Emerging Infectious Diseases Are Virulent Viruses—Are We Prepared? An Overview
Abstract
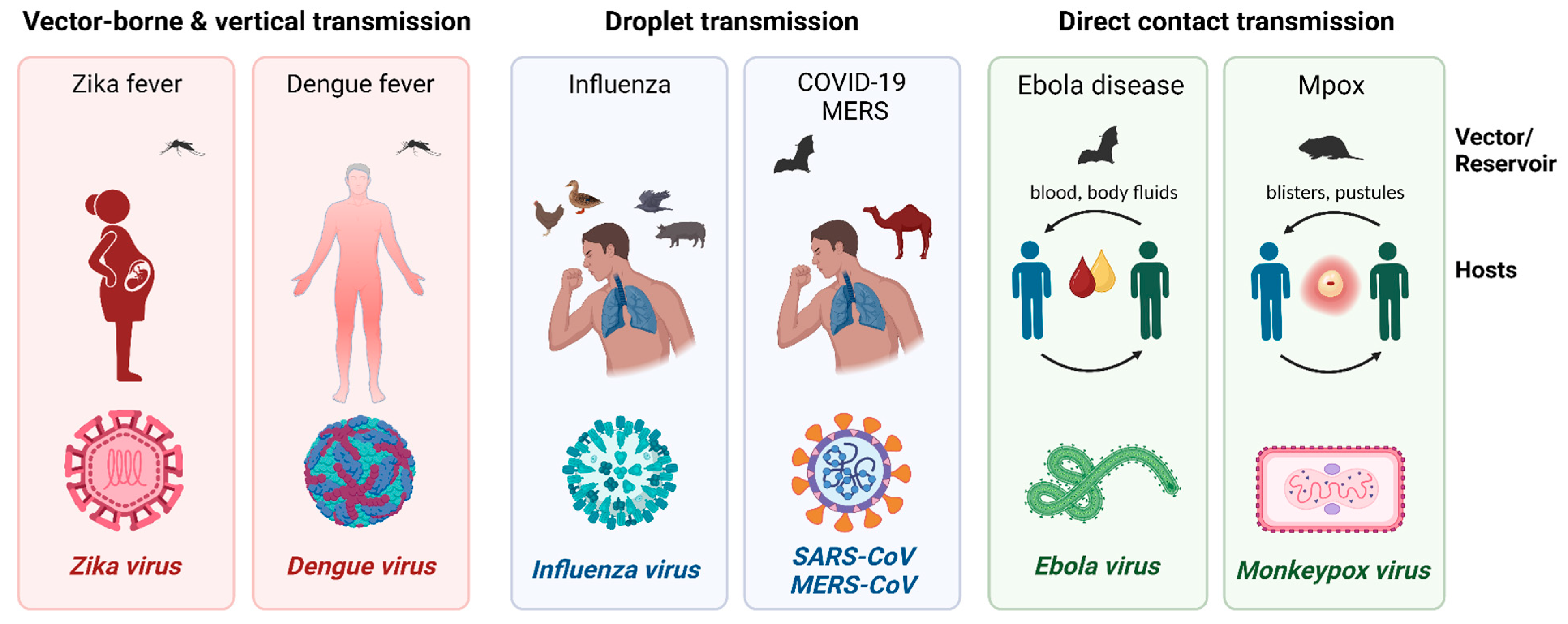
:1. Introduction
2. Emerging and Re-Emerging Viruses
2.1. Filoviridae: Ebola Virus and Marburg Virus
2.2. Flaviviridae: Zika Virus and Dengue Virus
2.3. Coronaviruses: MERS-CoV, SARS-CoV, and SARS-CoV-2
2.4. Avian Influenza (H5N1, H7N9)
2.5. Monkeypox Virus
3. Vaccination
3.1. History
3.2. Vaccination, Herd Immunity, and Public Awareness
3.3. Vaccine Hesitancy
3.4. Vaccine Development in Emerging Viruses
4. Clinical Manifestation and Treatment
4.1. Filoviridae: Ebolavirus and Marburg Virus
4.2. Zika Virus
4.3. Coronavirus: MERS-CoV, SARS-CoV, and SARS-CoV-2 (COVID-19)
4.3.1. MERS-CoV
4.3.2. SARS-CoV
4.3.3. SARS-CoV-2
4.4. Avian Influenza (H5N1, H7N9)
4.4.1. H5N1
4.4.2. H7N9
4.5. Monkeypox Virus (Mpox)
5. Surveillance Tools
5.1. Air Surveillance System
5.2. Viral Detection Methods
5.3. Potential Surveillance Location Identification
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeDuc, J.W.; Barry, M.A. SARS, the First Pandemic of the 21st Century1. EID J. 2004, 10, e26. [Google Scholar] [CrossRef]
- Sullivan, S.J.; Jacobson, R.M.; Dowdle, W.R.; Poland, G.A. 2009 H1N1 Influenza. Mayo Clin. Proc. 2010, 85, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Ebola Virus Disease. Available online: https://www.who.int/health-topics/ebola#tab=tab_1 (accessed on 23 August 2023).
- Marburg Virus Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/marburg-virus-disease (accessed on 23 August 2023).
- Jacob, S.T.; Crozier, I.; Fischer, W.A., 2nd; Hewlett, A.; Kraft, C.S.; Vega, M.A.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola virus disease. Nat. Rev. Dis. Prim. 2020, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- WHO Zika Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/zika-virus (accessed on 23 August 2023).
- World Health Organization (WHO). Dengue and Severe Dengue. Key Facts. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue#:~:text=Dengue%20(break%2Dbone%20fever),body%20aches%2C%20nausea%20and%20rash (accessed on 17 September 2023).
- WHO. Middle East Respiratory Syndrome Coronavirus (MERS-CoV). MERS Monthly Summary, November 2019. Available online: https://www.who.int/emergencies/mers-cov/en/ (accessed on 20 August 2023).
- Severe Acute Respiratory Syndrome (SARS). Available online: https://www.who.int/health-topics/severe-acute-respiratory-syndrome#tab=tab_1 (accessed on 20 August 2023).
- Influenza Type A Viruses|Avian Influenza. Available online: https://www.cdc.gov/flu/avianflu/influenza-a-virus-subtypes.htm (accessed on 15 August 2023).
- Sutton, T.C.; Finch, C.; Shao, H.; Angel, M.; Chen, H.; Capua, I.; Cattoli, G.; Monne, I.; Perez, D.R. Airborne transmission of highly pathogenic H7N1 influenza virus in ferrets. J. Virol. 2014, 88, 6623–6635. [Google Scholar] [CrossRef]
- Sun, H.; Li, F.; Liu, Q.; Du, J.; Liu, L.; Sun, H.; Li, C.; Liu, J.; Zhang, X.; Yang, J.; et al. Mink is a highly susceptible host species to circulating human and avian influenza viruses. Emerg. Microbes Infect. 2021, 10, 472–480. [Google Scholar] [CrossRef]
- Mpox (Monkeypox). Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 20 August 2023).
- Noda, T.; Sagara, H.; Suzuki, E.; Takada, A.; Kida, H.; Kawaoka, Y. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J. Virol. 2002, 76, 4855–4865. [Google Scholar] [CrossRef]
- Baseler, L.; Chertow, D.S.; Johnson, K.M.; Feldmann, H.; Morens, D.M. The Pathogenesis of Ebola Virus Disease. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 387–418. [Google Scholar] [CrossRef]
- Ebola Disease Distribution Map: Cases of Ebola Disease in Africa Since 1976. Available online: https://www.cdc.gov/vhf/ebola/history/distribution-map.html (accessed on 24 August 2023).
- Martin, P.; Laupland, K.B.; Frost, E.H.; Valiquette, L. Laboratory diagnosis of Ebola virus disease. Intensiv. Care Med. 2015, 41, 895–898. [Google Scholar] [CrossRef]
- Dudas, G.; Carvalho, L.M.; Bedford, T.; Tatem, A.J.; Baele, G.; Faria, N.R.; Park, D.J.; Ladner, J.T.; Arias, A.; Asogun, D.; et al. Virus genomes reveal factors that spread and sustained the Ebola epidemic. Nature 2017, 544, 309–315. [Google Scholar] [CrossRef]
- Tong, Y.-G.; The China Mobile Laboratory Testing Team in Sierra Leone; Shi, W.-F.; Liu, D.; Qian, J.; Liang, L.; Bo, X.-C.; Liu, J.; Ren, H.-G.; Fan, H.; et al. Genetic diversity and evolutionary dynamics of Ebola virus in Sierra Leone. Nature 2015, 524, 93–96. [Google Scholar] [CrossRef]
- World Health Organization. WHO Emergency Use Assessment and Listing for Ebola Virus Disease IVDs. Product: ReEBOV Antigen Rapid Test Kit; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Broadhurst, M.J.; Kelly, J.D.; Miller, A.; Semper, A.; Bailey, D.; Groppelli, E.; Simpson, A.; Brooks, T.; Hula, S.; Nyoni, W.; et al. ReEBOV Antigen Rapid Test kit for point-of-care and laboratory-based testing for Ebola virus disease: A field validation study. Lancet 2015, 386, 867–874. [Google Scholar] [CrossRef]
- Layqah, L.A.; Eissa, S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim. Acta 2019, 186, 224. [Google Scholar] [CrossRef]
- Brauburger, K.; Hume, A.J.; Mühlberger, E.; Olejnik, J. Forty-five years of marburg virus research. Viruses 2012, 4, 1878–1927. [Google Scholar] [CrossRef]
- Marburg Virus Disease Distribution Map. Available online: https://www.cdc.gov/vhf/marburg/outbreaks/distribution-map.html (accessed on 24 August 2023).
- Coelho, S.V.A.; Neris, R.L.S.; Papa, M.P.; Schnellrath, L.C.; Meuren, L.M.; Tschoeke, D.A.; Leomil, L.; Verçoza, B.R.F.; Miranda, M.; Thompson, F.L.; et al. Development of standard methods for Zika virus propagation, titration, and purification. J. Virol. Methods 2017, 246, 65–74. [Google Scholar] [CrossRef]
- Woolsey, C.; Cross, R.W.; Agans, K.N.; Borisevich, V.; Deer, D.J.; Geisbert, J.B.; Gerardi, C.; Latham, T.E.; Fenton, K.A.; Egan, M.A.; et al. A highly attenuated Vesiculovax vaccine rapidly protects nonhuman primates against lethal Marburg virus challenge. PLoS Negl. Trop. Dis. 2022, 16, e0010433. [Google Scholar] [CrossRef] [PubMed]
- Herrada, C.A.; Kabir, A.; Altamirano, R.; Asghar, W. Advances in Diagnostic Methods for Zika Virus Infection. J. Med. Devices 2018, 12, 040802. [Google Scholar] [CrossRef]
- Madanayake, N.H.; Rienzie, R.; Adassooriya, N.M. Electron microscopic methods for virus diagnosis. In Viral Infections and Antiviral Therapies; Elsevier: Amsterdam, The Netherlands, 2023; pp. 121–140. [Google Scholar] [CrossRef]
- Lim, J.K.; Seydou, Y.; Carabali, M.; Barro, A.; Dahourou, D.L.; Lee, K.S.; Nikiema, T.; Namkung, S.; Lee, J.-S.; Shin, M.Y.; et al. Clinical and epidemiologic characteristics associated with dengue during and outside the 2016 outbreak identified in health facility-based surveillance in Ouagadougou, Burkina Faso. PLoS Negl. Trop. Dis. 2019, 13, e0007882. [Google Scholar] [CrossRef] [PubMed]
- Needs, S.H.; Sirivisoot, S.; Jegouic, S.; Prommool, T.; Luangaram, P.; Srisawat, C.; Sriraksa, K.; Limpitikul, W.; Mairiang, D.; Malasit, P.; et al. Smartphone multiplex microcapillary diagnostics using Cygnus: Development and evaluation of rapid serotype-specific NS1 detection with dengue patient samples. PLoS Negl. Trop. Dis. 2022, 16, e0010266. [Google Scholar] [CrossRef]
- Masters, P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006, 66, 193–292. [Google Scholar] [CrossRef]
- Chong, Z.X.; Liew, W.P.P.; Ong, H.K.; Yong, C.Y.; Shit, C.S.; Ho, W.Y.; Ng, S.Y.; Yeap, S.K. Current diagnostic approaches to detect two important betacoronaviruses: Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Pathol.-Res. Pract. 2021, 225, 153565. [Google Scholar] [CrossRef]
- Singh, B.; Datta, B.; Ashish, A.; Dutta, G. A comprehensive review on current COVID-19 detection methods: From lab care to point of care diagnosis. Sens. Int. 2021, 2, 100119. [Google Scholar] [CrossRef] [PubMed]
- Massart, S.; Chiumenti, M.; De Jonghe, K.; Glover, R.; Haegeman, A.; Koloniuk, I.; Komínek, P.; Kreuze, J.; Kutnjak, D.; Lotos, L.; et al. Virus detection by high-throughput sequencing of small RNAs: Large-scale performance testing of sequence analysis strategies. Phytopathology 2019, 109, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Al Ahmad, M.; Mustafa, F.; Panicker, N.; Rizvi, T.A. Optical Detection of SARS-CoV-2 Utilizing Antigen-Antibody Binding Interactions. Sensors 2021, 21, 6596. [Google Scholar] [CrossRef]
- Moreira, E.A.; Yamauchi, Y.; Matthias, P. How Influenza Virus Uses Host Cell Pathways during Uncoating. Cells 2021, 10, 1722. [Google Scholar] [CrossRef] [PubMed]
- Korteweg, C.; Gu, J. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am. J. Pathol. 2008, 172, 1155–1170. [Google Scholar] [CrossRef]
- Lansiaux, E.; Jain, N.; Laivacuma, S.; Reinis, A. The virology of human monkeypox virus (hMPXV): A brief overview. Virus Res. 2022, 322, 198932. [Google Scholar] [CrossRef]
- Chelsky, Z.L.; Dittmann, D.; Blanke, T.; Chang, M.; Vormittag-Nocito, E.; Jennings, L.J. Validation Study of a Direct Real-Time PCR Protocol for Detection of Monkeypox Virus. J. Mol. Diagn. 2022, 24, 1155–1159. [Google Scholar] [CrossRef]
- Moss, B. “Poxviridae.” Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 2129–2159. [Google Scholar]
- Turley, P.K. Vaccine: From vacca, a cow. AJO-DO Clin. Companion 2021, 1, 5–6. [Google Scholar] [CrossRef]
- History of Smallpox. CDC. Available online: https://www.cdc.gov/smallpox/history/history.html (accessed on 15 September 2023).
- Carroll, D.S.; Emerson, G.L.; Li, Y.; Sammons, S.; Olson, V.; Frace, M.; Nakazawa, Y.; Czerny, C.P.; Tryland, M.; Kolodziejek, J.; et al. Chasing Jenner’s vaccine: Revisiting cowpox virus classification. PLoS ONE 2011, 6, e23086. [Google Scholar] [CrossRef]
- Patel, M.; Lee, A.D.; Redd, S.B.; Clemmons, N.S.; McNall, R.J.; Cohn, A.C.; Gastañaduy, P.A. Increase in Measles Cases—United States, January 1–April 26, 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 402–404. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Measles Cases and Outbreaks. Available online: https://www.cdc.gov/measles/cases-outbreaks.html (accessed on 16 September 2023).
- Patel, M.; Lee, A.D.; Clemmons, N.S.; Redd, S.B.; Poser, S.; Blog, D.; Zucker, J.R.; Leung, J.; Link-Gelles, R.; Pham, H.; et al. National Update on Measles Cases and Outbreaks—United States, January 1–October 1, 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 893–896. [Google Scholar] [CrossRef]
- O’reilly, K.M.; Lamoureux, C.; Molodecky, N.A.; Lyons, H.; Grassly, N.C.; Tallis, G. An assessment of the geographical risks of wild and vaccine-derived poliomyelitis outbreaks in Africa and Asia. BMC Infect. Dis. 2017, 17, 367. [Google Scholar] [CrossRef] [PubMed]
- Polio Vaccine: Vaccine-Derived Poliovirus. Available online: https://www.cdc.gov/vaccines/vpd/polio/hcp/vaccine-derived-poliovirus-faq.html (accessed on 15 September 2023).
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J.A. Vaccine hesitancy: An overview. Hum. Vaccines Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- FDA News Release: FDA Approves First COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed on 1 September 2023).
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccines Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef]
- Halperin, S.A.; Das, R.; Onorato, M.T.; Liu, K.; Martin, J.; Grant-Klein, R.J.; Nichols, R.; Coller, B.-A.; Helmond, F.A.; Simon, J.K. Immunogenicity, lot consistency, and extended safety of rVSVΔG-ZEBOV-GP vaccine: A phase 3 randomized, double-blind, placebo-controlled study in healthy adults. J. Infect. Dis. 2019, 220, 1127–1135. [Google Scholar] [CrossRef]
- Schieffelin, J. REVIVE (Response to the Ebola Virus Vaccine). 15 August 2023. Available online: https://clinicaltrials.gov/study/NCT05992480?cond=Ebola%20Virus%20Disease&intr=Vaccine&rank=3 (accessed on 28 August 2023).
- Study Record. Available online: https://clinicaltrials.gov/study/NCT05817422?cond=Marburg%20Virus%20Disease&term=vaccine&limit=25&page=1&rank=1 (accessed on 11 September 2023).
- Study Record. Available online: https://clinicaltrials.gov/study/NCT05469802?cond=Zika%20Virus&term=vaccine&limit=25&aggFilters=status:not%20rec%20act&rank=2 (accessed on 11 September 2023).
- Current U.S. Bird Flu Situations in Humans. Available online: https://www.cdc.gov/flu/avianflu/inhumans.htm#:~:text=Six%20main%20hemagglutinin%20(HA)%20subtypes,majority%20of%20infections%20in%20people (accessed on 15 August 2023).
- Zeng, X.; Tian, G.; Shi, J.; Deng, G.; Li, C.; Chen, H. Vaccination of poultry successfully eliminated human infection with H7N9 virus in China. Sci. China Life Sci. 2018, 61, 1465–1473. [Google Scholar] [CrossRef]
- Puthanakit, T.; Anugulruengkitt, S.; Jantarabenjakul, W. Prevention of Emerging Infections in Children. Pediatr. Clin. N. Am. 2022, 69, 185–202. [Google Scholar] [CrossRef]
- Key Facts about Seasonal Flu Vaccines. Available online: https://www.cdc.gov/flu/prevent/keyfacts.htm (accessed on 15 August 2023).
- Bah, E.I.; Lamah, M.C.; Fletcher, T.; Jacob, S.T.; Brett-Major, D.M.; Sall, A.A.; Shindo, N.; Fischer, W.A.; Lamontagne, F.; Saliou, S.M.; et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N. Engl. J. Med. 2015, 372, 40. [Google Scholar] [CrossRef]
- Schieffelin, J.S.; Shaffer, J.G.; Goba, A.; Gbakie, M.; Gire, S.K.; Colubri, A.; Sealfon, R.S.; Kanneh, L.; Moigboi, A.; Momoh, M.; et al. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N. Engl. J. Med. 2014, 371, 2092. [Google Scholar] [CrossRef]
- Chertow, D.S.; Kleine, C.; Edwards, J.K.; Scaini, R.; Giuliani, R.; Sprecher, A. Ebola virus disease in west africa—Clinical manifestations and management. N. Engl. J. Med. 2014, 371, 2054–2057. [Google Scholar] [CrossRef]
- Batra, S.; Ochani, R.K.; Diwan, M.N.; Yasmin, F.; Qureshi, S.S.; Bhimani, S.; Shaikh, S.; Tariq, M.A.; Ashraf, M.A.; Farooqi, H.A.; et al. Clinical aspects of Ebola virus disease: A review. Infez. Med. 2020, 28, 212–222. [Google Scholar] [PubMed]
- Comer, J.E.; Brasel, T.; Massey, S.; Beasley, D.W.; Cirimotich, C.M.; Sanford, D.C.; Chou, Y.-L.; Niemuth, N.A.; Novak, J.; Sabourin, C.L.; et al. Natural History of Marburg Virus Infection to Support Medical Countermeasure Development. Viruses 2022, 14, 2291. [Google Scholar] [CrossRef] [PubMed]
- Marburg Virus Disease. Available online: https://www.who.int/health-topics/marburg-virus-disease#tab=tab (accessed on 20 July 2023).
- Geisbert, T.W.; Young, H.A.; Jahrling, P.B.; Davis, K.J.; Kagan, E.; Hensley, L.E. Mechanisms underlying coagulation abnormalities in Ebola hemorrhagic fever: Overexpression of tissue factor in primate monocytes/macrophages is a key event. J. Infect. Dis. 2003, 188, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Rougeron, V.; Feldmann, H.; Grard, G.; Becker, S.; Leroy, E. Ebola and Marburg haemorrhagic fever. J. Clin. Virol. 2015, 64, 111–119. [Google Scholar] [CrossRef]
- Martines, R.B.; Ng, D.L.; Greer, P.W.; Rollin, P.E.; Zaki, S.R. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J. Pathol. 2015, 235, 153–174. [Google Scholar] [CrossRef]
- Rayaprolu, V.; Fulton, B.O.; Rafique, A.; Arturo, E.; Williams, D.; Hariharan, C.; Callaway, H.; Parvate, A.; Schendel, S.L.; Parekh, D.; et al. Structure of the Inmazeb cocktail and resistance to Ebola virus escape. Cell Host Microbe 2023, 31, 260–272.e7. [Google Scholar] [CrossRef]
- Misasi, J.; Sullivan, N.J. Immunotherapeutic strategies to target vulnerabilities in the Ebolavirus glycoprotein. Immunity 2021, 54, 412–436. [Google Scholar] [CrossRef]
- Brasil, P.; Calvet, G.A.; Siqueira, A.M.; Wakimoto, M.; De Sequeira, P.C.; Nobre, A.; Quintana, M.D.S.B.; De Mendonça, M.C.L.; Lupi, O.; De Souza, R.V.; et al. Zika Virus Outbreak in Rio de Janeiro, Brazil: Clinical Characterization, Epidemiological and Virological Aspects. PLoS Negl. Trop. Dis. 2016, 10, e0004636. [Google Scholar] [CrossRef]
- Derrington, S.M.; Cellura, A.P.; McDermott, L.E.; Gubitosi, T.; Sonstegard, A.M.; Chen, S.; Garg, A. Mucocutaneous Findings and Course in an Adult With Zika Virus Infection. JAMA Dermatol. 2016, 152, 691–693. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Zika Virus Disease Q & A. Available online: http://www.cdc.gov/zika/disease-qa.html (accessed on 20 August 2023).
- Parra, B.; Lizarazo, J.; Jiménez-Arango, J.A.; Zea-Vera, A.F.; González-Manrique, G.; Vargas, J.; Angarita, J.A.; Zuñiga, G.; Lopez-Gonzalez, R.; Beltran, C.L.; et al. Guillain-Barré Syndrome Associated with Zika Virus Infection in Colombia. N. Engl. J. Med. 2016, 375, 1513. [Google Scholar] [CrossRef]
- Adebayo, G.; Neumark, Y.; Gesser-Edelsburg, A.; Abu Ahmad, W.; Levine, H. Zika pandemic online trends, incidence and health risk communication: A time trend study. BMJ Glob. Health 2017, 2, e000296. [Google Scholar] [CrossRef] [PubMed]
- Kakooza-Mwesige, A.; Tshala-Katumbay, D.; Juliano, S.L. Viral infections of the central nervous system in Africa. Brain Res. Bull. 2019, 145, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Song, B.-H.; Yun, S.-I.; Woolley, M.; Lee, Y.-M. Zika virus: History, epidemiology, transmission, and clinical presentation. J. Neuroimmunol. 2017, 308, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika virus and birth defects—Reviewing the evidence for causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef]
- Brasil, P.; Vasconcelos, Z.; Kerin, T.; Gabaglia, C.R.; Ribeiro, I.P.; Bonaldo, M.C.; Damasceno, L.; Pone, M.V.; Pone, S.; Zin, A.; et al. Zika virus vertical transmission in children with confirmed antenatal exposure. Nat. Commun. 2020, 11, 3510. [Google Scholar] [CrossRef]
- Walker, C.L.; Little, M.-T.E.; Roby, J.A.; Armistead, B.; Gale, M.; Rajagopal, L.; Nelson, B.R.; Ehinger, N.; Mason, B.; Nayeri, U.; et al. Zika virus and the nonmicrocephalic fetus: Why we should still worry. Am. J. Obstet. Gynecol. 2019, 220, 45–56. [Google Scholar] [CrossRef]
- Vouga, M.; Baud, D. Imaging of congenital Zika virus infection: The route to identification of prognostic factors. Prenat. Diagn. 2016, 36, 799–811. [Google Scholar] [CrossRef]
- Zika Virus: Symptoms, Diagnosis, & Treatment. Available online: http://www.cdc.gov/zika/symptoms/index.html (accessed on 20 August 2023).
- Guery, B.; Poissy, J.; el Mansouf, L.; Séjourné, C.; Ettahar, N.; Lemaire, X.; Vuotto, F.; Goffard, A.; Behillil, S.; Enouf, V.; et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: A report of nosocomial transmission. Lancet 2013, 381, 2265–2272. [Google Scholar] [CrossRef]
- Clinical Management of Severe Acute Respiratory Infection When Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Infection Is Suspected, Updated January 2019. Available online: https://www.who.int/publications/i/item/WHO-MERS-Clinical-15-1-Revision-1 (accessed on 20 August 2023).
- Tai, D.Y. Pharmacologic Treatment of SARS: Current Knowledge and Recommendations. Ann. Acad. Med. Singap. 2007, 36, 438–443. [Google Scholar] [CrossRef]
- Wu, Y.; Kang, L.; Guo, Z.; Liu, J.; Liu, M.; Liang, W. Incubation Period of COVID-19 Caused by Unique SARS-CoV-2 Strains: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2228008. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Valdes, A.M.; Polidori, L.; Antonelli, M.; Penamakuri, S.; Nogal, A.; Louca, P.; May, A.; Figueiredo, J.C.; Hu, C.; et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: A prospective observational study from the ZOE COVID Study. Lancet 2022, 399, 1618. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.H.; Reiter, E.R.; French, E.; Costanzo, R.M. Decreasing Incidence of Chemosensory Changes by COVID-19 Variant. Otolaryngol. Head Neck Surg. 2023, 168, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.; van der Meer, N.J.; Arbous, M.S.; Gommers, D.A.; Kant, K.M.; Kaptein, F.H.; van Paassen, J.; Stals, M.A.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145. [Google Scholar] [CrossRef]
- World Health Organization Therapeutics and COVID-19: Living Guideline V. 13.1, Published on 1/12/23. Available online: https://app.magicapp.org/#/guideline/nBkO1E (accessed on 20 August 2023).
- Oner, A.F.; Bay, A.; Arslan, S.; Akdeniz, H.; Sahin, H.A.; Cesur, Y.; Epcacan, S.; Yilmaz, N.; Deger, I.; Kizilyildiz, B.; et al. Avian influenza A (H5N1) infection in eastern Turkey in 2006. N. Engl. J. Med. 2006, 355, 2179–2185. [Google Scholar] [CrossRef]
- Liem, N.T.; Tung, C.V.; Hien, N.D.; Hien, T.T.; Chau, N.Q.; Long, H.T.; Hien, N.T.; Mai, L.Q.; Taylor, W.R.; Wertheim, H.; et al. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004–2006. Clin. Infect. Dis. 2009, 48, 1639. [Google Scholar] [CrossRef]
- Gao, H.-N.; Lu, H.-Z.; Cao, B.; Du, B.; Shang, H.; Gan, J.-H.; Lu, S.-H.; Yang, Y.-D.; Fang, Q.; Shen, Y.-Z.; et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N. Engl. J. Med. 2013, 368, 2277–2285. [Google Scholar] [CrossRef]
- Ip, D.K.; Liao, Q.; Wu, P.; Gao, Z.; Cao, B.; Feng, L.; Xu, X.; Jiang, H.; Li, M.; Bao, J.; et al. Detection of mild to moderate influenza A/H7N9 infection by China’s national sentinel surveillance system for influenza-like illness: Case series. BMJ 2013, 346, f3693. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, S.; Song, Z.; Wang, W.; Hao, P.; Li, J.; Zhang, X.; Yen, H.-L.; Shi, B.; Li, T.; et al. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet 2013, 381, 2273–2279. [Google Scholar] [CrossRef]
- Minhaj, F.S.; Ogale, Y.P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C.M.; Wilkins, K.; Bachmann, L.; Chatelain, R.; et al. Monkeypox Outbreak—Nine States, May 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Cash-Goldwasser, S.; Labuda, S.M.; McCormick, D.W.; Rao, A.K.; McCollum, A.M.; Petersen, B.W.; Chodosh, J.; Brown, C.M.; Chan-Colenbrander, S.Y.; Dugdale, C.M.; et al. Ocular Monkeypox—United States, July–September 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Fontoura, D.D.S.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: Descriptive case series. BMJ 2022, 378, e072410. [Google Scholar] [CrossRef]
- Guidance for Tecovirimat Use. Available online: https://www.cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html (accessed on 20 August 2023).
- Semba, R.D. The Ocular complications of smallpox and smallpox immunization. Arch. Ophthalmol. 2003, 121, 715–719. [Google Scholar] [CrossRef]
- Pan, M.; Lednicky, J.; Wu, C. Collection, particle sizing and detection of airborne viruses. J. Appl. Microbiol. 2019, 127, 1596–1611. [Google Scholar] [CrossRef]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, 6558. [Google Scholar] [CrossRef]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, L.; Qi, W.; Liu, Y.; Lin, J. Challenges and Perspectives for Biosensing of Bioaerosol Containing Pathogenic Microorganisms. Micromachines 2021, 12, 798. [Google Scholar] [CrossRef]
- Li, Y.H.; Fan, Y.Z.; Jiang, L.; Wang, H.B. Aerosol and environmental surface monitoring for SARS-CoV-2 RNA in a designated hospital for severe COVID-19 patients. Epidemiol. Infect. 2020, 148, e154. [Google Scholar] [CrossRef]
- Li, J.; Leavey, A.; Wang, Y.; O’neil, C.; Wallace, M.A.; Burnham, C.-A.D.; Boon, A.C.; Babcock, H.; Biswas, P. Comparing the performance of 3 bioaerosol samplers for influenza virus. J. Aerosol Sci. 2017, 115, 133–145. [Google Scholar] [CrossRef]
- Kenny, L.C.; Thorpe, A.; Stacey, P. A collection of experimental data for aerosol monitoring cyclones. Aerosol Sci. Technol. 2017, 51, 1190–1200. [Google Scholar] [CrossRef]
- Sung, G.; Ahn, C.; Kulkarni, A.; Shin, W.G.; Kim, T. Highly efficient in-line wet cyclone air sampler for airborne virus detection. J. Mech. Sci. Technol. 2017, 31, 4363–4369. [Google Scholar] [CrossRef]
- Alonso, C.; Raynor, P.C.; Davies, P.R.; Torremorell, M. Concentration, Size Distribution, and Infectivity of Airborne Particles Carrying Swine Viruses. PLoS ONE 2015, 10, e0135675. [Google Scholar] [CrossRef]
- Lindsley, W.G.; Blachere, F.M.; Beezhold, D.H.; Thewlis, R.E.; Noorbakhsh, B.; Othumpangat, S.; Goldsmith, W.T.; McMillen, C.M.; Andrew, M.E.; Burrell, C.N.; et al. Viable influenza A virus in airborne particles expelled during coughs versus exhalations. Influenza Other Respir. Viruses 2016, 10, 404–413. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, M. Liquid impinger BioSampler’s performance for size-resolved viable bioaerosol particles. J. Aerosol Sci. 2017, 106, 34–42. [Google Scholar] [CrossRef]
- Kim, H.R.; An, S.; Hwang, J. An integrated system of air sampling and simultaneous enrichment for rapid biosensing of airborne coronavirus and influenza virus. Biosens. Bioelectron. 2020, 170, 112656. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; An, S.; Hwang, J. High air flow-rate electrostatic sampler for the rapid monitoring of airborne coronavirus and influenza viruses. J. Hazard. Mater. 2021, 412, 125219. [Google Scholar] [CrossRef]
- Hong, S.C.; Kang, J.S.; Lee, J.E.; Kim, S.S.; Jung, J.H. Continuous aerosol size separator using inertial microfluidics and its application to airborne bacteria and viruses. Lab Chip 2015, 15, 1889–1897. [Google Scholar] [CrossRef]
- Choi, J.; Hong, S.C.; Kim, W.; Jung, J.H. Highly Enriched, Controllable, Continuous Aerosol Sampling Using Inertial Microfluidics and Its Application to Real-Time Detection of Airborne Bacteria. ACS Sens. 2017, 2, 513–521. [Google Scholar] [CrossRef]
- Fronczek, C.F.; Yoon, J.-Y. Biosensors for Monitoring Airborne Pathogens. JALA J. Assoc. Lab. Autom. 2015, 20, 390–410. [Google Scholar] [CrossRef]
- Benbough, J.E. Some factors affecting the survival of airborne viruses. J. Gen. Virol. 1971, 10, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Leland, D.S.; Ginocchio, C.C. Role of cell culture for virus detection in the age of technology. Clin. Microbiol. Rev. 2007, 20, 49–78. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, Y.; Chen, Z.; Sun, W.; Liu, X.; Chen, L.; Sun, J.; Qiu, X.; Yu, D.; Zhang, L. Real-Time Detection of LAMP Products of African Swine Fever Virus Using Fluorescence and Surface Plasmon Resonance Method. Biosensors 2022, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-Y.; Buckley, A.; Van Geelen, A.; Lager, K.; Henao-Díaz, A.; Poonsuk, K.; Piñeyro, P.; Baum, D.; Ji, J.; Wang, C.; et al. Detection of pseudorabies virus antibody in swine oral fluid using a serum whole-virus indirect ELISA. J. Vet.-Diagn. Investig. 2020, 32, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Hajjaran, H.; Ebadizadeh, M.; Ataei-Pirkooh, A.; Mohebali, M.; Samimi-Rad, K.; Saberi, R.; Naddaf, S.R. Development of an Indirect Fluorescent Antibody (IFA) Assay for the Detection of Leishmania RNA Virus 2 (LRV2) in Leishmania Parasites. Iran. J. Parasitol. 2022, 17, 349–357. [Google Scholar] [CrossRef]
- Visser, M.; Burger, J.T.; Maree, H.J. Targeted virus detection in next-generation sequencing data using an automated e-probe based approach. Virology 2016, 495, 122–128. [Google Scholar] [CrossRef]
- Madera, S.; Kistler, A.; Ranaivoson, H.C.; Ahyong, V.; Andrianiaina, A.; Andry, S.; Raharinosy, V.; Randriambolamanantsoa, T.H.; Ravelomanantsoa, N.A.F.; Tato, C.M.; et al. Discovery and Genomic Characterization of a Novel Henipavirus, Angavokely Virus, from Fruit Bats in Madagascar. J. Virol. 2022, 96, e0092122. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 20 August 2023).
- Centers for Disease Control and Prevention (CDC). Swine influenza A (H1N1) infection in two children--Southern California, March-April 2009. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 400–402. [Google Scholar]
- Agboli, E.; Zahouli, J.B.Z.; Badolo, A.; Jöst, H. Mosquito-Associated Viruses and Their Related Mosquitoes in West Africa. Viruses 2021, 13, 891. [Google Scholar] [CrossRef]
- Shen-Gunther, J.; Cai, H.; Wang, Y. A Customized Monkeypox Virus Genomic Database (MPXV DB v1.0) for Rapid Sequence Analysis and Phylogenomic Discoveries in CLC Microbial Genomics. Viruses 2022, 15, 40. [Google Scholar] [CrossRef]
- Abdeldayem, O.M.; Dabbish, A.M.; Habashy, M.M.; Mostafa, M.K.; Elhefnawy, M.; Amin, L.; Al-Sakkari, E.G.; Ragab, A.; Rene, E.R. Viral outbreaks detection and surveillance using wastewater-based epidemiology, viral air sampling, and machine learning techniques: A comprehensive review and outlook. Sci. Total Environ. 2022, 803, 149834. [Google Scholar] [CrossRef]
- Mackuľak, T.; Gál, M.; Špalková, V.; Fehér, M.; Briestenská, K.; Mikušová, M.; Tomčíková, K.; Tamáš, M.; Škulcová, A.B. Wastewater-Based Epidemiology as an Early Warning System for the Spreading of SARS-CoV-2 and Its Mutations in the Population. Int. J. Environ. Res. Public Health 2021, 18, 5629. [Google Scholar] [CrossRef] [PubMed]
- Engler, O.; Savini, G.; Papa, A.; Figuerola, J.; Groschup, M.H.; Kampen, H.; Medlock, J.; Vaux, A.; Wilson, A.J.; Werner, D.; et al. European surveillance for west Nile virus in mosquito populations. Int. J. Environ. Res. Public Health 2013, 10, 4869–4895. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, L.; Medema, G. Surveillance of Influenza A and the pandemic influenza A (H1N1) 2009 in sewage and surface water in the Netherlands. J. Water Health 2011, 9, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Gourinat, A.-C.; O’connor, O.; Calvez, E.; Goarant, C.; Dupont-Rouzeyrol, M. Detection of zika virus in urine. Emerg. Infect. Dis. 2015, 21, 84–86. [Google Scholar] [CrossRef]
- Barzon, L.; Pacenti, M.; Franchin, E.; Pagni, S.; Martello, T.; Cattai, M.; Cusinato, R.; Palù, G. Excretion of west Nile virus in urine during acute infection. J. Infect. Dis. 2013, 208, 1086–1092. [Google Scholar] [CrossRef]
| Virus | Size (Diameter) | Shape | Geo-Regions | Detection Method | References |
|---|---|---|---|---|---|
| Ebola | 80 nm, varying length | rod-shaped | 17 countries (mainly Africa) | RT-PCR, LFA, NGS, antigen test | [3,14,15,16,17,18,19,20,21,22] |
| Marburg | <14,000 nm | rod-shaped | 11 countries (mainly Africa) | RT-PCR, ELISA | [23,24] |
| Zika | 40–43 nm | sphere | 89 countries | RT-PCR, ELISA, RT-LAMP | [25,26,27] |
| Dengue | 40–50 nm | sphere | >100 countries | RT-PCR, ELISA, immunoassay | [7,28,29,30] |
| MERS-CoV | 80–120 nm | sphere | 27 countries | RT-PCR, CRISPR, biosensor | [8,31,32,33] |
| SARS-CoV | 80–120 nm | sphere | China and four other countries | RT-PCR, CRISPR | [9,31,32] |
| SARS-CoV-2 | 80–120 nm | sphere | worldwide | RT-PCR, CRISPR, ELISA, LAMP, LFA, NGS, antigen/antibody test | [31,32,33,34,35] |
| Avian Influenza | 100 nm | sphere | worldwide | RT-PCR | [36,37] |
| Monkeypox | 200–250 nm (length) | brick-shaped | 110 countries | RT-PCR | [13,38,39] |
| Sampling Method | Collection Mechanism | Advantage | Disadvantage | References |
|---|---|---|---|---|
| Natural sedimentation | Gravity. Collect the settling bioaerosol using a nutrient agar plate or swabs | Low cost, easy operation, and little impact on microbial activity | Low efficiency; increases microbial risk resulting from cultured pathogenic microorganisms | [106,107] |
| Filtration | Bioaerosol collected on filter media through interception, impaction, and diffusion | High collection efficiency, low cost, and easy to operate | Easily blocked, low collection velocity due to the fragility of the filters, and the air environment may increase the sampling difficulty | [106,108] |
| Centrifugation/cyclone | Centrifugal force deviates bioaerosol into the collection wall or liquid | Compact size, continuous-flow collection, and ability to collect the virus in different particle sizes | Low collection efficiency for small bioaerosols (<1 µm), virus deactivation upon collection, and evaporation of the thin liquid film | [109,110] |
| Impaction | Bioaerosol first drawn into a nozzle with a vacuum pump, then impacted onto a solid collection media | Cost-effective and easy to use | Reduced collection efficiency due to the deposition of the bioaerosol on the impactor wall; decreased bioactivity due to the shear force on the particles | [103,111] |
| Impingement | Bioaerosol is sucked into a chamber through a nozzle and captured with liquid collection media | The virus can be detected without the elution process | Reduced viability due to the shear forces in the nozzle; particles adhere to the wall of the collection chamber | [112,113] |
| Electrostatic precipitators (ESPs) | Bioaerosols are charged by metal needles at the inlet of the ESP, then travel in the direction of collecting electrodes | High collection efficiency can enrich viral particles by more than 106-fold | Extra preparation steps for additional material are needed for viral enrichment | [114,115] |
| Microfluidics | Relies on differently structured microfluid chips to trap and concentrate particles | Low cost, easy integration, and automatic operation | Small sampling volume | [116,117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.J.; Song, H.A.; Pierson, S.L.; Shen-Gunther, J.; Xia, Q. Emerging Infectious Diseases Are Virulent Viruses—Are We Prepared? An Overview. Microorganisms 2023, 11, 2618. https://doi.org/10.3390/microorganisms11112618
Han JJ, Song HA, Pierson SL, Shen-Gunther J, Xia Q. Emerging Infectious Diseases Are Virulent Viruses—Are We Prepared? An Overview. Microorganisms. 2023; 11(11):2618. https://doi.org/10.3390/microorganisms11112618
Chicago/Turabian StyleHan, Jasmine J., Hannah A. Song, Sarah L. Pierson, Jane Shen-Gunther, and Qingqing Xia. 2023. "Emerging Infectious Diseases Are Virulent Viruses—Are We Prepared? An Overview" Microorganisms 11, no. 11: 2618. https://doi.org/10.3390/microorganisms11112618
APA StyleHan, J. J., Song, H. A., Pierson, S. L., Shen-Gunther, J., & Xia, Q. (2023). Emerging Infectious Diseases Are Virulent Viruses—Are We Prepared? An Overview. Microorganisms, 11(11), 2618. https://doi.org/10.3390/microorganisms11112618







