Effects of Maize–Crop Rotation on Soil Physicochemical Properties, Enzyme Activities, Microbial Biomass and Microbial Community Structure in Southwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Soil Sampling
2.3. Soil Physicochemical Property Analysis
2.4. Soil Enzyme Activity and Microbial Biomass Analysis
2.5. Soil Bacterial and Fungal DNA Extraction and Sequencing
2.6. Statistical Analysis
3. Results
3.1. Soil Physical and Chemical Properties
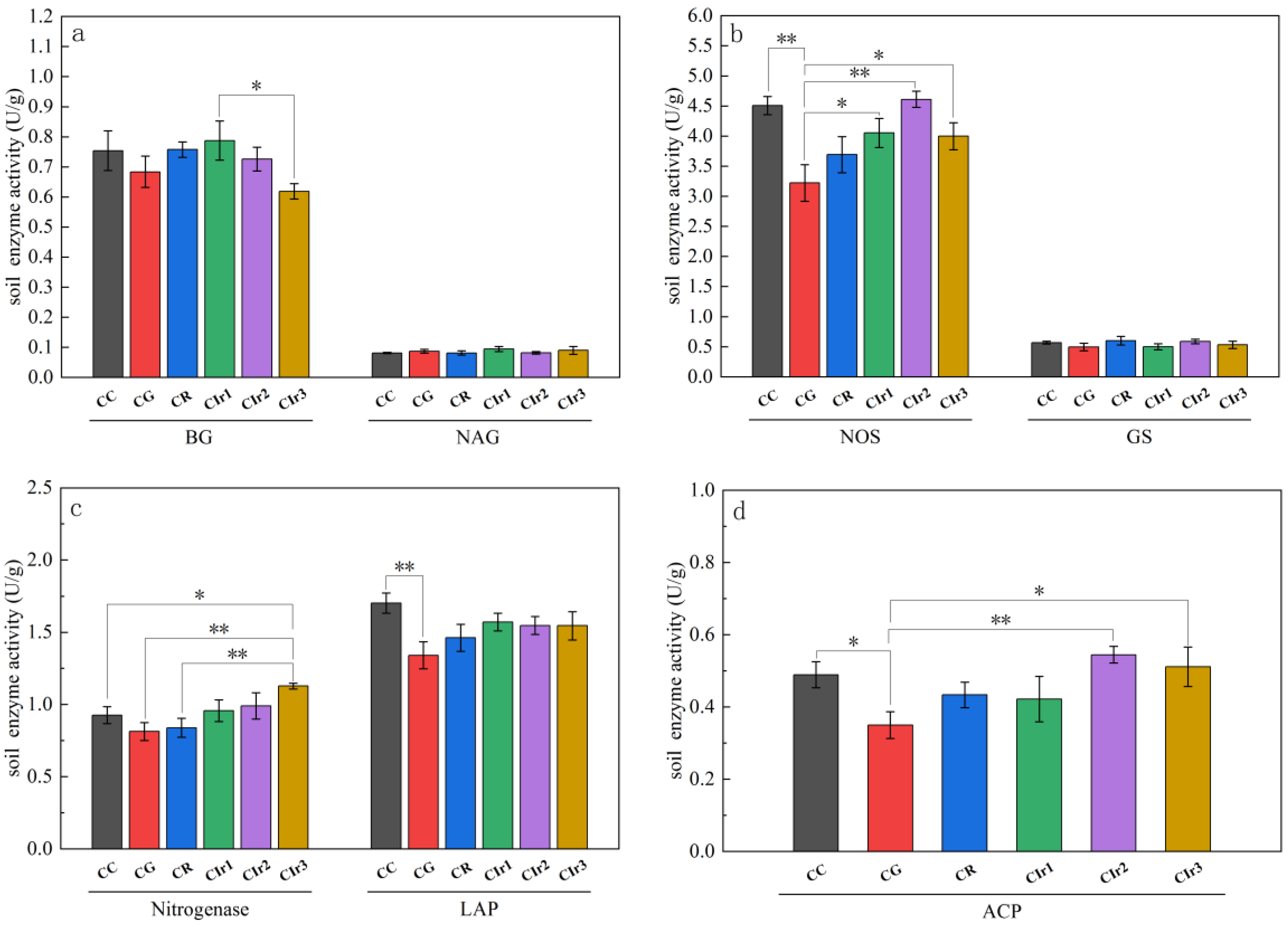
3.2. Soil Enzyme Activity
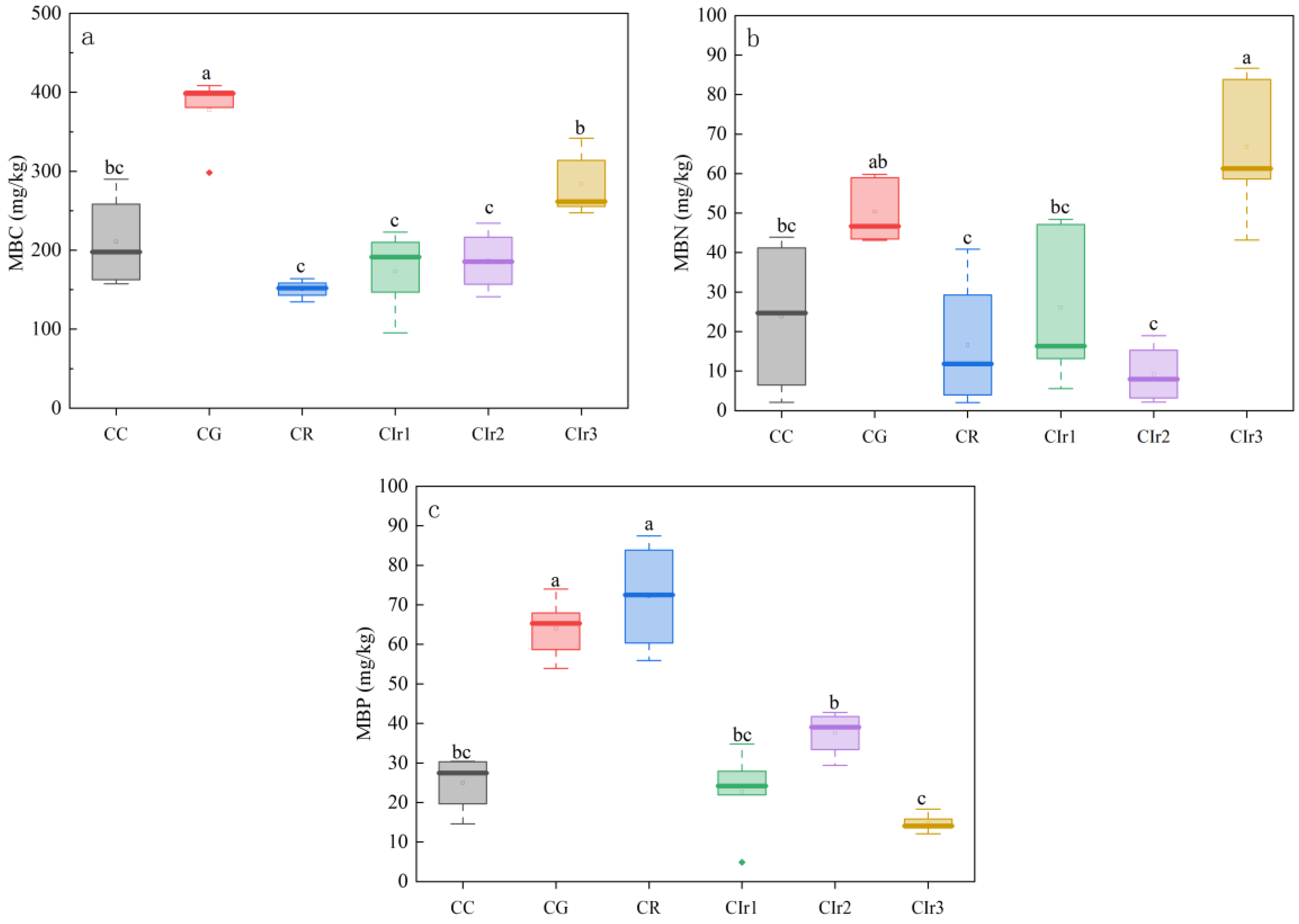
3.3. Soil Microbial Biomass
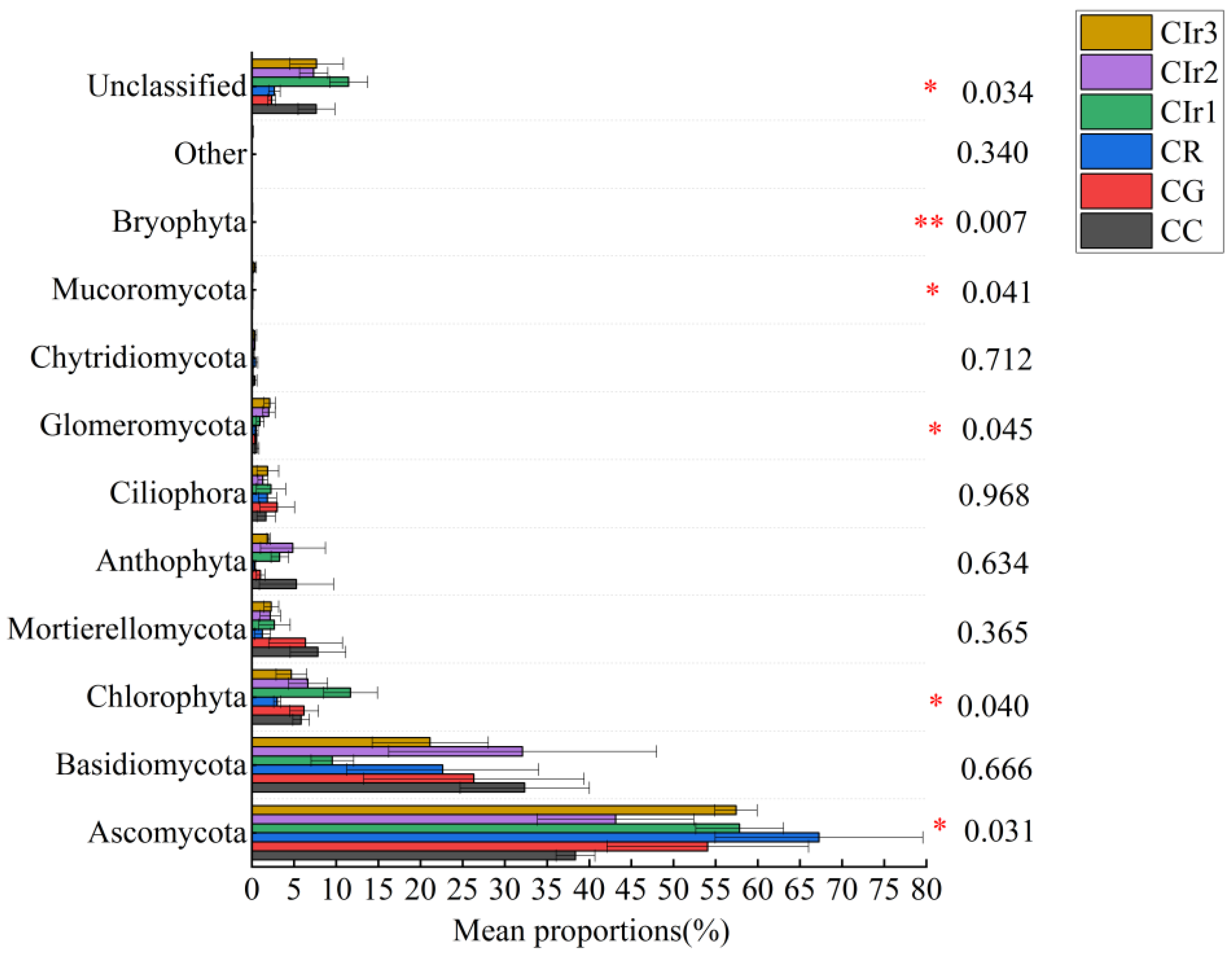
3.4. Microbial Community Diversity and Structure
3.5. Correlation of Soil Physical and Chemical Properties with Microbial Taxa Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salika, R.; Riffat, J. Abiotic stress responses in maize: A review. Acta Physiol. Plant. 2021, 43, 130. [Google Scholar] [CrossRef]
- Manisha, K.; Ruchi, S.; Devina, V.; Anil, G.; Harpreet, K.S.; Anupama, A.; Chahat, T.; Aastha, V.; Manisha, T.; Priyanka; et al. Maize: An underexploited golden cereal crop. Cereal Res. Commun. 2023, 51, 3–14. [Google Scholar] [CrossRef]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Q.; Zhou, Q.; Wang, C.; Bellingrath-Kimura, S.D.; Wu, W. Mapping the complex crop rotation systems in Southern China considering cropping intensity, crop diversity, and their seasonal dynamics. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2022, 15, 9584–9598. [Google Scholar] [CrossRef]
- Yao, Q.L.; Chen, F.B.; Liu, H.F.; Fang, P. Evolution of maize landraces in southwest China: Evidence from the globulin1 gene. Biochem. Syst. Ecol. 2015, 61, 54–61. [Google Scholar] [CrossRef]
- Meng, C.; Liu, H.; Wang, Y.; Li, Y.; Zhou, J.; Zhou, P.; Liu, X.; Li, Y.; Wu, J. Response of regional agricultural soil phosphorus status to net anthropogenic phosphorus input (NAPI) determined by soil pH value and organic matter content in subtropical China. Chemosphere 2018, 200, 487–494. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, W.; Wang, X.; Zhang, L.; Zhang, W.; Liu, D.; Chen, X. Agronomic, environmental, and ecosystem economic benefits of controlled-release nitrogen fertilizers for maize production in Southwest China. J. Clean. Prod. 2021, 312, 127611. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; Zhang, J.; Coulter, J.A.; Li, L.; Gan, Y. Diversifying crop rotation improves system robustness. Agron. Sustain. Dev. 2019, 39, 38. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, W.; Wang, X.; Lu, M.; Chadwick, D.; Zhang, Z.; Chen, X. Carbon footprint of maize production in tropical/subtropical region: A case study of Southwest China. Environ. Sci. Pollut. Res. 2021, 28, 28680–28691. [Google Scholar] [CrossRef]
- Garbelini, L.G.; Debiasi, H.; Junior, A.A.B.; Franchini, J.C.; Coelho, A.E.; Telles, T.S. Diversified crop rotations increase the yield and economic efficiency of grain production systems. Eur. J. Agron. 2022, 137, 126528. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Hazra, K.K.; Venkatesh, M.S.; Praharaj, C.S.; Kumar, N.; Nath, C.P.; Singh, U.; Singh, S.S. Grain legume inclusion in cereal–cereal rotation increased base crop productivity in the long run. Exp. Agric. 2020, 56, 142–158. [Google Scholar] [CrossRef]
- Yuan, M.; Bi, Y.D.; Han, D.W.; Wang, L.; Wang, L.X.; Fan, C.; Zhang, D.; Wang, Z.; Liang, W.W.; Zhu, Z.J.; et al. Long-term corn–soybean rotation and soil fertilization: Impacts on yield and agronomic traits. Agronomy 2022, 12, 2554. [Google Scholar] [CrossRef]
- Bak, G.R.; Lee, G.J.; Lee, J.T.; Jee, S.N. Crop rotation affects biological properties of rhizosphere soil and productivity of Kimchi cabbage (Brassica rapa ssp. pekinensis) compared to monoculture. Hortic. Environ. Biotechnol. 2022, 63, 613–625. [Google Scholar] [CrossRef]
- Rose, M.; Pahlmann, I.; Kage, H. Modified crop rotations for a sustainable intensification? A case study in a high-yielding environment with recurrent nitrogen surplus. Eur. J. Agron. 2023, 142, 126644. [Google Scholar] [CrossRef]
- Duan, C.; Shi, P.; Zong, N.; Wang, J.; Song, M.; Zhang, X. Feeding solution: Crop-livestock integration via crop-forage rotation in the southern Tibetan Plateau. Agric. Ecosyst. Environ. 2019, 284, 106589. [Google Scholar] [CrossRef]
- Holman, J.D.; Schlegel, A.; Obour, A.K.; Assefa, Y. Dryland cropping system impact on forage accumulation, nutritive value, and rainfall use efficiency. Crop Sci. 2020, 60, 3395–3409. [Google Scholar] [CrossRef]
- Koyama, A.; Dias, T.; Antunes, P.M. Application of plant–soil feedbacks in the selection of crop rotation sequences. Ecol. Appl. 2021, 32, e2501. [Google Scholar] [CrossRef]
- Degani, O.; Gordani, A.; Becher, P.; Chen, A.; Rabinovitz, O. Crop Rotation and minimal millage selectively affect maize growth promotion under Late Wilt Disease Stress. J. Fungi 2022, 8, 586. [Google Scholar] [CrossRef]
- Wang, P.C.; Ding, L.L.; Zou, C.; Zhang, Y.J.; Wang, M.Y. Rhizosphere element circling, multifunctionality, aboveground productivity and trade-offs are better predicted by rhizosphere rare taxa. Front. Plant Sci. 2022, 13, 985574. [Google Scholar] [CrossRef]
- Borase, D.; Nath, C.; Hazra, K.; Senthilkumar, M.; Singh, S.; Praharaj, C.; Singh, U.; Kumar, N. Long-term impact of diversified crop rotations and nutrient management practices on soil microbial functions and soil enzymes activity. Ecol. Indic. 2020, 114, 106322. [Google Scholar] [CrossRef]
- Song, D.L.; Dai, X.L.; Guo, T.F.; Cui, J.W.; Zhou, W.; Huang, S.M.; Shen, J.B.; Liang, G.Q.; He, P.; Wang, X.B.; et al. Organic amendment regulates soil microbial biomass and activity in wheat-maize and wheat-soybean rotation systems. Agric. Ecosyst. Environ. 2022, 333, 107974. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Wei, M.; Wang, Y.; Liang, Z.; Xia, P. Appropriate crop rotation alleviates continuous cropping barriers by changing rhizosphere microorganisms in Panax notoginseng. Rhizosphere 2022, 23, 100568. [Google Scholar] [CrossRef]
- Sofi, J.A.; Lone, A.H.; Ganie, M.A.; Dar, N.A.; Bhat, S.A.; Mukhtar, M.; Dar, M.A.; Ramzan, S. Soil microbiological activity and carbon dynamics in the current climate change scenarios: A review. Pedosphere 2016, 26, 577–591. [Google Scholar] [CrossRef]
- Donhauser, J.; Frey, B. Alpine soil microbial ecology in a changing world. FEMS Microbiol. Ecol. 2018, 94, fiy099. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Wu, X.; Duan, C.; Smith, A.R.; Jones, D.L. Traits of dominant species and soil properties co-regulate soil microbial communities across land restoration types in a subtropical plateau region of Southwest China. Ecol. Eng. 2020, 153, 105897. [Google Scholar] [CrossRef]
- Singh, S.R.; Yadav, P.; Singh, D.; Bahadur, L.; Singh, S.P.; Yadav, A.S.; Mishra, A.; Yadav, P.P.; Kumar, S. Impact of different cropping systems on the land nutrient index, microbial diversity, and soil quality. Land Degrad. Dev. 2021, 32, 3973–3991. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, Y.; Wu, C.; Yang, J.; Hu, Y.; Shi, H. Comprehensive assessment of paddy soil quality under land consolidation: A novel perspective of microbiology. PeerJ 2019, 7, e7351. [Google Scholar] [CrossRef]
- Djemiel, C.; Dequiedt, S.; Karimi, B.; Cottin, A.; Horrigue, W.; Bailly, A.; Boutaleb, A.; Sadet-Bourgeteau, S.; Maron, P.A.; Prévost-Bouré, N.C.; et al. Potential of meta-omics to provide modern microbial indicators for monitoring soil quality and securing food production. Front. Microbiol. 2022, 13, 889788. [Google Scholar] [CrossRef] [PubMed]
- Maguire, V.G.; Bordenave, C.D.; Nieva, A.S.; Llames, M.E.; Colavolpe, M.B.; Gárriz, A.; Ruiz, O.A. Soil bacterial and fungal community structure of a rice monoculture and rice-pasture rotation systems. Appl. Soil Ecol. 2020, 151, 153535. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Zhang, K.; Jeong, J.; Zeng, Z.; Zang, H. Does crop rotation yield more in China? A meta-analysis. Field Crops Res. 2020, 245, 107659. [Google Scholar] [CrossRef]
- Ma, P.; Lan, Y.; Lyu, T.; Li, F.; Yang, Z.; Sun, Y.; Ma, J. Nitrogen fate and efficiency of fertilizer application under a rapeseed–wheat–rice rotation system in southwest China. Agronomy 2021, 11, 258. [Google Scholar] [CrossRef]
- Xu, C.X.; Du, W.F.; Zhang, S.Q.; Zhang, Y.; Yang, R.Q.; Liu, X.Z. Using stable isotopes to assess food source and trophic level of terrestrial animals in Mawan cave of Fenggang in Guizhou. Chin. J. Ecol. 2020, 39, 2024–2032. [Google Scholar] [CrossRef]
- Liu, D.D.; Min, L.I.; Liu, R.J. Recent advances in the study of plant growth-promoting rhizobacteria in China. Chin. J. Ecol. 2016, 35, 815–824. [Google Scholar] [CrossRef]
- Feng, W.H.; Feng, Z.W.; Wei, K.; Rao, Y. Analysis of fatty acid composition agronomic and economic characters about 10 rape varieties in Guizhou. Seed 2016, 35, 108–112. [Google Scholar]
- Ding, L.L.; Wang, P.C.; Zhang, W.; Zhang, Y.; Li, S.J.; Wei, X.; Chen, X.; Zhang, Y.J.; Yang, F.L. Shrub encroachment shapes soil nutrient concentration, stoichiometry and carbon storage in an abandoned subalpine grassland. Sustainability 2019, 11, 1732. [Google Scholar] [CrossRef]
- Ding, L.L.; Wang, P.C. Afforestation suppresses soil nitrogen availability and soil multifunctionality on a subtropical grassland. Sci. Total. Environ. 2021, 761, 143663. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Yang, Z.P.; Xu, M.G.; Zheng, S.X.; Nie, J.; Gao, J.S.; Liao, Y.L.; Xie, J. Effects of long-term winter planted green manure on physical properties of reddish paddy soil under a double-rice cropping system. J. Integr. Agr. 2012, 11, 655–664. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, M.; Zhao, X.; Hao, B.; Zheng, P. Effects of green manure rotation on soil properties and yield and quality of silage maize in saline-alkali soils. Chin. J. Eco-Agric. 2018, 26, 856–864. [Google Scholar]
- Madari, B.; Machado, P.L.; Torres, E.; de Andrade, A.G.; Valencia, L.I. No tillage and crop rotation effects on soil aggregation and organic carbon in a Rhodic Ferralsol from southern Brazil. Soil Tillage Res. 2005, 80, 185–200. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.D.; Tian, X.H. Effect of straw-returning on the storage and distribution of different active fractions of soil organic carbon. Chin. J. Appl. Ecol. 2014, 25, 3491. [Google Scholar]
- Aziz, I.; Mahmood, T.; Islam, K.R. Effect of long term no-till and conventional tillage practices on soil quality. Soil Tillage Res. 2013, 131, 28–35. [Google Scholar] [CrossRef]
- Mazzoncini, M.; Antichi, D.; Di Bene, C.; Risaliti, R.; Petri, M.; Bonari, E. Soil carbon and nitrogen changes after 28 years of no-tillage management under Mediterranean conditions. Eur. J. Agron. 2016, 77, 156–165. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Cui, S.; Yang, Q.; Yang, X.; Li, J.; Shen, Y. Developing sustainable cropping systems by integrating crop rotation with conservation tillage practices on the Loess Plateau, a long-term imperative. Field Crops Res. 2018, 222, 164–179. [Google Scholar] [CrossRef]
- Svensson KS, S.; Lewan, E.; Clarholm, M. Effects of a ryegrass catch crop on microbial biomass and mineral nitrogen in an arable soil during winter. Swed. J. Agric. Res. 1994, 24, 31–38. [Google Scholar]
- Kramberger, B.; Lukac, B.; Gruskovnjak, D.; Gselman, A. Effects of Italian ryegrass and date of plow-in on soil mineral nitrogen and sugarbeet yield and quality. Agron. J. 2008, 100, 1332–1338. [Google Scholar] [CrossRef]
- Johnston, D.; Laidlaw, A.; Theodoridou, K.; Ferris, C. Performance and nutrient utilisation of dairy cows offered silages produced from three successive harvests of either a red clover–perennial ryegrass sward or a perennial ryegrass sward. Ir. J. Agric. Food Res. 2020, 59, 42–55. [Google Scholar] [CrossRef]
- Wu, L.; Peng, M.; Qiao, S.; Ma, X. Assessing impacts of rainfall intensity and slope on dissolved and adsorbed nitrogen loss under bare loessial soil by simulated rainfalls. Catena 2018, 170, 51–63. [Google Scholar] [CrossRef]
- Mäkelä, P.S.A.; Wasonga, D.O.; Hernandez, A.S.; Santanen, A. Seedling growth and phosphorus uptake in response to different phosphorus sources. Agronomy 2020, 10, 1089. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [PubMed]
- Matocha, C.J.; Karathanasis, T.D.; Murdock, L.W.; Grove, J.H.; Goodman, J.; Call, D. Influence of ryegrass on physico-chemical properties of a fragipan soil. Geoderma 2018, 317, 32–38. [Google Scholar] [CrossRef]
- Sudhamsu, J.; Crane, B.R. Bacterial nitric oxide synthases: What are they good for? Trends Microbiol. 2009, 17, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Siczek, A.; Lipiec, J. Soybean nodulation and nitrogen fixation in response to soil compaction and surface straw mulching. Soil Tillage Res. 2011, 114, 50–56. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Tian, J.; Lim, B.L.; Yan, X.; Liao, H. Overexpressing AtPAP15 enhances phosphorus efficiency in Soybean. Plant Physiol. 2009, 151, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, N.; Lin, C.; He, H.; Chen, G. Interaction between BSM-contaminated soils and Italian ryegrass. J. Environ. Sci. Health Part B 2012, 47, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.D.; Park, J.; Tran, H.T.; Del Vecchio, H.A.; Ying, S.; Zins, J.L.; Patel, K.; McKnight, T.D.; Plaxton, W.C. The secreted purple acid phosphatase isozymes AtPAP12 and AtPAP26 play a pivotal role in extracellular phosphate-scavenging by Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 6531–6542. [Google Scholar] [CrossRef]
- Daniel, W.; Anna, N.; Grażyna, P.; Agnieszka, M. Effects of pulp and Na-bentonite amendments on the mobility of trace elements, soil enzyme activities and microbial parameters under ex situ-aided phytostabilization. PLoS ONE 2017, 12, e0169688. [Google Scholar] [CrossRef]
- Del Vecchio, H.A.; Ying, S.; Park, J.; Knowles, V.L.; Kanno, S.; Tanoi, K.; She, Y.; Plaxton, W.C. The cell wall-targeted purple acid phosphatase AtPAP25 is critical for acclimation of Arabidopsis thaliana to nutritional phosphorus deprivation. Plant J. 2015, 80, 569–581. [Google Scholar] [CrossRef]
- Huang, S.; Li, J.; Wang, J. Clay minerals change the toxic effect of cadmium on the activities of Leucine Aminopeptidase. Adsorpt. Sci. Technol. 2021, 2021, 1024085. [Google Scholar] [CrossRef]
- Audu, V.; Rasche, F.; Mårtensson, L.M.D.; Emmerling, C. Perennial cereal grain cultivation: Implication on soil organic matter and related soil microbial parameters. Appl. Soil Ecol. 2022, 174, 104414. [Google Scholar] [CrossRef]
- Powlson, D.; Prookes, P.; Christensen, B. Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Baligar, V.C.; Calvert, D.V. Microbiological and biochemical indexing systems for assessing quality of acid soils. Adv. Agron. 2003, 78, 89–138. [Google Scholar] [CrossRef]
- Yang, R.J.; Ma, H.L.; Yang, Q.F.; Niu, J.Y. Effects of planting density and nitrogen application rate on soil microbial activity under wheat/forage rape multiple cropping. Chin. J. Appl. Ecol. 2007, 18, 113–117. [Google Scholar]
- Stark, C.; Condron, L.M.; Stewart, A.; Di, H.J.; O’Callaghan, M. Effects of past and current crop management on soil microbial biomass and activity. Biol. Fertil. Soils 2007, 43, 531–540. [Google Scholar] [CrossRef][Green Version]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 2010, 42, 926–934. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, J.; Zhang, X.; Gao, L. Effects of summer catch crop, residue management, soil temperature and water on the succeeding cucumber rhizosphere nitrogen mineralization in intensive production systems. Nutr. Cycl. Agroecosyst. 2010, 88, 429–446. [Google Scholar] [CrossRef]
- Yu, D.; Wen, Z.; Li, X.; Song, X.; Wu, H.; Yang, P. Effects of straw return on bacterial communities in a wheat-maize rotation system in the North China Plain. PLoS ONE 2018, 13, e0198087. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Liu, X.; Zhao, X.; Lu, D.; Zhou, J.; Li, C. Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice–wheat cropping system. Soil Tillage Res. 2017, 165, 121–127. [Google Scholar] [CrossRef]
- Małgorzata, I.; Inga, K.; Lidia, W. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef]
- Yu, J.Y.; Liu, Y.H.; Wang, Z.Y.; Huang, X.H.; Chai, D.; Gu, Y.F.; Zhao, K.; Yu, X.M.; Shuai, Z.B.; Liu, H.J.; et al. The cropping obstacle of garlic was associated with changes in soil physicochemical properties, enzymatic activities and bacterial and fungal communities. Front. Microbiol. 2022, 13, 828196. [Google Scholar] [CrossRef]
- Álvaro-Fuentes, J.; Arrúe, J.L.; Gracia, R.; López, M.V. Tillage and cropping intensification effects on soil aggregation: Temporal dynamics and controlling factors under semiarid conditions. Geoderma 2008, 145, 390–396. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Hilton, S.L.; Bending, G.D.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J. Changes in activity and structure of the soil microbial community after application of azoxystrobin or pirimicarb and an organic amendment to an agricultural soil. Appl. Soil Ecol. 2016, 106, 47–57. [Google Scholar] [CrossRef]
- Bucher, A.; Lanyon, L. Evaluating soil management with microbial community-level physiological profiles. Appl. Soil Ecol. 2005, 29, 59–71. [Google Scholar] [CrossRef]
- Lipson, D.A.; Schmidt, S.K. Seasonal changes in an alpine soil bacterial community in the colorado rocky mountains. Appl. Environ. Microbiol. 2004, 70, 2867–2879. [Google Scholar] [CrossRef]
- Chaudhry, V.; Rehman, A.; Mishra, A.; Chauhan, P.S.; Nautiyal, C.S. Changes in bacterial community structure of agricultural land due to long-term organic and chemical amendments. Microb. Ecol. 2012, 64, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- He, H.B.; Li, W.X.; Zhang, Y.W.; Cheng, J.K.; Jia, X.Y.; Li, S.; Yang, H.R.; Chen, B.M.; Xin, G.R. Effects of Italian ryegrass residues as green manure on soil properties and bacterial communities under an Italian ryegrass (Lolium multiflorum L.)-rice (Oryza sativa L.) rotation. Soil Tillage Res. 2020, 196, 104487. [Google Scholar] [CrossRef]
- Wang, G.H.; Liu, J.J.; Yu, Z.H.; Wang, X.Z.; Jin, J.; Liu, X.B. Research progress of acidobacteria ecology in soils. Biotechnol. Bull. 2016, 32, 14–20. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Ma, T.; Raza, W.; Li, J.; Howland, J.G.; Huang, Q.; Shen, Q. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Appl. Soil Ecol. 2017, 112, 42–50. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Zheng, Z. Distribution of microbial biomass and activity within soil aggregates as affected by tea plantation age. Catena 2017, 153, 1–8. [Google Scholar] [CrossRef]
- Ligi, T.; Oopkaup, K.; Truu, M.; Preem, J.-K.; Nõlvak, H.; Mitsch, W.J.; Mander, Ü.; Truu, J. Characterization of bacterial communities in soil and sediment of a created riverine wetland complex using high-throughput 16S rRNA amplicon sequencing. Ecol. Eng. 2014, 72, 56–66. [Google Scholar] [CrossRef]
- DeBruyn, J.M.; Nixon, L.T.; Fawaz, M.N.; Johnson, A.M.; Radosevich, M. Global Biogeography and Quantitative Seasonal Dynamics of Gemmatimonadetes in Soil. Appl. Environ. Microbiol. 2011, 77, 6295–6300. [Google Scholar] [CrossRef] [PubMed]
- Logue, J.B.; Lindström, E.S. Species sorting affects bacterioplankton community composition as determined by 16S rDNA and 16S rRNA fingerprints. ISME J. 2010, 4, 729–738. [Google Scholar] [CrossRef]
- Tao, J.M.; Liu, X.D.; Liang, Y.L.; Niu, J.J.; Xiao, Y.H.; Gu, Y.B.; Ma, L.Y.; Meng, D.L.; Zhang, Y.G.; Huang, W.K.; et al. Maize growth responses to soil microbes and soil properties after fertilization with different green manures. Appl. Microbiol. Biotechnol. 2016, 101, 1289–1299. [Google Scholar] [CrossRef]
- Wu, W.; Liu, B.; Li, H.J.; Li, S.; Chen, Z.Z. Effect of pH and salinity on sulfate reduction by microorganism. Chin. J. Environ. Eng. 2011, 5, 2527–2531. [Google Scholar]
- Baker, B.J.; Lazar, C.S.; Teske, A.P.; Dick, G.J. Genomic resolution of linkages in carbon, nitrogen, and sulfur cycling among widespread estuary sediment bacteria. Microbiome 2015, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, Z.; Liu, R.; Wu, J.; Zeng, Q.; Qi, Y. Rhizosphere bacterial community characteristics over different years of sugarcane ratooning in consecutive monoculture. Biomed. Res. Int. 2019, 2019, 4943150. [Google Scholar] [CrossRef] [PubMed]
- Komaki, H. Recent progress of reclassification of the Genus Streptomyces. Microorganisms 2023, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Kang, J.P.; Park, J.K.; Li, J.; Chen, L.; Yang, D.C. Rhodanobacter ginsengiterrae sp. nov., an antagonistic bacterium against root rot fungal pathogen Fusarium solani, isolated from ginseng rhizospheric soil. Arch. Microbiol. 2018, 200, 1457–1463. [Google Scholar] [CrossRef]
- Carlson, H.K.; Price, M.N.; Callaghan, M.; Aaring, A.; Chakraborty, R.; Liu, H.; Kuehl, J.V.; Arkin, A.P.; Deutschbauer, A.M. The selective pressures on the microbial community in a metal-contaminated aquifer. ISME J. 2018, 13, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; He, P.; Xu, S.; Li, S.; Wang, Y.; Zhang, W.; Li, X.; Shang, H.; Zeng, L.; Zheng, S.J. Banana disease-suppressive soil drives Bacillus assembled to defense Fusarium wilt of banana. Front. Microbiol. 2023, 14, 1211301. [Google Scholar] [CrossRef]
- Ren, H.; Wang, H.; Qi, X.; Yu, Z.; Zheng, X.; Zhang, S.; Wang, Z.; Zhang, M.; Ahmed, T.; Li, B. The damage caused by decline disease in bayberry plants through changes in soil properties, rhizosphere microbial community structure and metabolites. Plants 2021, 10, 2083. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Huertas, J.; Cuartero, J.; Ros, M.; Pascual, J.A.; Parras-Alcántara, L.; González-Rosado, M.; Özbolat, O.; Zornoza, R.; Egea-Cortines, M.; Hurtado-Navarro, M.; et al. How binomial (traditional rainfed olive grove—Crocus sativus) crops impact the soil bacterial community and enhance microbial capacities. J. Environ. Manag. 2023, 345, 118572. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhou, D.; Wan, R.; Wang, C.; Xie, J.; Ma, C.; Li, Y. HPLC and high-throughput sequencing revealed higher tea-leaves quality, soil fertility and microbial community diversity in ancient tea plantations: Compared with modern tea plantations. BMC Plant Biol. 2022, 22, 239. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Zhang, X.; Li, Q.; Huang, Z. Bamboo-based agroforestry changes phytoremediation efficiency by affecting soil properties in rhizosphere and non-rhizosphere in heavy metal-polluted soil (Cd/Zn/Cu). J. Soils Sediments 2022, 23, 368–378. [Google Scholar] [CrossRef]
- Pertile, M.; Sousa, R.M.S.; Mendes, L.W.; Antunes, J.E.L.; Oliveira, L.M.d.S.; de Araujo, F.F.; Melo, V.M.M.; Araujo, A.S.F. Response of soil bacterial communities to the application of the herbicides imazethapyr and flumyzin. Eur. J. Soil Biol. 2021, 102, 103252. [Google Scholar] [CrossRef]
- Ji, L.F.; Ni, K.; Ma, L.F.; Chen, Z.J.; Zhao, Y.Y.; Ruan, J.Y.; Guo, S.W. Effect of different fertilizer regimes on the fungal community of acidic tea-garden soil. Acta Ecol. Sin. 2018, 38, 8158–8166. [Google Scholar]
- Han, L.; Zhou, X.; Zhao, Y.; Wu, L.; Ping, X.; He, Y.; Peng, S.; He, X.; Du, Y. First report of Plectosphaerella plurivora causing root rot disease in Panax notoginseng in China. J. Phytopathol. 2020, 168, 375–379. [Google Scholar] [CrossRef]
- Rivera-Mendez, W.; Fuentes-Alfaro, R.; López, K.C.; Aguilar-Ulloa, W.; Zúñiga-Vega, C.; Brenes-Madriz, J. Biological control of Setophoma terrestris isolated from onion rhizosphere in Costa Rica. Arch. Phytopathol. Plant Prot. 2018, 52, 813–824. [Google Scholar] [CrossRef]
- Yajima, Y.; Tojo, M.; Chen, B.; Hoshino, T. Typhula cf. subvariabilis, new snow mould in Antarctica. Mycology 2017, 8, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, E.; Hanaka, A. Mortierella Species as the Plant Growth-Promoting Fungi Present in the Agricultural Soils. Agriculture 2020, 11, 7. [Google Scholar] [CrossRef]
- Sun, B.-T.; Akutse, K.S.; Xia, X.-F.; Chen, J.-H.; Ai, X.; Tang, Y.; Wang, Q.; Feng, B.-W.; Goettel, M.S.; You, M.-S. Endophytic effects of Aspergillus oryzae on radish (Raphanus sativus) and its herbivore, Plutella xylostella. Planta 2018, 248, 705–714. [Google Scholar] [CrossRef]
- Yadav, A.N.; Verma, P.; Kumar, V.; Sangwan, P.; Mishra, S.; Panjiar, N.; Gupta, V.K.; Saxena, A.K. Biodiversity of the genus penicillium in different habitats. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier Science: Amsterdam, The Netherlands, 2018; pp. 3–18. [Google Scholar]
- Kivlin, S.N.; Hawkes, C.V. Tree species, spatial heterogeneity, and seasonality drive soil fungal abundance, richness, and composition in Neotropical rainforests. Environ. Microbiol. 2016, 18, 4662–4673. [Google Scholar] [CrossRef]


| Treatments | Plant | One Rotation | Two Rotations | Three Rotations | |||
|---|---|---|---|---|---|---|---|
| Sowing Date | Harvest Date | Sowing Date | Harvest Date | Sowing Date | Harvest Date | ||
| CC | Zea mays | May 2017 | September 2017 | May 2018 | September 2018 | May 2019 | September 2019 |
| CG | Allium sativum | --- | --- | --- | --- | October 2018 | April 2019 |
| Zea mays | --- | --- | --- | --- | May 2019 | September 2019 | |
| CR | Brassica napus | --- | --- | --- | --- | October 2018 | April 2019 |
| Zea mays | --- | --- | --- | --- | May 2019 | September 2019 | |
| Cir1 | Lolium multiflorum | --- | --- | --- | --- | October 2018 | April 2019 |
| Zea mays | --- | --- | --- | --- | May 2019 | September 2019 | |
| Cir2 | Lolium multiflorum | --- | --- | October 2017 | April 2018 | October 2018 | April 2019 |
| Zea mays | --- | --- | May 2018 | September 2018 | May 2019 | September 2019 | |
| Cir3 | Lolium multiflorum | October 2016 | April 2017 | October 2017 | April 2018 | October 2018 | April 2019 |
| Zea mays | May 2017 | September 2017 | May 2018 | September 2018 | May 2019 | September 2019 | |
| Indicator | Treatments | p-Value | |||||
|---|---|---|---|---|---|---|---|
| CC | CG | Cr | CIr1 | CIr2 | CIr3 | ||
| WC (%) | 21.38 ± 1.22 b | 22.82 ± 1.00 ab | 23.22 ± 1.00 ab | 20.05 ± 1.00 b | 24.01 ± 2.08 ab | 26.23 ± 1.00 a | <0.05 |
| pH | 5.32 ± 0.05 bc | 5.22 ± 0.10 c | 6.05 ± 0.31 a | 5.22 ± 0.10 c | 4.76 ± 0.11 c | 6.04 ± 0.31 a | <0.05 |
| SOC (g kg−1) | 23.13 ± 0.66 a | 19.74 ± 0.72 bc | 21.91 ± 0.53 ab | 18.93 ± 0.55 c | 16.27 ± 0.96 d | 17.59 ± 0.95 cd | <0.05 |
| TN (g kg−1) | 2.18 ± 0.04 a | 1.96 ± 0.07 bc | 2.00 ± 0.06 ab | 1.74 ± 0.02 d | 1.85 ± 0.09 bcd | 1.78 ± 0.05 cd | <0.05 |
| TP (g kg−1) | 0.54 ± 0.02 d | 0.77 ± 0.02 b | 1.03 ± 0.04 a | 0.79 ± 0.02 b | 0.68 ± 0.01 c | 0.56 ± 0.02 d | <0.05 |
| AP (mg kg−1) | 7.60 ± 1.48 c | 10.83 ± 0.95 bc | 49.73 ± 7.12 a | 11.30 ± 2.49 bc | 19.49 ± 0.98 b | 4.17 ± 0.88 c | <0.05 |
| NO3−-N (mg kg−1) | 12.26 ± 2.42 b | 15.35 ± 3.22 b | 5.57 ± 4.78 c | 15.93 ± 3.05 b | 33.56 ± 4.42 a | 4.59 ± 1.27 c | <0.05 |
| NH4 +-N (mg kg−1) | 2.57 ± 0.27 c | 4.29 ± 2.12 c | 6.02 ± 1.92 c | 11.32 ± 5.84 b | 19.87 ± 4.93 a | 19.37 ± 0.85 a | <0.05 |
| Indicator | Treatments | |||||
|---|---|---|---|---|---|---|
| CC | CG | Cr | CIr1 | CIr2 | CIr3 | |
| SOC | 0.49 | 0.60 | 0.46 | 0.54 | 0.50 | 0.48 |
| TN | 0.50 | 0.56 | 0.53 | 0.49 | 0.43 | 0.56 |
| TP | 0.55 | 0.53 | 0.56 | 0.28 | 0.44 | 0.32 |
| AP | 0.44 | 0.46 | 0.41 | 0.34 | 0.56 | 0.49 |
| NO3−-N | 0.38 | 0.30 | 0.61 | 0.55 | 0.57 | 0.49 |
| NH4+-N | 0.34 | 0.58 | 0.3 | 0.43 | 0.39 | 0.44 |
| Degree of membership | 2.71 | 3.03 | 2.87 | 2.63 | 2.89 | 2.78 |
| Ranking | 5 | 1 | 3 | 6 | 2 | 4 |
| Indicator | Treatments | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| CC | CG | CR | CIr1 | CIr2 | CIr3 | |||
| Bacterial | Shannon | 10.16 ± 0.15 ab | 9.57 ± 0.10 bc | 10.38 ± 0.16 ab | 9.81 ± 0.15 abc | 9.16 ± 0.53 c | 10.52 ± 0.19 a | 0.013 |
| Chao1 | 6080 ± 224 a | 5660 ± 153 a | 4852 ± 1624 a | 5056 ± 198 a | 4354 ± 447 a | 6401 ± 425 a | 0.363 | |
| Fungi | Shannon | 5.63 ± 1.01 a | 5.20 ± 0.65 a | 6.15 ± 0.42 a | 6.56 ± 1.30 a | 4.52 ± 0.51 a | 6.25 ± 0.65 a | >0.05 |
| Chao1 | 1101 ± 33 a | 1068 ± 72 ab | 1044 ± 62 ab | 970 ± 19 ab | 954 ± 38 c | 1069 ± 46 ab | 0.046 | |
| Indicator | Bacterial | Fungal | ||||||
|---|---|---|---|---|---|---|---|---|
| Chao 1 | Shannon | Chao 1 | Shannon | |||||
| r | p | r | p | r | p | r | p | |
| WC | 0.365 | p > 0.05 | 0.431 * | 0.036 | 0.168 | p > 0.05 | 0.236 | p > 0.05 |
| pH | 0.508 * | 0.011 | 0.526 ** | 0.008 | 0.102 | p > 0.05 | 0.189 | p > 0.05 |
| SOC | 0.498 * | 0.013 | 0.446 * | 0.029 | −0.002 | p > 0.05 | 0.143 | p > 0.05 |
| TN | 0.124 | p > 0.05 | −0.089 | p > 0.05 | −0.053 | p > 0.05 | −0.103 | p > 0.05 |
| TP | 0.151 | p > 0.05 | 0.082 | p > 0.05 | −0.34 | p > 0.05 | −0.027 | p > 0.05 |
| AP | 0.168 | p > 0.05 | 0.095 | p > 0.05 | −0.397 | p > 0.05 | −0.105 | p > 0.05 |
| NO3−-N | −0.469 * | 0.021 | −0.581 ** | 0.003 | −0.518 ** | 0.01 | −0.283 | p > 0.05 |
| NH4+-N | −0.624 ** | 0.001 | −0.591 ** | 0.002 | −0.366 | p > 0.05 | −0.418 * | 0.042 |
| MBC | 0.129 | p > 0.05 | −0.025 | p > 0.05 | 0.214 | p > 0.05 | −0.075 | p > 0.05 |
| MBN | 0.311 | p > 0.05 | 0.256 | p > 0.05 | 0.271 | p > 0.05 | 0.018 | p > 0.05 |
| MBP | 0.076 | p > 0.05 | −0.137 | p > 0.05 | −0.252 | p > 0.05 | −0.126 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Xie, W.; Ding, L.; Zhuo, Y.; Gao, Y.; Li, J.; Zhao, L. Effects of Maize–Crop Rotation on Soil Physicochemical Properties, Enzyme Activities, Microbial Biomass and Microbial Community Structure in Southwest China. Microorganisms 2023, 11, 2621. https://doi.org/10.3390/microorganisms11112621
Wang P, Xie W, Ding L, Zhuo Y, Gao Y, Li J, Zhao L. Effects of Maize–Crop Rotation on Soil Physicochemical Properties, Enzyme Activities, Microbial Biomass and Microbial Community Structure in Southwest China. Microorganisms. 2023; 11(11):2621. https://doi.org/10.3390/microorganisms11112621
Chicago/Turabian StyleWang, Puchang, Wenhui Xie, Leilei Ding, Yingping Zhuo, Yang Gao, Junqin Li, and Lili Zhao. 2023. "Effects of Maize–Crop Rotation on Soil Physicochemical Properties, Enzyme Activities, Microbial Biomass and Microbial Community Structure in Southwest China" Microorganisms 11, no. 11: 2621. https://doi.org/10.3390/microorganisms11112621
APA StyleWang, P., Xie, W., Ding, L., Zhuo, Y., Gao, Y., Li, J., & Zhao, L. (2023). Effects of Maize–Crop Rotation on Soil Physicochemical Properties, Enzyme Activities, Microbial Biomass and Microbial Community Structure in Southwest China. Microorganisms, 11(11), 2621. https://doi.org/10.3390/microorganisms11112621





