Endoscopic Diagnosis and Therapy for Epstein–Barr Virus-Associated Gastric Cancer
Abstract
1. Introduction
2. Clinical Features of EBVaGC
3. Location of EBVaGC and Helicobacter pylori (Hp)-Related Gastritis
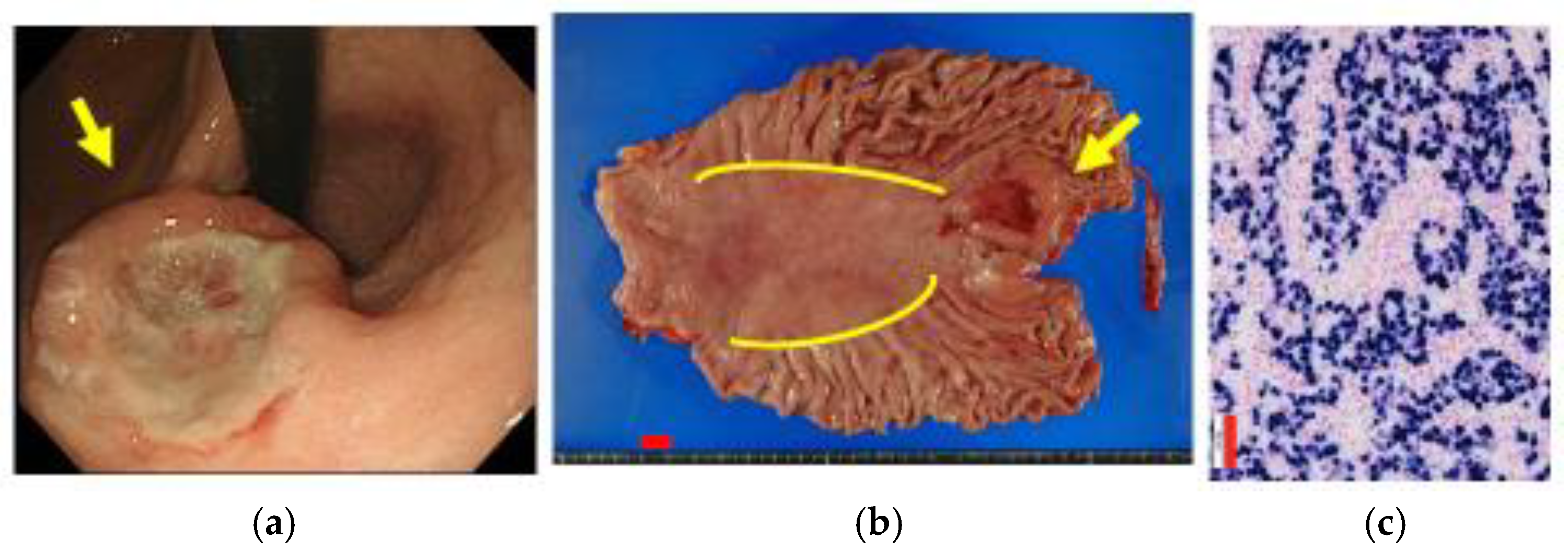
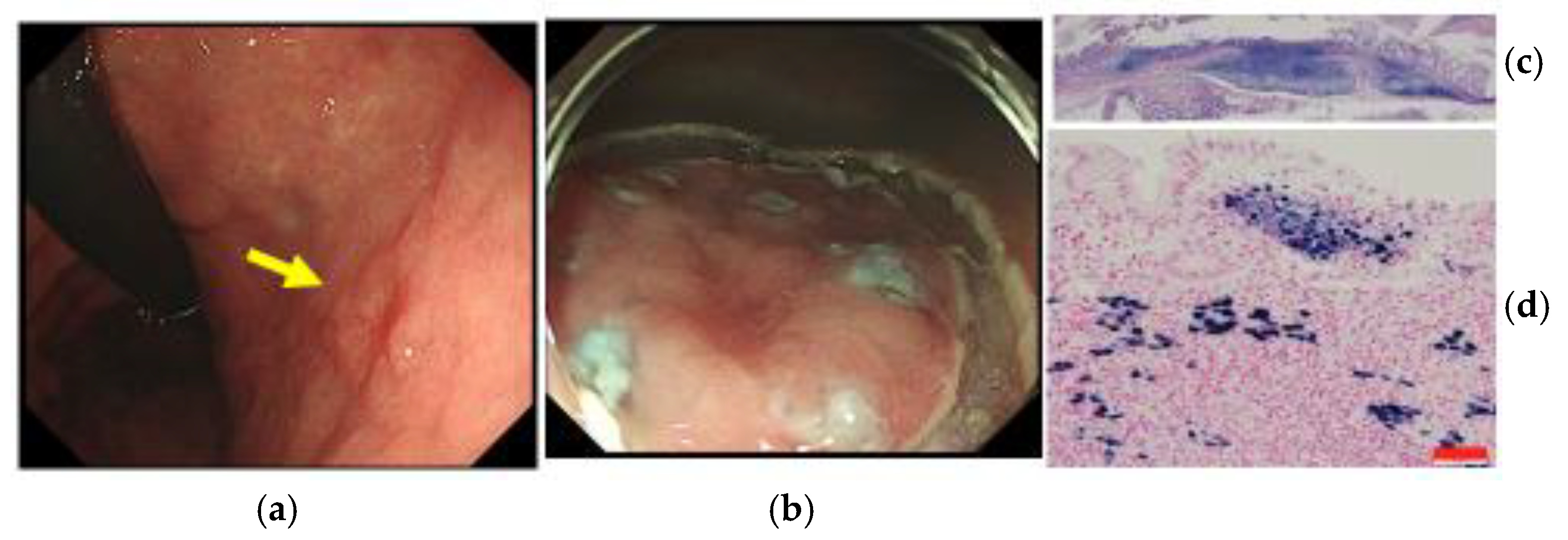
4. Endoscopic Diagnosis of EBVaGC
5. Endoscopic Therapy against EBVaGC
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burke, A.P.; Yen, T.S.; Shekitka, K.M.; Sobin, L.H. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod. Pathol. 1990, 3, 377–380. [Google Scholar] [PubMed]
- Shibata, D.; Weiss, L. Epstein-Barr virus-associated gastric adenocarcinoma. Am. J. Pathol. 1992, 140, 769–774. [Google Scholar] [PubMed]
- Imai, S.; Koizumi, S.; Sugiura, N.; Tokunaga, M.; Uemura, Y.; Yamamoto, N.; Tanaka, S.; Sato, E.; Osato, T. Gastric carcinoma: Monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc. Natl. Acad. Sci. USA 1994, 91, 9131–9135. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Tokunaga, M.; Land, C.E. Epstein-Barr virus involvement in gastric cancer: Biomarker for lymph node metastasis. Cancer Epidemiol. Biomark. Prev. 1998, 7, 449–450. [Google Scholar]
- Camargo, M.C.; Kim, W.H.; Chiaravalli, A.M.; Kim, K.M.; Corvalan, A.H.; Matsuo, K.; Yu, J.; Sung, J.J.; Herrera-Goepfert, R.; Meneses-Gonzalez, F.; et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: An international pooled analysis. Gut 2014, 63, 236–243. [Google Scholar] [CrossRef]
- Yanai, H.; Chihara, D.; Harano, M.; Sakaguchi, E.; Murakami, T.; Nishikawa, J. Epstein-Barr virus-associated early gastric cancer treated with endoscopic submucosal dissection: A possible candidate for extended criteria of endoscopic submucosal dissection. Intern. Med. 2019, 58, 3247–3250. [Google Scholar] [CrossRef]
- Yanai, H.; Yahara, N.; Furuya, T.; Hayashi, H.; Murakami, T.; Shimokawa, Y.; Sugihara, S. Long-term survival of patient with Epstein-Barr virus-positive gastric cancer treated with chemotherapy: Case report. J. Gastrointest. Cancer 2016, 47, 107–110. [Google Scholar] [CrossRef]
- Yanai, H.; Nishikawa, J.; Mizugaki, Y.; Shimizu, N.; Takada, K.; Matsusaki, K.; Toda, T.; Matsumoto, Y.; Tada, M.; Okita, K. Endoscopic and pathologic features of Epstein-Barr virus-associated gastric carcinoma. Gastrointest. Endosc. 1997, 45, 236–242. [Google Scholar] [CrossRef]
- Nishikawa, J.; Yanai, H.; Hirano, A.; Okamoto, T.; Nakamura, H.; Matsusaki, K.; Kawano, T.; Miura, O.; Okita, K. High prevalence of Epstein-Barr virus in gastric carcinoma after Billroth-II reconstruction. Scand. J. Gastroenterol. 2002, 37, 825–829. [Google Scholar] [CrossRef]
- Nishikawa, J.; Yanai, H.; Mizugaki, Y.; Takada, K.; Tada, M.; Okita, K. Hypoechoic submucosal nodules: A sign of Epstein-Barr virus-associated early gastric cancer. J. Gastroenterol. Hepatol. 1998, 13, 585–590. [Google Scholar] [CrossRef]
- Yanagi, A.; Nishikawa, J.; Shimokuri, K.; Shuto, T.; Takagi, T.; Takagi, F.; Kobayashi, Y.; Yamamoto, M.; Miura, O.; Yanai, H.; et al. Clinicopathologic characteristics of Epstein-Barr virus-associated gastric cancer over the past decade in Japan. Microorganisms 2019, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, M.; Georges, D.; Clifford, G.M.; Martel, C. Estimating the global burden of Epstein-Barr virus-associated gastric cancer: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, J.; Tokunaga, M.; Satoh, E.; Tanaka, S.; Land, C.E. Morphological characteristics of Epstein-Barr virus-related early gastric carcinoma: A case-control study. Pathol. Int. 1997, 47, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Srivastava, A.; Lee, J.; Kim, Y.S.; Kim, K.M.; Kang, W.K.; Kim, M.; Kim, S.; Park, C.K.; Kim, S. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology 2010, 139, 84–92. [Google Scholar] [CrossRef]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, J.; Xiao, L.; Tang, F.; Zhang, Z.; Zhang, Y.; Feng, Z.; Jiang, Y.; Shao, C. Accumulation mechanisms of CD4+ CD25+ FOXP3+ regulatory T cells in EBV-associated gastric carcinoma. Sci. Rep. 2015, 5, 18057. [Google Scholar] [CrossRef]
- Yanai, H.; Murakami, T.; Yoshiyama, H.; Takeuchi, H.; Nishikawa, J.; Nakamura, H.; Okita, K.; Miura, O.; Shimizu, N.; Takada, K. Epstein-Barr virus-associated gastric carcinoma and atrophic gastritis. J. Clin. Gastroenterol. 1999, 29, 39–43. [Google Scholar] [CrossRef]
- Kaizaki, Y.; Sakurai, S.; Chong, J.; Fukayama, M. Atrophic gastritis, Epstein-Barr virus infection, and Epstein-Barr virus-associated gastric carcinoma. Gastric Cancer 1999, 2, 101–108. [Google Scholar] [CrossRef][Green Version]
- Camargo, M.C.; Kim, K.M.; Matsuo, K.; Torres, J.; Liao, L.M.; Morgan, D.; Michel, A.; Waterboer, T.; Zabaleta, J.; Dominguez, R.L.; et al. Anti-Helicobacter pylori antibody profiles in Epstein-Barr virus-positive and -negative gastric cancer. Helicobacter 2016, 21, 153–157. [Google Scholar] [CrossRef]
- Imai, S.; Nishikawa, J.; Takada, K. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 1998, 72, 4371–4378. [Google Scholar] [CrossRef]
- Kartica, A.V.; Iizasa, H.; Ding, D.; Kanehiro, Y.; Tajima, Y.; Kaji, S.; Yanai, H.; Yoshiyama, H. Application of biopsy samples used for Helicobacter pylori urease test to predict Epstein-Barr virus-associated cancer. Microorganisms 2020, 8, 923. [Google Scholar] [CrossRef] [PubMed]
- Fukayama, M.; Kunita, A.; Kaneda, A. Gastritis-infection-cancer sequence of Epstein-Barr virus-associated gastric cancer. Adv. Exp. Med. Biol. 2018, 1045, 437–457. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Chihara, D.; Harano, M.; Sakaguchi, K.; Murakami, T.; Nishikawa, J. Endoscopic and pathologic motifs for the clinical diagnosis of Epstein–Barr virus-associated gastric cancer. DEN Open 2021, 1, e7. [Google Scholar] [CrossRef] [PubMed]
- Osumi, H.; Kawachi, H.; Yoshio, T.; Fujisaki, J. Clinical impact of Epstein-Barr virus status on the incidence of lymph node metastasis in early gastric cancer. Dig. Endosc. 2020, 32, 316–322. [Google Scholar] [CrossRef]
- Nagaya, T.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Endoscopic near infrared photoimmunotherapy using a fiber optic diffuser for peritoneal dissemination of gastric cancer. Cancer Sci. 2018, 109, 1902–1908. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanai, H.; Fujiwara, J.; Toyama, E.; Okuda, H.; Miura, O.; Kaino, S.; Nishikawa, J. Endoscopic Diagnosis and Therapy for Epstein–Barr Virus-Associated Gastric Cancer. Microorganisms 2023, 11, 2619. https://doi.org/10.3390/microorganisms11112619
Yanai H, Fujiwara J, Toyama E, Okuda H, Miura O, Kaino S, Nishikawa J. Endoscopic Diagnosis and Therapy for Epstein–Barr Virus-Associated Gastric Cancer. Microorganisms. 2023; 11(11):2619. https://doi.org/10.3390/microorganisms11112619
Chicago/Turabian StyleYanai, Hideo, Junko Fujiwara, Eiichiro Toyama, Hiroshi Okuda, Osamu Miura, Seiji Kaino, and Jun Nishikawa. 2023. "Endoscopic Diagnosis and Therapy for Epstein–Barr Virus-Associated Gastric Cancer" Microorganisms 11, no. 11: 2619. https://doi.org/10.3390/microorganisms11112619
APA StyleYanai, H., Fujiwara, J., Toyama, E., Okuda, H., Miura, O., Kaino, S., & Nishikawa, J. (2023). Endoscopic Diagnosis and Therapy for Epstein–Barr Virus-Associated Gastric Cancer. Microorganisms, 11(11), 2619. https://doi.org/10.3390/microorganisms11112619





