Analysis of Mould Exposure of Immunosuppressed Patients at a German University Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Vote
2.2. Airborne Microbial Measurement
2.3. Weather Data Collection
2.4. Detection of Aspergillus-Infections Using Galactomannan Antigen Detections
2.5. Laboratory Testing of Patient Specimen
2.6. Fungal Culture
2.7. Laboratory Panfungal, Aspergillus and Mucorales PCR Assays
2.8. Retrospective Analysis of Nosocomial Aspergillus Infections in Immunocompromised Patients
3. Results
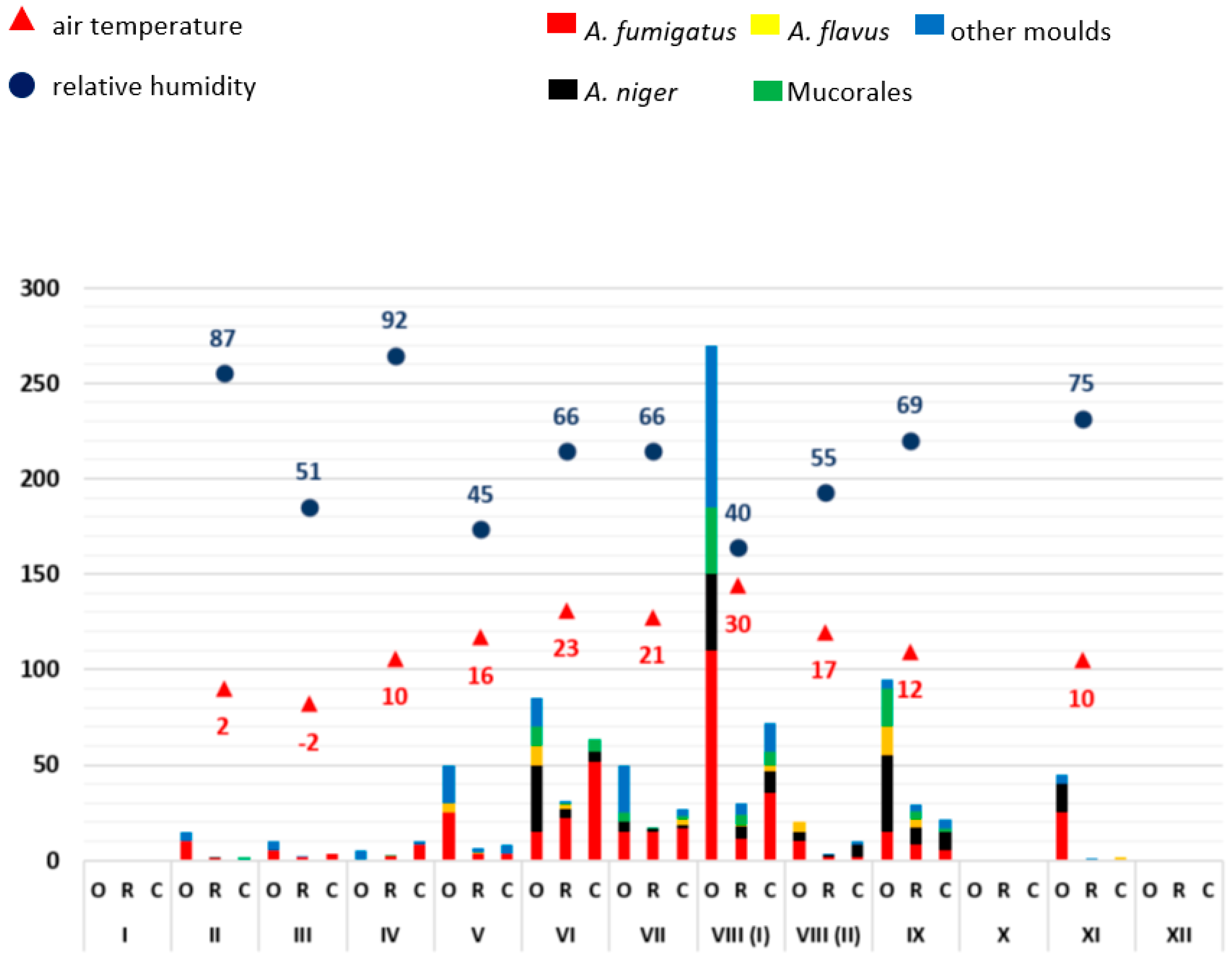
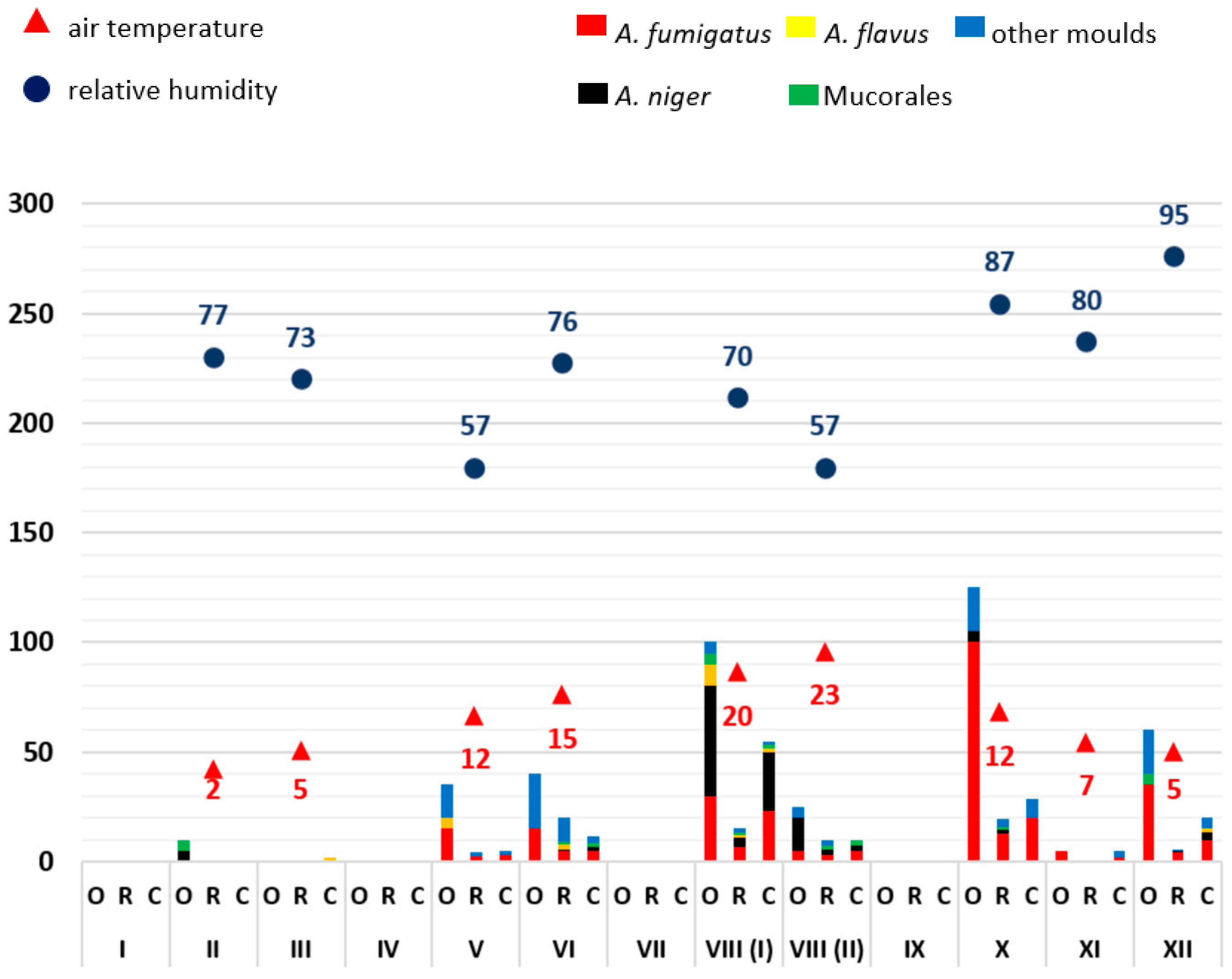
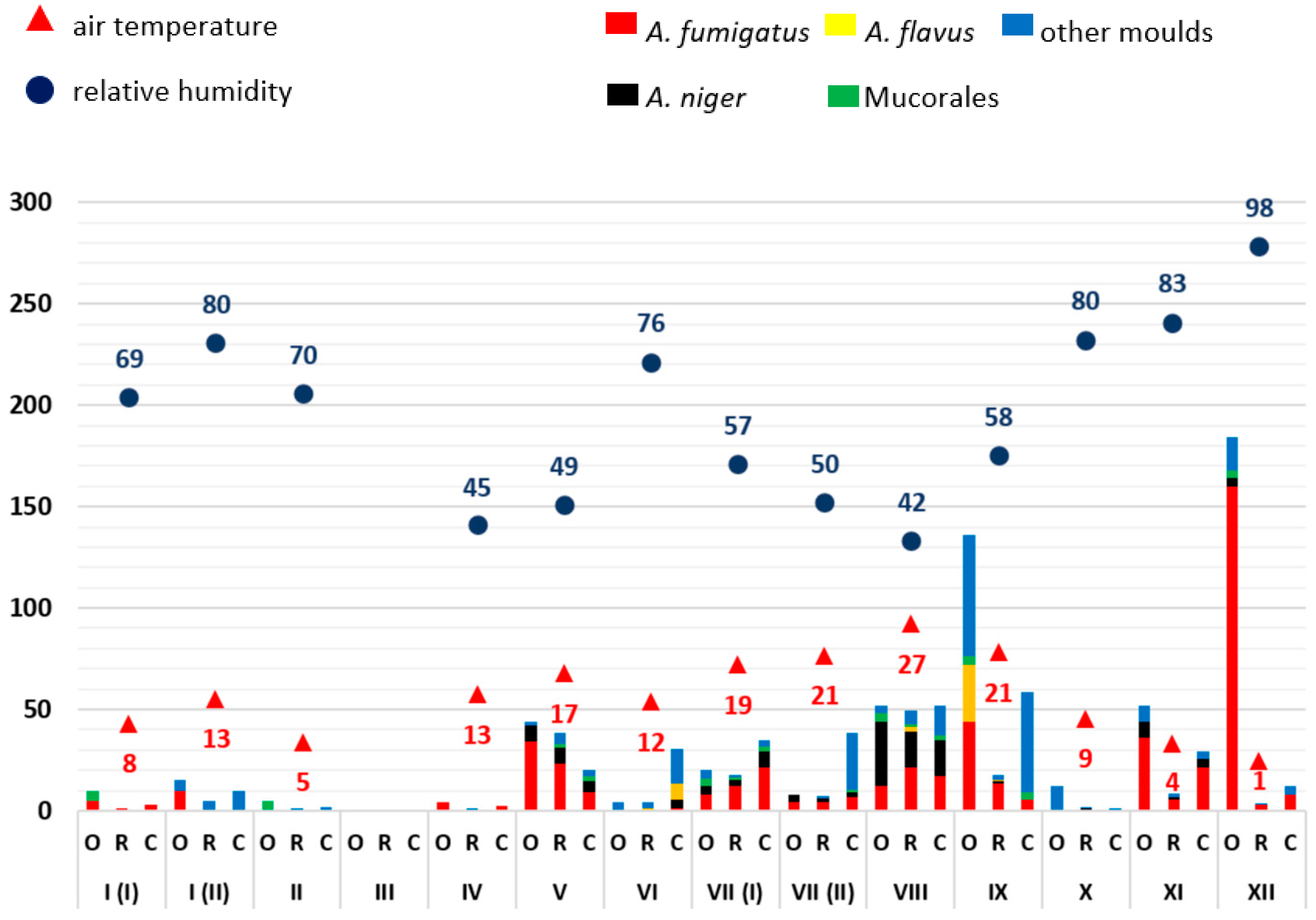
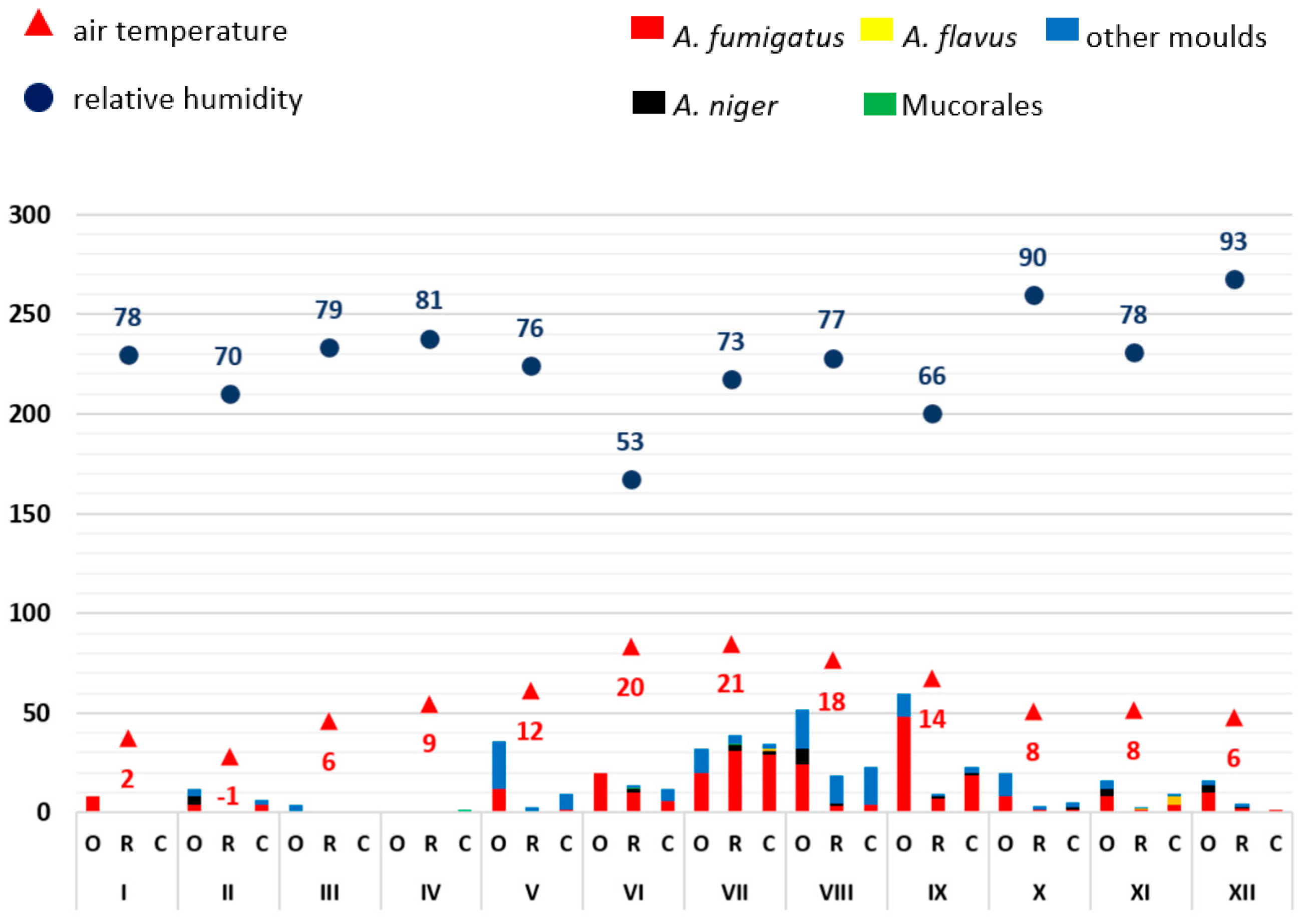
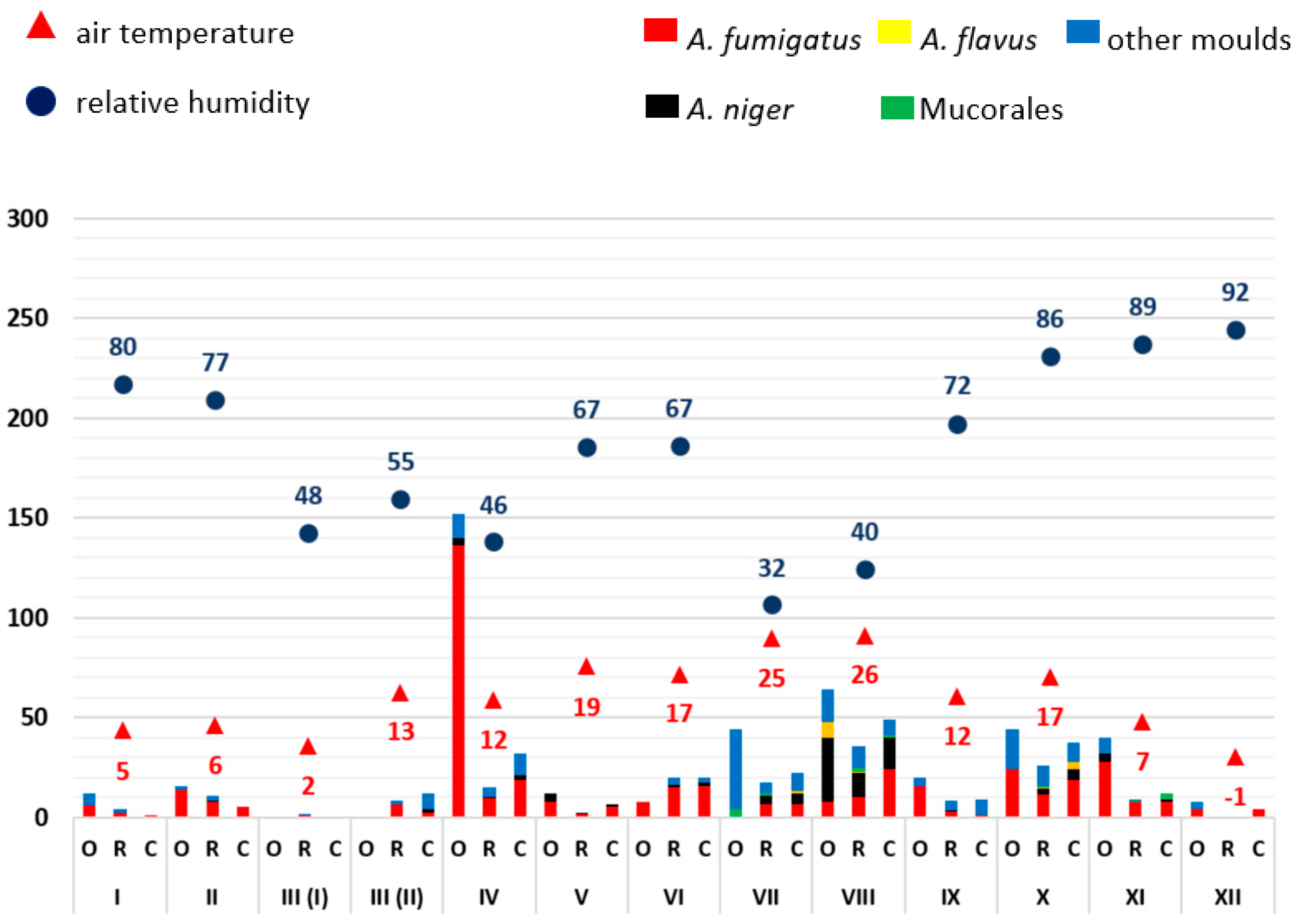
3.1. Monthly Mould Load Depending on Temperature and Air Humidity
3.2. Analysis of the Frequency of Aspergillus Antigen Detection and Mould Infections
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robert Koch-Institut (RKI). Schimmelpilzbelastung in Innenräumen—Befunderhebung, gesundheitliche Bewertung und Maßnahmen. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2007, 50, 1308–1323. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.; Yagüe, G.; Segovia, M.; Catalán, V. A study of air microbe levels in different areas of a hospital. Curr. Microbiol. 2009, 59, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kolstad, H.A.; Brauer, C.; Iversen, M.; Sigsgaard, T.; Mikkelsen, S. Do indoor molds in nonindustrial environments threaten workers’ health? A review of the epidemiologic evidence. Epidemiol. Rev. 2002, 24, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Innenraumlufthygiene-Kommission des Umweltbundesamtes. Leitfaden zur Vorbeugung, Erfassung und Sanierung von Schimmelbefall in Gebäuden (Schimmelpilz-Leitfaden); Umweltbundesamt: Berlin, Germany, 2017; Available online: https://www.umweltbundesamt.de/www.umweltbundesamt.de/schimmelleitfaden (accessed on 22 October 2023).
- Chabi, M.L.; Goracci, A.; Roche, N.; Paugam, A.; Lupo, A.; Revel, M.P. Pulmonary aspergillosis. Diagn. Interv. Imaging. 2015, 96, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Wiesmüller, G.A.; Heinzow, B.; Aurbach, U.; Bergmann, K.-C.; Bufe, A.; Buzina, W.; Cornely, O.A.; Engelhart, S.; Fischer, G.; Gabrio, T.; et al. S2k-Leitlinie Medizinisch Klinische Diagnostik bei Schimmelpilzexposition in Innenräumen; AWMF-Register-Nr. 161/001, Stand 11.04.2016, Gültig bis 10.04.2021. Available online: https://register.awmf.org/de/leitlinien/detail/161-001 (accessed on 4 April 2023).
- Bundesanstalt für Arbeitsschutz und Arbeitsmedizin. Technische Regeln für Biologische Arbeitsstoffe—Einstufung von Pilzen in Risikogruppen (TRBA 460), Juli 2016 GMBl. Nr. 29/30 vom 22.07.2016, S. 562, 5. Änderung: GMBl. Nr. 16-24 vom 20.03.2023, S. 330. Available online: https://www.baua.de/DE/Angebote/Regelwerk/TRBA/TRBA-460.html (accessed on 22 October 2023).
- Denham, S.T.; Wambaugh, M.A.; Brown, J.C.S. How Environmental Fungi Cause a Range of Clinical Outcomes in Susceptible Hosts. J. Mol. Biol. 2019, 431, 2982–3009. [Google Scholar] [CrossRef] [PubMed]
- Kommission für Krankenhaushygiene und Infektionsprävention beim Robert Koch-Institut (RKI). Anforderungen an die Hygiene bei der medizinischen Versorgung von immunsupprimierten Patienten. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2010, 53, 357–388. [Google Scholar] [CrossRef] [PubMed]
- Rácil, Z.; Kocmanová, I.; Wagnerová, B.; Winterová, J.; Mayer, J. Vyuzití detekce galaktomannanu pro casnou diagnostiku invazivní aspergilózy [Contribution of galactomannan antigen detection to early diagnosis of invasive aspergillosis]. Klin. Mikrobiol. Infekc. Lek. 2007, 13, 176–183. [Google Scholar] [PubMed]
- Einsele, H.; Loeffler, J. Contribution of new diagnostic approaches to antifungal treatment plans in high-risk haematology patients. Clin. Microbiol. Infect. 2008, 14 (Suppl. S4), 37–45. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, V.; Walsh, T.J.; Hope, W.; Cortez, K.J. Galactomannan antigen detection in the diagnosis of invasive aspergillosis. Expert Rev. Mol. Diagn. 2007, 7, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Foy, P.C.; van Burik, J.A.; Weisdorf, D.J. Galactomannan antigen enzyme-linked immunosorbent assay for diagnosis of invasive aspergillosis after hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2007, 13, 440–443. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bundesministerium für Arbeit und Soziales. Verordnung über Sicherheit und Gesundheitsschutz bei Tätigkeiten mit Biologischen Arbeitsstoffen (Biostoffverordnung—BioStoffV). 2013. Available online: https://www.gesetze-im-internet.de/biostoffv_2013/BJNR251410013.html (accessed on 4 April 2023).
- Feller, W. An Introduction to Probability Theory and Its Applications. Band 1; Wiley: New York, NY, USA, 1950; ISBN 0-471-25708-7. [Google Scholar]
- Deutscher Wetterdienst (DWD). Bundesoberbehörde im Geschäftsbereich des Bundesministeriums für Verkehr und Digitale Infrastruktur, Frankfurter Straße 135, 63067 Offenbach, (CDC—Clima Data Center). Available online: https://cdc.dwd.de/portal/202209231028/view1 (accessed on 4 April 2023).
- Arndt, T. Galaktomannan-Test. In Lexikon der Medizinischen Laboratoriumsdiagnostik; Gressner, A.M., Arndt, T., Eds.; Springer Reference Medizin; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Haase, G.; Hamprecht, A.; Held, J.; Kurzai, O.; Rath, P.M.; Schwarz, R.; Steinmann, J.; Bader, O.; Behrens-Baumann, W.; Gräser, Y.; et al. Mikrobiologisch-Infektiologische Qualitätsstandards (MiQ) der Deutschen Gesellschaft für Hygiene und Mikrobiologie (DGHM) e.v., Pilzinfektionen Teil I und II, 14-15/2021; Urban & Fischer: München, Germany; Jena, Germany, 2021. [Google Scholar]
- Khot, P.D.; Ko, D.L.; Fredricks, D.N. Sequencing and analysis of fungal rRNA operons for development of broad-range fungal PCR assays. Appl. Environ. Microbiol. 2009, 75, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.; Goldenberger, D.; Schmidt, F.; Weisser, M.; Wehrle-Wieland, E.; Einsele, H.; Frei, R.; Löffler, J. Development and application of two independent real-time PCR assays to detect clinically relevant Mucorales species. J. Med. Microbiol. 2016, 65, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch-Institut (RKI); Nationales Referenzzentrum für Surveillance (NRZ). Definitionen nosokomialer Infektionen für die Surveillance im Krankenhaus-Infektions-Surveillance-System (KISS-Definitionen). 07/2017. Available online: https://www.rki.de/DE/Content/Infekt/Krankenhaushygiene/Nosokomiale_Infektionen/Downloads/RKI_Definitionen_nosokomialer_Infektionen_E-Book.pdf?__blob=publicationFile (accessed on 22 October 2023).
- van Burik, J.A.; Magee, P.T. Aspects of fungal pathogenesis in humans. Annu. Rev. Microbiol. 2001, 55, 743–772. [Google Scholar] [CrossRef] [PubMed]
- Belizario, J.A.; Lopes, L.G.; Pires, R.H. Fungi in the indoor air of critical hospital areas: A review. Aerobiologia 2021, 37, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Martins-Diniz, J.N.; da Silva, R.A.; Miranda, E.T.; Mendes-Giannini, M.J. Monitoramento de fungos anemófilos e de leveduras em unidade hospitalar [Monitoring of airborne fungus and yeast species in a hospital unit]. Rev. Saude Publica 2005, 39, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.Y.; Burge, H.A.; Chang, J.C. Review of quantitative standards and guidelines for fungi in indoor air. J. Air Waste Manag. Assoc. 1996, 46, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Viscoli, C.; Castagnola, E. Geoclimatic factors and invasive aspergillosis after allogeneic hematopoietic stem cell transplantation: New perspectives for patient management? Clin. Infect. Dis. 2010, 50, 1598–1600. [Google Scholar] [CrossRef] [PubMed]
| 2018 | |||||||||||||
| Month | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | Total |
| Patients a (n) | 87 | 74 | 99 | 84 | 86 | 75 | 93 | 85 | 79 | 88 | 79 | 77 | 1006 |
| Patient days a (n) | 715 | 631 | 804 | 680 | 739 | 756 | 852 | 732 | 725 | 867 | 841 | 676 | 9018 |
| Aspergillus AG detection (n) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Aspergillus culture pos. (n) | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Aspergillus PCR pos. (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucorales PCR pos. (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mould infections NI b (n) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Mould infections EORTC (n) c | 0 | 0 | 0 | 0 | 0 | 1 d | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 2019 | |||||||||||||
| Month | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | Total |
| Patients a (n) | 70 | 71 | 76 | 77 | 74 | 76 | 75 | 79 | 77 | 77 | 86 | 81 | 919 |
| Patient days a (n) | 724 | 735 | 794 | 810 | 789 | 743 | 735 | 852 | 885 | 713 | 844 | 729 | 9353 |
| Aspergillus AG detection (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aspergillus culture pos. (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 |
| Aspergillus PCR pos. (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucorales PCR pos. (n) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Mould infections NI b (n) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Mould infections EORTC (n) c | 0 | 0 | 0 | 0 | 0 | 0 | 1 d | 0 | 0 | 0 | 1 d | 0 | 2 |
| 2020 | |||||||||||||
| Month | 01 | 02 | 03 | 04 e | 05 e | 06 | 07 | 08 | 09 | 10 | 11 | 12 | Total |
| Patients a (n) | 77 | 68 | 87 | 70 | 74 | 77 | 81 | 62 | 60 | 69 | 50 | 56 | 831 |
| Patient days a (n) | 789 | 744 | 800 | 642 | 663 | 689 | 777 | 764 | 698 | 790 | 414 | 567 | 8337 |
| Aspergillus AG detection (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Aspergillus culture pos. (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Aspergillus PCR pos. (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Mucorales PCR pos. (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mould infections NI b (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Mould infections EORTC (n) c | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 d | 0 | 0 | 0 | 0 | 1 |
| 2021 | |||||||||||||
| Month | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 f | Total |
| Patients a (n) | 56 | 52 | 70 | 64 | 61 | 75 | 74 | 79 | 80 | 88 | 49 | 40 | 788 |
| Patient days a (n) | 590 | 532 | 686 | 616 | 613 | 792 | 693 | 792 | 861 | 841 | 508 | 269 | 7793 |
| Aspergillus AG detection (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aspergillus culture pos. (n) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| Aspergillus PCR pos. (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucorales PCR pos. (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mould infections NI b (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mould infections EORTC (n) c | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2022 | |||||||||||||
| Month | 01 f | 02 f | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | Total |
| Patients a (n) | 56 | 45 | 37 | 46 | 59 | 75 | 76 | 59 | 71 | 71 | 80 | 80 | 755 |
| Patient days a (n) | 470 | 388 | 263 | 388 | 601 | 689 | 793 | 614 | 731 | 630 | 692 | 740 | 6999 |
| Aspergillus AG detection (n) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 3 |
| Aspergillus culture pos. (n) | 0 | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 5 |
| Aspergillus PCR pos. (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 3 |
| Mucorales PCR pos. (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mould infections NI b (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mould infections EORTC (n) c | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 g | 0 | 0 | 0 | 1 d | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puhlmann, D.; Bergmann, D.; Besier, S.; Hogardt, M.; Wichelhaus, T.A.; Langhans, S.; Hack, D.; Reinheimer, C.; Vehreschild, M.J.G.T.; Jung, J.; et al. Analysis of Mould Exposure of Immunosuppressed Patients at a German University Hospital. Microorganisms 2023, 11, 2652. https://doi.org/10.3390/microorganisms11112652
Puhlmann D, Bergmann D, Besier S, Hogardt M, Wichelhaus TA, Langhans S, Hack D, Reinheimer C, Vehreschild MJGT, Jung J, et al. Analysis of Mould Exposure of Immunosuppressed Patients at a German University Hospital. Microorganisms. 2023; 11(11):2652. https://doi.org/10.3390/microorganisms11112652
Chicago/Turabian StylePuhlmann, Danuta, Dominic Bergmann, Silke Besier, Michael Hogardt, Thomas A. Wichelhaus, Sabine Langhans, Daniel Hack, Claudia Reinheimer, Maria J. G. T. Vehreschild, Jens Jung, and et al. 2023. "Analysis of Mould Exposure of Immunosuppressed Patients at a German University Hospital" Microorganisms 11, no. 11: 2652. https://doi.org/10.3390/microorganisms11112652
APA StylePuhlmann, D., Bergmann, D., Besier, S., Hogardt, M., Wichelhaus, T. A., Langhans, S., Hack, D., Reinheimer, C., Vehreschild, M. J. G. T., Jung, J., & Kempf, V. A. J. (2023). Analysis of Mould Exposure of Immunosuppressed Patients at a German University Hospital. Microorganisms, 11(11), 2652. https://doi.org/10.3390/microorganisms11112652





