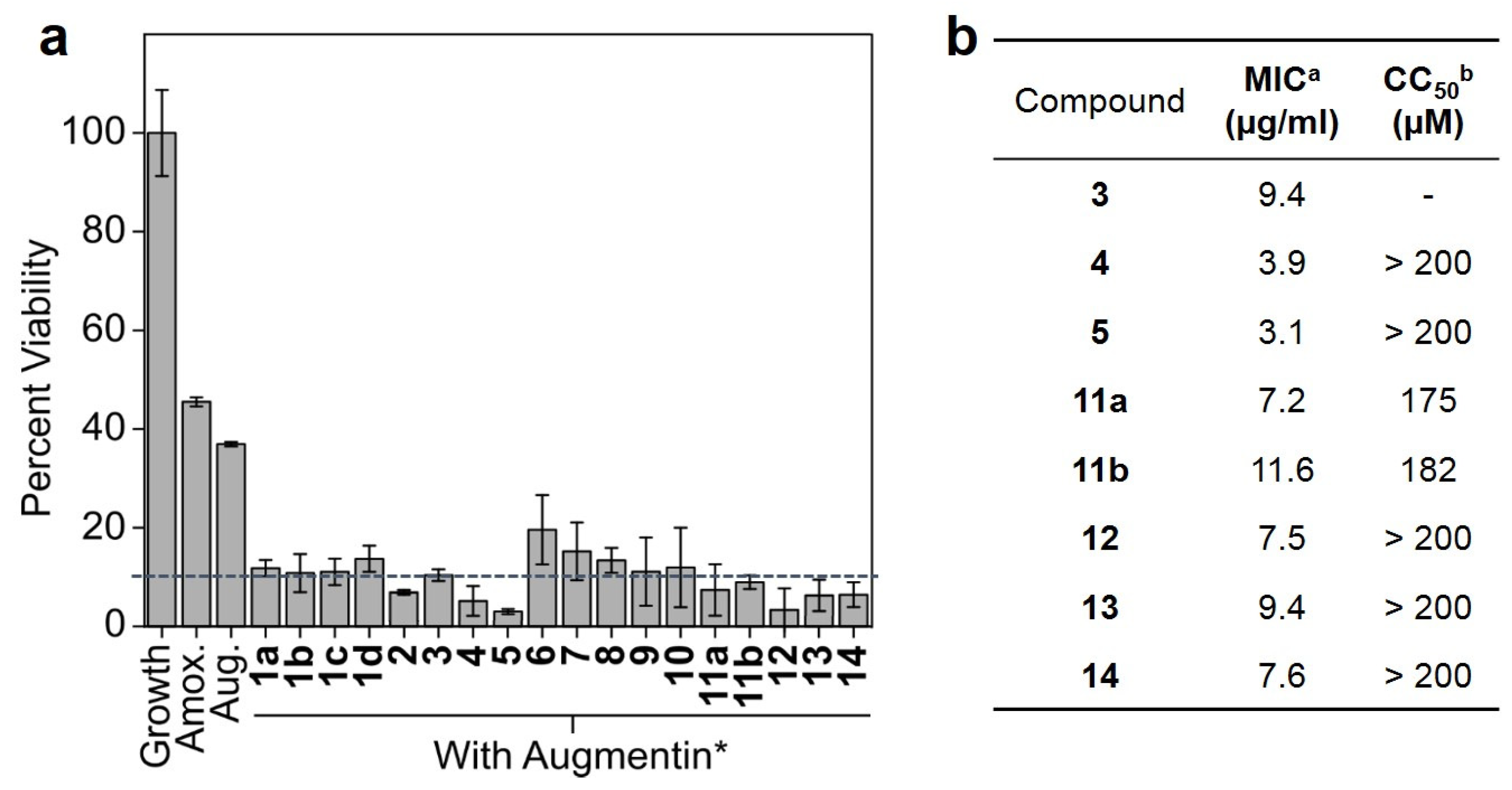
2.7. Minimum Inhibitory Concentration (MIC) Assay
2.8. In Vivo Treatment of the Murine Skin Wound Model
2.9. Statistical Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, R.E.; Hatfield, K.M.; Wolford, H.; Samore, M.H.; Scott, R.D.; Reddy, S.C.; Olubajo, B.; Paul, P.; Jernigan, J.A.; Baggs, J. National Estimates of Healthcare Costs Associated With Multidrug-Resistant Bacterial Infections Among Hospitalized Patients in the United States. Clin. Infect. Dis. 2021, 72, S17–S26. [Google Scholar] [CrossRef]
- Lisa, M.N.; Palacios, A.R.; Aitha, M.; Gonzalez, M.M.; Moreno, D.M.; Crowder, M.W.; Bonomo, R.A.; Spencer, J.; Tierney, D.L.; Llarrull, L.I.; et al. A general reaction mechanism for carbapenem hydrolysis by mononuclear and binuclear metallo-beta-lactamases. Nat. Commun. 2017, 8, 538. [Google Scholar] [CrossRef]
- Salimraj, R.; Hinchliffe, P.; Kosmopoulou, M.; Tyrrell, J.M.; Brem, J.; van Berkel, S.S.; Verma, A.; Owens, R.J.; McDonough, M.A.; Walsh, T.R.; et al. Crystal structures of VIM-1 complexes explain active site heterogeneity in VIM-class metallo-beta-lactamases. FEBS J. 2019, 286, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Carcione, D.; Siracusa, C.; Sulejmani, A.; Leoni, V.; Intra, J. Old and New Beta-Lactamase Inhibitors: Molecular Structure, Mechanism of Action, and Clinical Use. Antibiotics 2021, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.B.; Moussa, S.H.; McLeod, S.M.; Durand-Reville, T.; Miller, A.A. Durlobactam, a New Diazabicyclooctane beta-Lactamase Inhibitor for the Treatment of Acinetobacter Infections in Combination with Sulbactam. Front. Microbiol. 2021, 12, 709974. [Google Scholar] [CrossRef]
- Aitha, M.; Marts, A.R.; Bergstrom, A.; Moller, A.J.; Moritz, L.; Turner, L.; Nix, J.C.; Bonomo, R.A.; Page, R.C.; Tierney, D.L.; et al. Biochemical, mechanistic, and spectroscopic characterization of metallo-beta-lactamase VIM-2. Biochemistry 2014, 53, 7321–7331. [Google Scholar] [CrossRef] [PubMed]
- Mojica, M.F.; Rossi, M.-A.; Vila, A.J.; Bonomo, R.A. The urgent need for metallo-β-lactamase inhibitors: An unattended global threat. Lancet Infect. Dis. 2022, 22, e28–e34. [Google Scholar] [CrossRef]
- Ju, L.C.; Cheng, Z.; Fast, W.; Bonomo, R.A.; Crowder, M.W. The Continuing Challenge of Metallo-beta-Lactamase Inhibition: Mechanism Matters. Trends Pharmacol. Sci. 2018, 39, 635–647. [Google Scholar] [CrossRef]
- Nyborg, J.K.; Peersen, O.B. That zincing feeling: The effects of EDTA on the behaviour of zinc-binding transcriptional regulators. Biochem. J. 2004, 381, e3–e4. [Google Scholar] [CrossRef]
- Palzkill, T. Metallo-beta-lactamase structure and function. Ann. N. Y. Acad. Sci. 2013, 1277, 91–104. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Ding, S.; Murakami, E.; Imamura, K.; Fuchigami, S.; Hashiguchi, R.; Yutani, K.; Mori, H.; Suzuki, S.; Arakawa, Y.; et al. A demetallation method for IMP-1 metallo-beta-lactamase with restored enzymatic activity upon addition of metal ion(s). Chembiochem 2011, 12, 1979–1983. [Google Scholar] [CrossRef] [PubMed]
- Paul-Soto, R.; Bauer, R.; Frere, J.M.; Galleni, M.; Meyer-Klaucke, W.; Nolting, H.; Rossolini, G.M.; de Seny, D.; Hernandez-Valladares, M.; Zeppezauer, M.; et al. Mono- and binuclear Zn2+-beta-lactamase. Role of the conserved cysteine in the catalytic mechanism. J. Biol. Chem. 1999, 274, 13242–13249. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.; Reid-Yu, S.A.; Wang, W.; King, D.T.; De Pascale, G.; Strynadka, N.C.; Walsh, T.R.; Coombes, B.K.; Wright, G.D. Aspergillomarasmine A overcomes metallo-beta-lactamase antibiotic resistance. Nature 2014, 510, 503–506. [Google Scholar] [CrossRef]
- Rotondo, C.M.; Sychantha, D.; Koteva, K.; Wright, G.D. Suppression of beta-Lactam Resistance by Aspergillomarasmine A Is Influenced by both the Metallo-beta-Lactamase Target and the Antibiotic Partner. Antimicrob. Agents Chemother. 2020, 64, e01386-19. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, A.; Katko, A.; Adkins, Z.; Hill, J.; Cheng, Z.; Burnett, M.; Yang, H.; Aitha, M.; Mehaffey, M.R.; Brodbelt, J.S.; et al. Probing the Interaction of Aspergillomarasmine A with Metallo-beta-lactamases NDM-1, VIM-2, and IMP-7. ACS Infect. Dis. 2018, 4, 135–145. [Google Scholar] [CrossRef]
- Bahr, G.; Vitor-Horen, L.; Bethel, C.R.; Bonomo, R.A.; Gonzalez, L.J.; Vila, A.J. Clinical Evolution of New Delhi Metallo-beta-Lactamase (NDM) Optimizes Resistance under Zn(II) Deprivation. Antimicrob. Agents Chemother. 2018, 62, e01849-17. [Google Scholar] [CrossRef] [PubMed]
- Bahr, G.; Gonzalez, L.J.; Vila, A.J. Metallo-beta-lactamases in the Age of Multidrug Resistance: From Structure and Mechanism to Evolution, Dissemination, and Inhibitor Design. Chem. Rev. 2021, 121, 7957–8094. [Google Scholar] [CrossRef]
- Tsivkovski, R.; Totrov, M.; Lomovskaya, O. Biochemical Characterization of QPX7728, a New Ultrabroad-Spectrum Beta-Lactamase Inhibitor of Serine and Metallo-Beta-Lactamases. Antimicrob. Agents Chemother. 2020, 64, e00130-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Trout, R.E.L.; Chu, G.H.; McGarry, D.; Jackson, R.W.; Hamrick, J.C.; Daigle, D.M.; Cusick, S.M.; Pozzi, C.; De Luca, F.; et al. Discovery of Taniborbactam (VNRX-5133): A Broad-Spectrum Serine- and Metallo-beta-lactamase Inhibitor for Carbapenem-Resistant Bacterial Infections. J. Med. Chem. 2020, 63, 2789–2801. [Google Scholar] [CrossRef] [PubMed]
- Hecker, S.J.; Reddy, K.R.; Lomovskaya, O.; Griffith, D.C.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Sun, D.; Sabet, M.; Tarazi, Z.; et al. Discovery of Cyclic Boronic Acid QPX7728, an Ultrabroad-Spectrum Inhibitor of Serine and Metallo-beta-lactamases. J. Med. Chem. 2020, 63, 7491–7507. [Google Scholar] [CrossRef] [PubMed]
- Riviere, G.; Oueslati, S.; Gayral, M.; Crechet, J.B.; Nhiri, N.; Jacquet, E.; Cintrat, J.C.; Giraud, F.; van Heijenoort, C.; Lescop, E.; et al. NMR Characterization of the Influence of Zinc(II) Ions on the Structural and Dynamic Behavior of the New Delhi Metallo-beta-Lactamase-1 and on the Binding with Flavonols as Inhibitors. ACS Omega 2020, 5, 10466–10480. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, X.; Gao, Q.; Lv, C.; Xu, J.; Jin, H.; Zhu, H. Quercetin increases the antioxidant capacity of the ovary in menopausal rats and in ovarian granulosa cell culture in vitro. J. Ovarian Res. 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Maalik, A.; Khan, F.A.; Mumtaz, A.; Mehmood, A.; Azhar, S.; Atif, M.; Karim, S.; Altaf, Y.; Tariq, I. Pharmacological Applications of Quercetin and its Derivatives: A Short Review. Trop. J. Pharm. Res. 2014, 13, 1561–1566. [Google Scholar] [CrossRef]
- Halevas, E.; Mavroidi, B.; Pelecanou, M.; Hatzidimitriou, A.G. Structurally characterized zinc complexes of flavonoids chrysin and quercetin with antioxidant potential. Inorganica Chim. Acta 2021, 523, 120407. [Google Scholar] [CrossRef]
- Bhuiya, S.; Haque, L.; Pradhan, A.B.; Das, S. Inhibitory effects of the dietary flavonoid quercetin on the enzyme activity of zinc(II)-dependent yeast alcohol dehydrogenase: Spectroscopic and molecular docking studies. Int. J. Biol. Macromol. 2017, 95, 177–184. [Google Scholar] [CrossRef]
- Parellada, J.; Suarez, G.; Guinea, M. Inhibition of zinc metallopeptidases by flavonoids and related phenolic compounds: Structure-activity relationships. J. Enzym. Inhib. 1998, 13, 347–359. [Google Scholar] [CrossRef]
- Parellada, J.; Guinea, M. Flavonoid inhibitors of trypsin and leucine aminopeptidase: A proposed mathematical model for IC50 estimation. J. Nat. Prod. 1995, 58, 823–829. [Google Scholar] [CrossRef]
- Denny, B.J.; Lambert, P.A.; West, P.W. The flavonoid galangin inhibits the L1 metallo-beta-lactamase from Stenotrophomonas maltophilia. FEMS Microbiol. Lett. 2002, 208, 21–24. [Google Scholar] [CrossRef]
- Hossion, A.M.; Zamami, Y.; Kandahary, R.K.; Tsuchiya, T.; Ogawa, W.; Iwado, A.; Sasaki, K. Quercetin diacylglycoside analogues showing dual inhibition of DNA gyrase and topoisomerase IV as novel antibacterial agents. J. Med. Chem. 2011, 54, 3686–3703. [Google Scholar] [CrossRef]
- Vipin, C.; Saptami, K.; Fida, F.; Mujeeburahiman, M.; Rao, S.S.; Athmika; Arun, A.B.; Rekha, P.D. Potential synergistic activity of quercetin with antibiotics against multidrug-resistant clinical strains of Pseudomonas aeruginosa. PLoS ONE 2020, 15, e0241304. [Google Scholar] [CrossRef]
- Lin, R.D.; Chin, Y.P.; Hou, W.C.; Lee, M.H. The effects of antibiotics combined with natural polyphenols against clinical methicillin-resistant Staphylococcus aureus (MRSA). Planta Med. 2008, 74, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Sahyon, H.A.; Ramadan, E.N.M.; Mashaly, M.M.A. Synergistic Effect of Quercetin in Combination with Sulfamethoxazole as New Antibacterial Agent: In Vitro and In Vivo Study. Pharm. Chem. J. 2019, 53, 803–813. [Google Scholar] [CrossRef]
- Hirai, I.; Okuno, M.; Katsuma, R.; Arita, N.; Tachibana, M.; Yamamoto, Y. Characterisation of anti-Staphylococcus aureus activity of quercetin. Int. J. Food Sci. Technol. 2010, 45, 1250–1254. [Google Scholar] [CrossRef]
- Abreu, A.C.; Serra, S.C.; Borges, A.; Saavedra, M.J.; McBain, A.J.; Salgado, A.J.; Simoes, M. Combinatorial Activity of Flavonoids with Antibiotics Against Drug-Resistant Staphylococcus aureus. Microb. Drug Resist. 2015, 21, 600–609. [Google Scholar] [CrossRef]
- Siriwong, S.; Thumanu, K.; Hengpratom, T.; Eumkeb, G. Synergy and Mode of Action of Ceftazidime plus Quercetin or Luteolin on Streptococcus pyogenes. Evid. Based Complement. Altern. Med. 2015, 2015, 759459. [Google Scholar] [CrossRef]
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC Pharmacol. Toxicol. 2016, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Dai, C.; Shen, Z.; Tang, Q.; Wang, H.; Zhai, B.; Zhao, L.; Hao, Z. Mechanism of Synergy Between Tetracycline and Quercetin Against Antibiotic Resistant Escherichia coli. Front. Microbiol. 2019, 10, 2536. [Google Scholar] [CrossRef]
- Pal, A.; Tripathi, A. Demonstration of bactericidal and synergistic activity of quercetin with meropenem among pathogenic carbapenem resistant Escherichia coli and Klebsiella pneumoniae. Microb. Pathog. 2020, 143, 104120. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Jung, M.; Park, K.-H.; Chong, Y. Quercetin-Pivaloxymethyl Conjugate Potentiates Antibiotics againstPseudomonas aeruginosaandAcinetobacter baumannii. Bull. Korean Chem. Soc. 2018, 39, 879–881. [Google Scholar] [CrossRef]
- Poirel, L.; Naas, T.; Nicolas, D.; Collet, L.; Bellais, S.; Cavallo, J.-D.; Nordmann, P. Characterization of VIM-2, a Carbapenem-Hydrolyzing Metallo-β-Lactamase and Its Plasmid- and Integron-Borne Gene from a Pseudomonas aeruginosa Clinical Isolate in France. Antimicrob. Agents Chemother. 2000, 44, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Docquier, J.D.; Lamotte-Brasseur, J.; Galleni, M.; Amicosante, G.; Frere, J.M.; Rossolini, G.M. On functional and structural heterogeneity of VIM-type metallo-beta-lactamases. J. Antimicrob. Chemother. 2003, 51, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Woodford, N. The beta-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 2006, 14, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, S.; Bogaerts, P.; Dortet, L.; Bernabeu, S.; Ben Lakhal, H.; Longshaw, C.; Glupczynski, Y.; Naas, T. In vitro Activity of Cefiderocol and Comparators against Carbapenem-Resistant Gram-Negative Pathogens from France and Belgium. Antibiotics 2022, 11, 1352. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Chazotte, B. Labeling Golgi with fluorescent ceramides. Cold Spring Harb. Protoc. 2012, 2012. [Google Scholar] [CrossRef]
- Shannon, K.; Phillips, I. beta-Lactamase detection by three simple methods: Intralactam, nitrocefin and acidimetric. J. Antimicrob. Chemother. 1980, 6, 617–621. [Google Scholar] [CrossRef]
- Shin, W.S.; Bergstrom, A.; Bonomo, R.A.; Crowder, M.W.; Muthyala, R.; Sham, Y.Y. Discovery of 1-Hydroxypyridine-2(1H)-thione-6-carboxylic Acid as a First-in-Class Low-Cytotoxic Nanomolar Metallo beta-Lactamase Inhibitor. ChemMedChem 2017, 12, 845–849. [Google Scholar] [CrossRef]
- O’Callaghan, C.H.; Morris, A.; Kirby, S.M.; Shingler, A.H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1972, 1, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Woodall, C.A. Electroporation of E. coli. E. coli Plasmid Vectors: Methods and Applications; Humana Press: Totowa, NJ, USA, 2003; Volume 235. [Google Scholar]
- Bio-Rad. Impregnated Disks for Detecting Metallo-Β-Lactamase (MBL). 2012. Available online: https://commerce.bio-rad.com/webroot/web/pdf/inserts/CDG/en/67936_2012_10_EN.pdf (accessed on 23 January 2023).
- Yong, D.; Lee, K.; Yum, J.H.; Shin, H.B.; Rossolini, G.M.; Chong, Y. Imipenem-EDTA disk method for differentiation of metallo-beta-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 2002, 40, 3798–3801. [Google Scholar] [CrossRef]
- Pitout, J.D.; Gregson, D.B.; Poirel, L.; McClure, J.A.; Le, P.; Church, D.L. Detection of Pseudomonas aeruginosa producing metallo-beta-lactamases in a large centralized laboratory. J. Clin. Microbiol. 2005, 43, 3129–3135. [Google Scholar] [CrossRef]
- EUCAST. MIC Determination of Non-Fastidious and Fastidious Organisms. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination (accessed on 30 January 2023).
- CLSI. M100 Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Liu, Y.; Guo, M. Studies on transition metal-quercetin complexes using electrospray ionization tandem mass spectrometry. Molecules 2015, 20, 8583–8594. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, J.E. Molecular aspects on the interaction of quercetin and its metal complexes with DNA. Int. J. Biol. Macromol. 2011, 48, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Yildirim, S. Transformation of Acinetobacter baumannii: Electroporation. Acinetobacter Baumannii 2019, 1946, 69–74. [Google Scholar] [CrossRef]
- Pseudomonas Aeruginosa (ATCC® 19660™). Available online: https://genomes.atcc.org/genomes/4a6672c8744d4cdb?tab=annotations-tab (accessed on 13 February 2023).
- Leiros, H.K.; Edvardsen, K.S.; Bjerga, G.E.; Samuelsen, O. Structural and biochemical characterization of VIM-26 shows that Leu224 has implications for the substrate specificity of VIM metallo-beta-lactamases. FEBS J. 2015, 282, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.; Cheng, B.; Gao, L.; Qin, C.; Zhang, L.; Zhang, X.; Wang, J.; Wan, Y. Discovery of Quercetin and Its Analogs as Potent OXA-48 Beta-Lactamase Inhibitors. Front. Pharmacol. 2022, 13, 926104. [Google Scholar] [CrossRef]
- Kaye, K.S.; Petty, L.A.; Shorr, A.F.; Zilberberg, M.D. Current Epidemiology, Etiology, and Burden of Acute Skin Infections in the United States. Clin. Infect. Dis. 2019, 68, S193–S199. [Google Scholar] [CrossRef] [PubMed]
- Youn, C.; Archer, N.K.; Miller, L.S. Research Techniques Made Simple: Mouse Bacterial Skin Infection Models for Immunity Research. J. Investig. Dermatol. 2020, 140, 1488–1497.e1481. [Google Scholar] [CrossRef]
- Dryden, M.S. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 2010, 65 (Suppl. 3), iii35–iii44. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Kharkwal, G.B.; Tanaka, M.; Huang, Y.Y.; Bil de Arce, V.J.; Hamblin, M.R. Animal models of external traumatic wound infections. Virulence 2011, 2, 296–315. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Karimi, M.; Sadasivam, M.; Antunes-Melo, W.C.; Carrasco, E.; Hamblin, M.R. In-vivo monitoring of infectious diseases in living animals using bioluminescence imaging. Virulence 2018, 9, 28–63. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benin, B.M.; Hillyer, T.; Crugnale, A.S.; Fulk, A.; Thomas, C.A.; Crowder, M.W.; Smith, M.A.; Shin, W.S. Taxifolin as a Metallo-β-Lactamase Inhibitor in Combination with Augmentin against Verona Imipenemase 2 Expressing Pseudomonas aeruginosa. Microorganisms 2023, 11, 2653. https://doi.org/10.3390/microorganisms11112653
Benin BM, Hillyer T, Crugnale AS, Fulk A, Thomas CA, Crowder MW, Smith MA, Shin WS. Taxifolin as a Metallo-β-Lactamase Inhibitor in Combination with Augmentin against Verona Imipenemase 2 Expressing Pseudomonas aeruginosa. Microorganisms. 2023; 11(11):2653. https://doi.org/10.3390/microorganisms11112653
Chicago/Turabian StyleBenin, Bogdan M., Trae Hillyer, Aylin S. Crugnale, Andrew Fulk, Caitlyn A. Thomas, Michael W. Crowder, Matthew A. Smith, and Woo Shik Shin. 2023. "Taxifolin as a Metallo-β-Lactamase Inhibitor in Combination with Augmentin against Verona Imipenemase 2 Expressing Pseudomonas aeruginosa" Microorganisms 11, no. 11: 2653. https://doi.org/10.3390/microorganisms11112653
APA StyleBenin, B. M., Hillyer, T., Crugnale, A. S., Fulk, A., Thomas, C. A., Crowder, M. W., Smith, M. A., & Shin, W. S. (2023). Taxifolin as a Metallo-β-Lactamase Inhibitor in Combination with Augmentin against Verona Imipenemase 2 Expressing Pseudomonas aeruginosa. Microorganisms, 11(11), 2653. https://doi.org/10.3390/microorganisms11112653





