Cultivating Resilience in Dryland Soils: An Assisted Migration Approach to Biological Soil Crust Restoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biocrust Collection
2.2. Biocrust Cultivation
2.3. Sampling Cultivated Biocrust
2.4. Molecular Methods and Bioinformatic
2.5. Statistical Analyses
3. Results
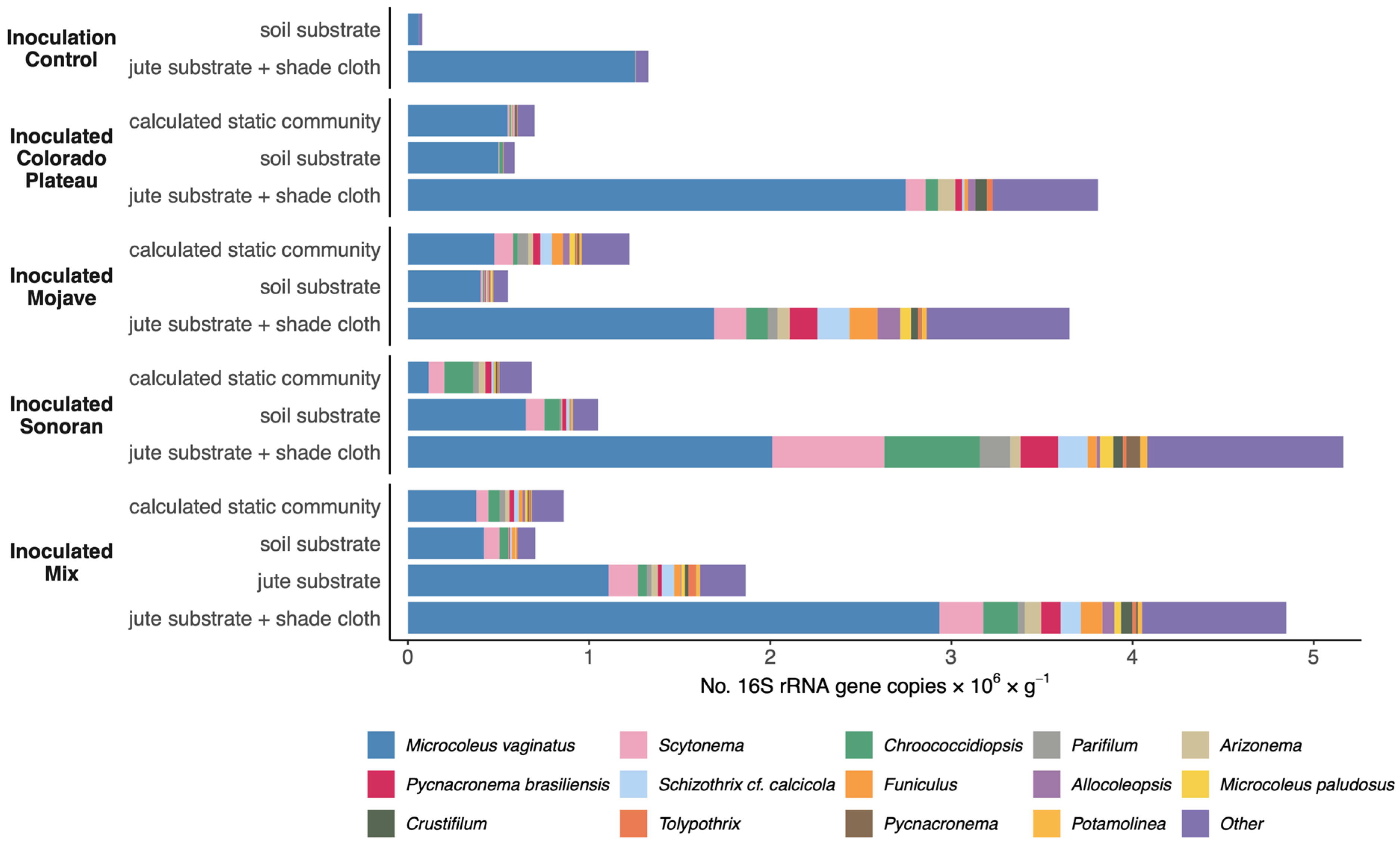
3.1. Cultivated Cyanobacterial Community Composition
3.2. Indicator Cyanobacteria in Cultivated Mixed Biocrust
4. Discussion
4.1. Tradeoffs in Non-Local Sourcing of Biocrust Inocula
4.2. Shade Cloth Enhances Biocrust Cultivation
4.3. Translation to Restoration
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safriel, U.; Adeel, Z.; Niemeijer, D.; Puigdefabregas, J.; White, R.; Lal, R.; Winslow, M.; Ziedler, J.; Prince, S.; Archer, E.; et al. Dryland Systems. In Ecosystems and Human Well-Being: Current State and Trends; Hassan, R., Scholes, R., Ash, N., Eds.; Island Press: Washington DC, USA, 2005; Volume 1. [Google Scholar]
- FAO and Intergovernmental Technical Panel on Soils. Status of the World’s Soil Resources (SWSR)-Main Report; Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils: Rome, Italy, 2015. [Google Scholar]
- Feng, S.; Fu, Q. Expansion of Global Drylands under a Warming Climate. Atmos. Chem. Phys. 2013, 13, 10081–10094. [Google Scholar] [CrossRef]
- Hoover, D.L.; Bestelmeyer, B.; Grimm, N.B.; Huxman, T.E.; Reed, S.C.; Sala, O.; Seastedt, T.R.; Wilmer, H.; Ferrenberg, S. Traversing the Wasteland: A Framework for Assessing Ecological Threats to Drylands. Bioscience 2020, 70, 35–47. [Google Scholar] [CrossRef]
- Sena, A.; Ebi, K. When Land Is Under Pressure Health Is Under Stress. Int. J. Environ. Res. Public Health 2021, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.F.; Maestre, F.T.; Kemp, P.R.; Stafford-Smith, D.M.; Lambin, E. Natural and Human Dimensions of Land Degradation in Drylands: Causes and Consequences. Terrestrial Ecosystems in a Changing World; Global Change: Berlin, Germany, 2007; pp. 247–257. [Google Scholar] [CrossRef]
- Shackelford, N.; Paterno, G.B.; Winkler, D.E.; Erickson, T.E.; Leger, E.A.; Svejcar, L.N.; Breed, M.F.; Faist, A.M.; Harrison, P.A.; Curran, M.F.; et al. Drivers of Seedling Establishment Success in Dryland Restoration Efforts. Nat. Ecol. Evol. 2021, 5, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Copeland, S.M.; Bradford, J.B.; Duniway, M.C.; Schuster, R.M. Potential Impacts of Overlapping Land-Use and Climate in a Sensitive Dryland: A Case Study of the Colorado Plateau, USA. Ecosphere 2017, 8, e01823. [Google Scholar] [CrossRef]
- Weber, B.; Belnap, J.; Büdel, B.; Antoninka, A.J.; Barger, N.N.; Chaudhary, V.B.; Darrouzet-Nardi, A.; Eldridge, D.J.; Faist, A.M.; Ferrenberg, S.; et al. What Is a Biocrust? A Refined, Contemporary Definition for a Broadening Research Community. Biol. Rev. 2022, 97, 1768–1785. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, D.J.; Reed, S.; Travers, S.K.; Bowker, M.A.; Maestre, F.T.; Ding, J.; Havrilla, C.; Rodriguez-Caballero, E.; Barger, N.; Weber, B.; et al. The Pervasive and Multifaceted Influence of Biocrusts on Water in the World’s Drylands. Glob. Chang. Biol. 2020, 26, 6003–6014. [Google Scholar] [CrossRef]
- Sancho, L.G.; Belnap, J.; Colesie, C.; Raggio, J.; Weber, B. Carbon Budgets of Biological Soil Crusts at Micro-, Meso-, and Global Scales. In Biological Soil Crusts: An Organizing Principle in Drylands; Springer: Berlin/Heidelberg, Germany, 2016; pp. 287–304. [Google Scholar] [CrossRef]
- Barger, N.N.; Weber, B.; Garcia-Pichel, F.; Zaady, E.; Belnap, J. Patterns and Controls on Nitrogen Cycling of Biological Soil Crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Springer: Berlin/Heidelberg, Germany, 2016; pp. 257–285. [Google Scholar] [CrossRef]
- Belnap, J.; Büdel, B. Biological Soil Crusts as Soil Stabilizers. In Biological Soil Crusts: An Organizing Principle in Drylands; Springer: Berlin/Heidelberg, Germany, 2016; pp. 305–320. [Google Scholar] [CrossRef]
- Phillips, M.L.; McNellis, B.E.; Howell, A.; Lauria, C.M.; Belnap, J.; Reed, S.C. Biocrusts Mediate a New Mechanism for Land Degradation under a Changing Climate. Nat. Clim. Chang. 2022, 12, 71–76. [Google Scholar] [CrossRef]
- Reed, S.C.; Coe, K.K.; Sparks, J.P.; Housman, D.C.; Zelikova, T.J.; Belnap, J. Changes to Dryland Rainfall Result in Rapid Moss Mortality and Altered Soil Fertility. Nat. Clim. Chang. 2012, 2, 752–755. [Google Scholar] [CrossRef]
- Ladrón De Guevara, M.; Maestre, F.T. Ecology and Responses to Climate Change of Biocrust-Forming Mosses in Drylands. J. Exp. Bot. 2022, 73, 4380–4395. [Google Scholar] [CrossRef]
- Ferrenberg, S.; Reed, S.C.; Belnap, J.; Schlesinger, W.H. Climate Change and Physical Disturbance Cause Similar Community Shifts in Biological Soil Crusts. Proc. Natl. Acad. Sci. USA 2015, 112, 12116–12121. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Bowker, M.; Zhang, Y.; Belnap, J. Natural Recovery of Biological Soil Crusts After Disturbance. In Biological Soil Crusts: An Organizing Principle in Drylands; Springer: Berlin/Heidelberg, Germany, 2016; pp. 479–498. [Google Scholar] [CrossRef]
- Zhao, Y.; Bowker, M.A.; Zhang, Y.; Zaady, E. Enhanced Recovery of Biological Soil Crusts After Disturbance. In Biological Soil Crusts: An Organizing Principle in Drylands; Springer: Berlin/Heidelberg, Germany, 2016; pp. 499–523. [Google Scholar] [CrossRef]
- Adelizzi, R.; O’Brien, E.A.; Hoellrich, M.; Rudgers, J.A.; Mann, M.; Fernandes, V.M.C.; Darrouzet-Nardi, A.; Stricker, E. Disturbance to Biocrusts Decreased Cyanobacteria, N-Fixer Abundance, and Grass Leaf N but Increased Fungal Abundance. Ecology 2022, 103, e3656. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Zhao, Y.; Gao, L.; Yang, Q.; Yang, K. Moss-Dominated Biocrusts Improve the Structural Diversity of Underlying Soil Microbial Communities by Increasing Soil Stability and Fertility in the Loess Plateau Region of China. Eur. J. Soil Biol. 2019, 95, 103120. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Eldridge, D.J.; Bowker, M.A.; Ochoa, V.; Gozalo, B.; Berdugo, M.; Val, J.; Singh, B.K. Biocrust-Forming Mosses Mitigate the Negative Impacts of Increasing Aridity on Ecosystem Multifunctionality in Drylands. N. Phytol. 2016, 209, 1540–1552. [Google Scholar] [CrossRef]
- Steven, B.; Phillips, M.L.; Belnap, J.; Gallegos-Graves, L.V.; Kuske, C.R.; Reed, S.C. Resistance, Resilience, and Recovery of Dryland Soil Bacterial Communities Across Multiple Disturbances. Front. Microbiol. 2021, 12, 648455. [Google Scholar] [CrossRef]
- Belnap, J.; Eldridge, D. Disturbance and Recovery of Biological Soil Crusts. Ecol. Stud. 2001, 150, 363–383. [Google Scholar] [CrossRef]
- Giraldo-Silva, A.; Nelson, C.; Barger, N.N.; Garcia-Pichel, F. Nursing Biocrusts: Isolation, Cultivation, and Fitness Test of Indigenous Cyanobacteria. Restor. Ecol. 2019, 27, 793–803. [Google Scholar] [CrossRef]
- Xiao, J.; Lan, S.; Farías, M.E.; Qian, L.; Xia, L.; Song, S.; Wu, L. The Living Forms of Microcoleus Vaginatus and Their Contributions to the Aggregate Structure of Biocrusts. FEMS Microbiol. Ecol. 2023, 99, fiad040. [Google Scholar] [CrossRef]
- Rajeev, L.; Da Rocha, U.N.; Klitgord, N.; Luning, E.G.; Fortney, J.; Axen, S.D.; Shih, P.M.; Bouskill, N.J.; Bowen, B.P.; Kerfeld, C.A.; et al. Dynamic Cyanobacterial Response to Hydration and Dehydration in a Desert Biological Soil Crust. ISME J. 2013, 7, 2178–2191. [Google Scholar] [CrossRef]
- Nelson, C.; Garcia-Pichel, F. Beneficial Cyanosphere Heterotrophs Accelerate Establishment of Cyanobacterial Biocrust. Appl. Environ. Microbiol. 2021, 87, e0123621. [Google Scholar] [CrossRef]
- Román, J.R.; Roncero-Ramos, B.; Chamizo, S.; Rodríguez-Caballero, E.; Cantón, Y. Restoring Soil Functions by Means of Cyanobacteria Inoculation: Importance of Soil Conditions and Species Selection. Land Degrad. Dev. 2018, 29, 3184–3193. [Google Scholar] [CrossRef]
- Bethany, J.; Giraldo-Silva, A.; Nelson, C.; Barger, N.N.; Garcia-Pichel, F. Optimizing the Production of Nursery-Based Biological Soil Crusts for Restoration of Arid Land Soils. Appl. Environ. Microbiol. 2019, 85, e00735-19. [Google Scholar] [CrossRef]
- Ayuso, S.V.; Silva, A.G.; Nelson, C.; Barger, N.N.; Garcia-Pichel, F. Microbial Nursery Production of High-Quality Biological Soil Crust Biomass for Restoration of Degraded Dryland Soils. Appl. Environ. Microbiol. 2017, 83, e02179-16. [Google Scholar] [CrossRef]
- Antoninka, A.; Bowker, M.A.; Barger, N.N.; Belnap, J.; Giraldo-Silva, A.; Reed, S.C.; Garcia-Pichel, F.; Duniway, M.C. Addressing Barriers to Improve Biocrust Colonization and Establishment in Dryland Restoration. Restor. Ecol. 2020, 28, S150–S159. [Google Scholar] [CrossRef]
- Faist, A.M.; Antoninka, A.J.; Belnap, J.; Bowker, M.A.; Duniway, M.C.; Garcia-Pichel, F.; Nelson, C.; Reed, S.C.; Giraldo-Silva, A.; Velasco-Ayuso, S.; et al. Inoculation and Habitat Amelioration Efforts in Biological Soil Crust Recovery Vary by Desert and Soil Texture. Restor. Ecol. 2020, 28, S96–S105. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Loza, V.; Marusenko, Y.; Mateo, P.; Potrafka, R.M. Temperature Drives the Continental-Scale Distribution of Key Microbes in Topsoil Communities. Science 2013, 340, 1574–1577. [Google Scholar] [CrossRef]
- Moreira, C.; Fernandes, V.; Giraldo-Silva, A.; Roush, D.; Garcia-Pichel, F. Coleofasciculaceae, a Monophyletic Home for the Microcoleus Steenstrupii Complex and Other Desiccation-Tolerant Filamentous Cyanobacteria. J. Phycol. 2021, 57, 1563–1579. [Google Scholar] [CrossRef]
- Butterfield, B.J.; Copeland, S.M.; Munson, S.M.; Roybal, C.M.; Wood, T.E. Prestoration: Using Species in Restoration That Will Persist Now and into the Future. Restor. Ecol. 2017, 25, S155–S163. [Google Scholar] [CrossRef]
- Schwinning, S.; Belnap, J.; Bowling, D.R.; Ehleringer, J.R. Sensitivity of the Colorado Plateau to Change: Climate, Ecosystems, and Society. Ecol. Soc. 2008, 13, 1–28. [Google Scholar] [CrossRef]
- Winkler, D.E.; Backer, D.M.; Belnap, J.; Bradford, J.B.; Butterfield, B.J.; Copeland, S.M.; Duniway, M.C.; Faist, A.M.; Fick, S.E.; Jensen, S.L.; et al. Beyond Traditional Ecological Restoration on the Colorado Plateau. Restor. Ecol. 2018, 26, 1055–1060. [Google Scholar] [CrossRef]
- Farrell, H.L.; Munson, S.M.; Butterfield, B.J.; Duniway, M.C.; Faist, A.M.; Gornish, E.S.; Havrilla, C.A.; Larios, L.; Reed, S.C.; Rowe, H.I.; et al. Soil Surface Treatments and Precipitation Timing Determine Seedling Development across Southwestern US Restoration Sites. Ecol. Appl. 2023, 33, e2834. [Google Scholar] [CrossRef] [PubMed]
- Balazs, K.R.; Kramer, A.T.; Munson, S.M.; Talkington, N.; Still, S.; Butterfield, B.J. The Right Trait in the Right Place at the Right Time: Matching Traits to Environment Improves Restoration Outcomes. Ecol. Appl. 2020, 30, e02110. [Google Scholar] [CrossRef] [PubMed]
- Havrilla, C.A.; Munson, S.M.; McCormick, M.L.; Laushman, K.M.; Balazs, K.R.; Butterfield, B.J. RestoreNet: An Emerging Restoration Network Reveals Controls on Seeding Success across Dryland Ecosystems. J. Appl. Ecol. 2020, 57, 2191–2202. [Google Scholar] [CrossRef]
- Harrison, P.A. Climate Change and the Suitability of Local and Non-Local Species for Ecosystem Restoration. Ecol. Manag. Restor. 2021, 22, 75–91. [Google Scholar] [CrossRef]
- Young, K.E.; Grover, H.S.; Bowker, M.A. Altering Biocrusts for an Altered Climate. New Phytol. 2016, 210, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Antoninka, A.; Chuckran, P.F.; Mau, R.L.; Slate, M.L.; Mishler, B.D.; Oliver, M.J.; Coe, K.K.; Stark, L.R.; Fisher, K.M.; Bowker, M.A. Responses of Biocrust and Associated Soil Bacteria to Novel Climates Are Not Tightly Coupled. Front. Microbiol. 2022, 13, 1177. [Google Scholar] [CrossRef]
- Birnbaum, C.; Bradshaw, L.E.; Ruthrof, K.X.; Fontaine, J.B. Topsoil Stockpiling in Restoration: Impact of Storage Time on Plant Growth and Symbiotic Soil Biota. Restoration 2017, 35, 3. [Google Scholar] [CrossRef]
- Tucker, C.; Antoninka, A.; Day, N.; Poff, B.; Reed, S. Biological Soil Crust Salvage for Dryland Restoration: An Opportunity for Natural Resource Restoration. Restor. Ecol. 2020, 28, S9–S16. [Google Scholar] [CrossRef]
- Official Series Description-THOROUGHFARE Series. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/T/THOROUGHFARE.html (accessed on 14 July 2023).
- Kettler, T.A.; Doran, J.W.; Gilbert, T.L. Simplified Method for Soil Particle-Size Determination to Accompany Soil-Quality Analyses. Soil Sci. Soc. Am. J. 2001, 65, 849–852. [Google Scholar] [CrossRef]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Wong, J.; Heiner, C.; Oh, S.; Theirot, C.; Gulati, A.; McGill, S.; Dougherty, M.K. High-throughput amplicon sequencing of the full-length 16S rRNA gene with single-nucleotide resolution. Nucelic Acids Res. 2019, 47, e103. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Roush, D.; Giraldo-Silva, A.; Garcia-Pichel, F. Cydrasil 3, a Curated 16S RRNA Gene Reference Package and Web App for Cyanobacterial Phylogenetic Placement. Sci. Data 2021, 8, 230. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v3: An Online Tool for the Display and Annotation of Phylogenetic and Other Trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef]
- Matsen, F.A.; Kodner, R.B.; Armbrust, E.V. Pplacer: Linear Time Maximum-Likelihood and Bayesian Phylogenetic Placement of Sequences onto a Fixed Reference Tree. BMC Bioinform. 2010, 11, 538. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Hewitt, N.; Klenk, N.; Smith, A.L.; Bazely, D.R.; Yan, N.; Wood, S.; MacLellan, J.I.; Lipsig-Mumme, C.; Henriques, I. Taking Stock of the Assisted Migration Debate. Biol. Conserv. 2011, 144, 2560–2572. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B.H. Resistance, Resilience, and Redundancy in Microbial Communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef]
- Mueller, J.M.; Hellmann, J.J. An Assessment of Invasion Risk from Assisted Migration. Conserv. Biol. 2008, 22, 562–567. [Google Scholar] [CrossRef]
- Martínez-Hidalgo, P.; Maymon, M.; Pule-Meulenberg, F.; Hirsch, A.M. Engineering Root Microbiomes for Healthier Crops and Soils Using Beneficial, Environmentally Safe Bacteria. Can. J. Microbiol. 2019, 65, 91–104. [Google Scholar] [CrossRef]
- Tariq, M.; Jameel, F.; Ijaz, U.; Abdullah, M.; Rashid, K. Biofertilizer Microorganisms Accompanying Pathogenic Attributes: A Potential Threat. Physiol. Mol. Biol. Plants 2022, 28, 77. [Google Scholar] [CrossRef]
- Bethany, J.; Johnson, S.L.; Garcia-Pichel, F. High Impact of Bacterial Predation on Cyanobacteria in Soil Biocrusts. Nat. Commun. 2022, 13, 4835. [Google Scholar] [CrossRef]
- Giraldo-Silva, A.; Fernandes, V.M.C.; Bethany, J.; Garcia-Pichel, F. Niche Partitioning with Temperature among Heterocystous Cyanobacteria (Scytonema spp., Nostoc spp., and Tolypothrix spp.) from Biological Soil Crusts. Microorganisms 2020, 8, 396. [Google Scholar] [CrossRef]
- Baldanta, S.; Arnal, R.; Blanco-Rivero, A.; Guevara, G.; Navarro Llorens, J.M. First Characterization of Cultivable Extremophile Chroococcidiopsis Isolates from a Solar Panel. Front. Microbiol. 2023, 14, 982422. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Ye, T.; Li, X.; Wang, G. Protection and Damage Repair Mechanisms Contributed To the Survival of Chroococcidiopsis Sp. Exposed To a Mars-Like Near Space Environment. Microbiol. Spectr. 2022, 10, e0344022. [Google Scholar] [CrossRef] [PubMed]
- Chock, T.; Antoninka, A.J.; Faist, A.M.; Bowker, M.A.; Belnap, J.; Barger, N.N. Responses of Biological Soil Crusts to Rehabilitation Strategies. J. Arid. Environ. 2019, 163, 77–85. [Google Scholar] [CrossRef]
- Bowker, M.A.; Antoninka, A.J.; Chuckran, P.F. Improving Field Success of Biocrust Rehabilitation Materials: Hardening the Organisms or Softening the Environment? Restor. Ecol. 2020, 28, S177–S186. [Google Scholar] [CrossRef]
- Downing, W.M.; Krawchuk, M.A.; Meigs, G.W.; Haire, S.L.; Coop, J.D.; Walker, R.B.; Whitman, E.; Chong, G.; Miller, C. Influence of Fire Refugia Spatial Pattern on Post-Fire Forest Recovery in Oregon’s Blue Mountains. Landsc. Ecol. 2019, 34, 771–792. [Google Scholar] [CrossRef]
- Bowker, M.A. Biological Soil Crust Rehabilitation in Theory and Practice: An Underexploited Opportunity. Restor. Ecol. 2007, 15, 13–23. [Google Scholar] [CrossRef]
- Nelson, C.; Giraldo-Silva, A.; Garcia-Pichel, F. A Fog-Irrigated Soil Substrate System Unifies and Optimizes Cyanobacterial Biocrust Inoculum Production. Appl. Environ. Microbiol. 2020, 86, e00624-20. [Google Scholar] [CrossRef] [PubMed]
- Bu, C.; Li, R.; Wang, C.; Bowker, M.A. Successful Field Cultivation of Moss Biocrusts on Disturbed Soil Surfaces in the Short Term. Plant Soil 2017, 429, 227–240. [Google Scholar] [CrossRef]
- Fick, S.E.; Barger, N.; Tatarko, J.; Duniway, M.C. Induced Biological Soil Crust Controls on Wind Erodibility and Dust (PM10) Emissions. Earth Surf. Process. Landf. 2019, 45, 224–236. [Google Scholar] [CrossRef]
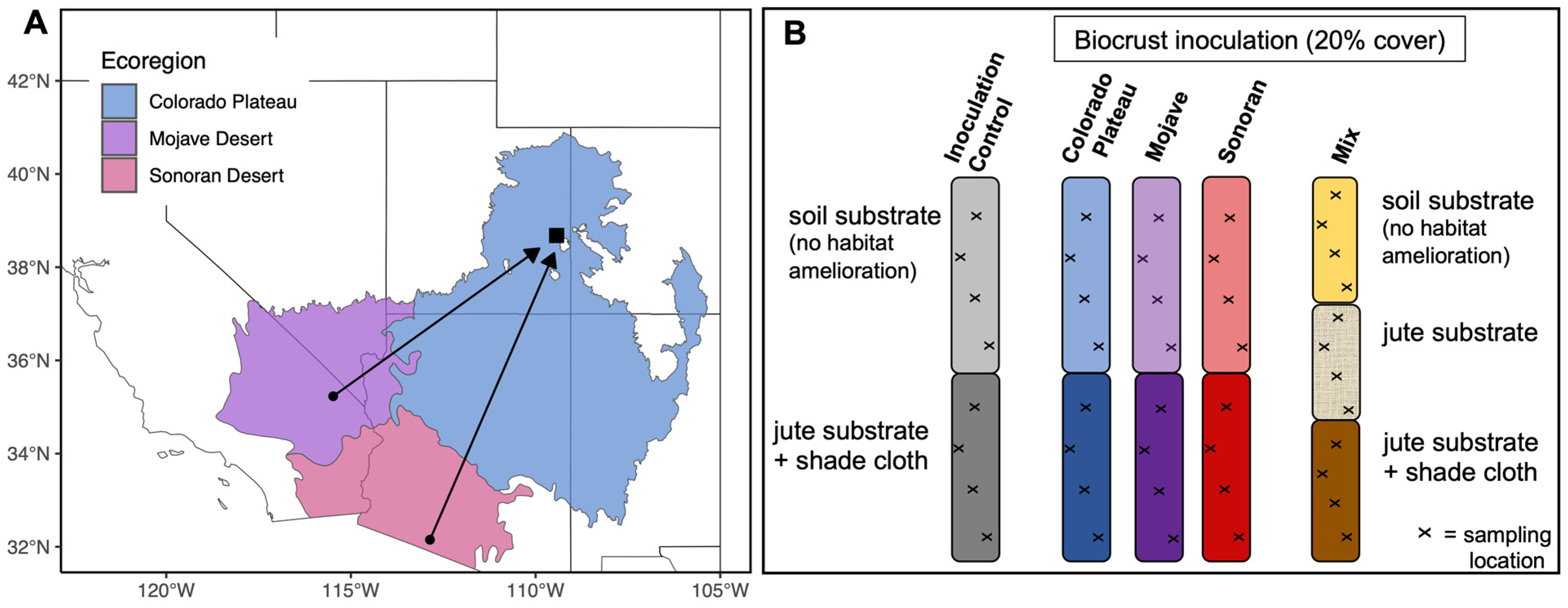



| Site Characteristics | Colorado Plateau 1 | Colorado Plateau 2 | Mojave Desert 3 | Sonoran Desert 4 |
|---|---|---|---|---|
| Elevation (m) | ~1500 | ~1350 | ~920 | ~240 |
| Annual average precipitation (cm) | 21.7 | 22.7 | 13.9 | 12.6 |
| Annual average Min–Max Temperature (°C) | 6.4–21.8 | 4.8–21.8 | 14.8–23.4 | 15.1–30.6 |
| Soil type 5 | Aridisol Pocum series | Entisol Thoroughfare series | Aridisol Tonopah series | Aridisol Gunsight series |
| Biological Soil Crust (Biocrust) Community | Moss- and cyanobacteria-dominant | Moss- and cyanobacteria-dominant | Lichen- and cyanobacteria-dominant | Lichen- and cyanobacteria-dominant |
| Ecological Site 6 | Semidesert Shallow Sandy Loam (Blackbrush) | Desert Sandy Loam (Fourwing Saltbush) | Arid Active Alluvial Fans | Limy Upland and Desert Pavements |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jech, S.D.; Day, N.; Barger, N.N.; Antoninka, A.; Bowker, M.A.; Reed, S.; Tucker, C. Cultivating Resilience in Dryland Soils: An Assisted Migration Approach to Biological Soil Crust Restoration. Microorganisms 2023, 11, 2570. https://doi.org/10.3390/microorganisms11102570
Jech SD, Day N, Barger NN, Antoninka A, Bowker MA, Reed S, Tucker C. Cultivating Resilience in Dryland Soils: An Assisted Migration Approach to Biological Soil Crust Restoration. Microorganisms. 2023; 11(10):2570. https://doi.org/10.3390/microorganisms11102570
Chicago/Turabian StyleJech, Sierra D., Natalie Day, Nichole N. Barger, Anita Antoninka, Matthew A. Bowker, Sasha Reed, and Colin Tucker. 2023. "Cultivating Resilience in Dryland Soils: An Assisted Migration Approach to Biological Soil Crust Restoration" Microorganisms 11, no. 10: 2570. https://doi.org/10.3390/microorganisms11102570
APA StyleJech, S. D., Day, N., Barger, N. N., Antoninka, A., Bowker, M. A., Reed, S., & Tucker, C. (2023). Cultivating Resilience in Dryland Soils: An Assisted Migration Approach to Biological Soil Crust Restoration. Microorganisms, 11(10), 2570. https://doi.org/10.3390/microorganisms11102570





