In Vivo Production, Development and Storage of Oscheius myriophila (Nematoda: Rhabditida) in Galleria mellonella (Lepidoptera: Pyralidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. G. mellonella Production
2.2. O. myriophila Strain MC5-2014 Propagation
2.3. Quantification of IJs
2.4. Bioassays
In Vivo Production
2.5. Development of O. myriophila
2.6. Fixation of EPNs
2.7. Cleared of EPNs
2.8. Storage of IJs of O. myriophila
2.9. IJ Pathogenicity
2.10. Statistical Analysis
3. Results
3.1. In Vivo Production
3.2. Development of O. myriophila
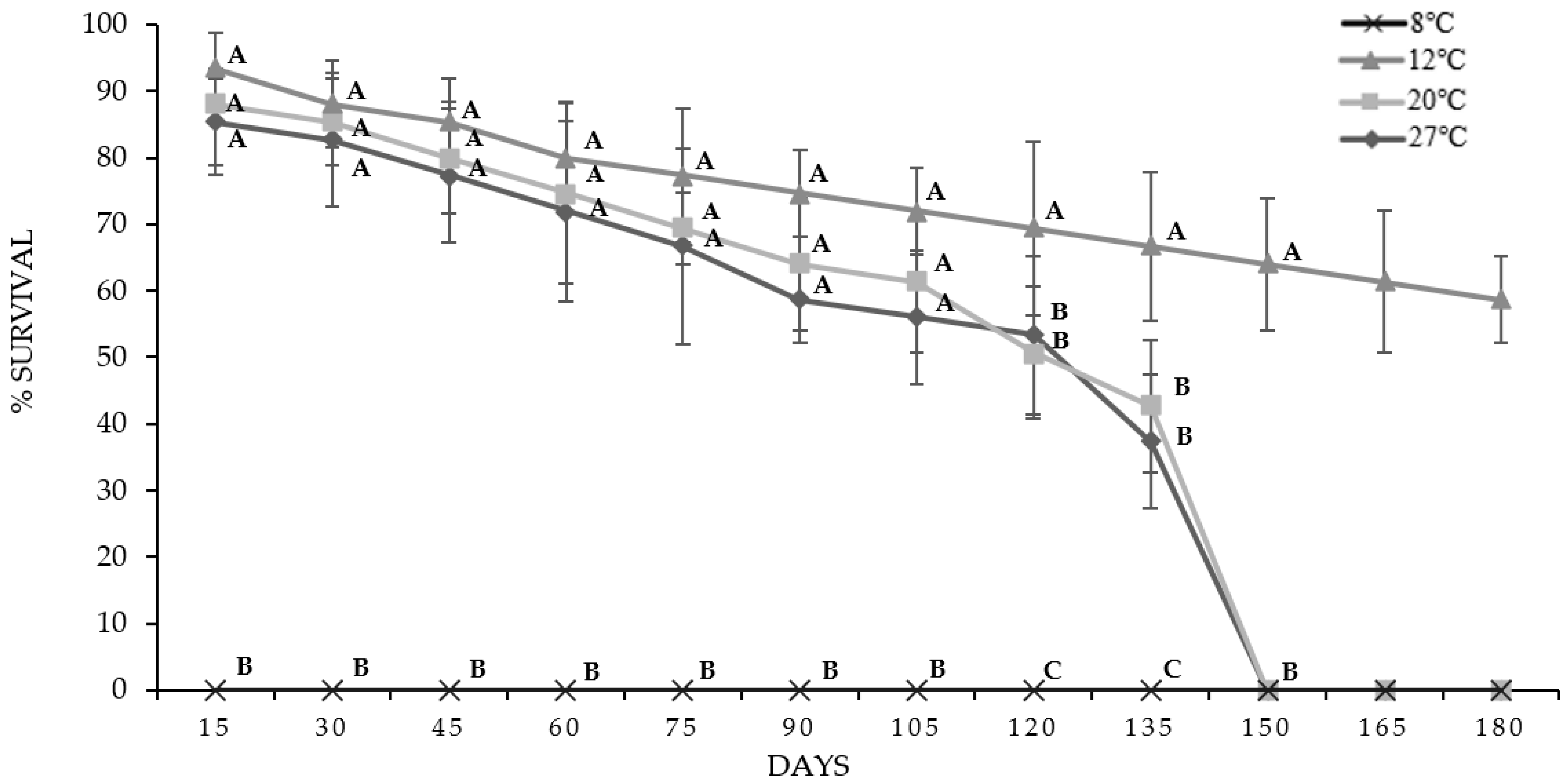
3.3. Storage IJs of O. myriophila
Pathogenicity of IJs
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shapiro-Ilan, D.; Gaugler, R. Production technology for entomopathogenic nematodes and their bacterial symbionts. J. Ind. Microbiol. Biotechnol. 2002, 28, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Travassos, L. Sobre O Genera Oxystomatium. Boletin Biol. 1927, 5, 20–21. [Google Scholar]
- Poinar, G. Description and biology of a new insect parasitic Rhabditoid, Heterorhabditis bacteriophora N. Gen., N. Sp. (Rhabditida; Heterorhabditidae N. Fam.). Nematologica 1975, 21, 463–470. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.; Hazir, S.; Glazer, I. Basic and applied research: Entomopathogenic nematodes. In Microbial Control of Insect and Mite Pests; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 91–105. [Google Scholar] [CrossRef]
- Picoaga, A.; Abelleira, A.; Mansilla, J.; Areeiro, E. The Entomopathogenic Nematodes and Their Application for Biological Control of Insect Pests. Vitic. Enol. Prof. 2007, 113, 42–46. [Google Scholar]
- Zhang, C.; Liu, J.; Xu, M.; Sun, J.; Yang, S.; An, X.; Gao, G.; Lin, M.; Lai, R.; He, Z.; et al. Heterorhabditidoides chongmingensis gen. nov., sp. nov. (Rhabditida: Rhabditidae), a novel menber of the entomopathogenic nematodes. J. Invertebr. Pathol. 2008, 98, 153–168. [Google Scholar] [CrossRef]
- Abebe, E.; Jumba, M.; Bonner, K.; Gray, V.; Morris, K.; Thomas, W. An entomopathogenic Caenorhabditis briggsae. J. Exp. Biol. 2010, 213, 3223–3229. [Google Scholar] [CrossRef]
- Torrini, G.; Mazza, G.; Carletti, B.; Benvenuti, C.; Roversi, P.; Fanelli, E.; Luca, F.; Troccoli, A.; Tarasco, E.O. onirici sp. n. (Nematoda: Rhabditidae): A new entomopathogenic nematode from an Italian cave. Zootaxa 2015, 3937, 533–548. [Google Scholar] [CrossRef]
- Seenivasan, N.; Sivakumar, M. Screening for environmental stress-tolerant entomopathogenic nematodes virulent against cotton bollworms. Phytoparasitica 2014, 42, 165–177. [Google Scholar] [CrossRef]
- Castro-Ortega, I.; Caspeta-Mandujano, J.; Suárez-Rodríguez, R.; Peña-Chora, G.; Ramírez-Trujillo, J.; Cruz-Pérez, K.; Sosa, I.; Hernández-Velázquez, V. Oscheius myriophila (Nematoda: Rhabditida) isolated in sugar cane soils in Mexico with potential to be used as entomopathogenic nematode. J. Nematol. 2020, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, S.; Xu, M.; Sun, J.; Liu, H.; Liu, J.; Liu, H.; Kan, F.; Sun, J.; Lai, R.; et al. Serratia nematodiphila sp. nov., associated symbiotically with the entomopathogenic nematode Heterorhabditidoides chongmingensis (Rhabditida: Rhabditidae). Int. J. Syst. Evol. Microbiol. 2009, 59, 1603–1608. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.; Gaugler, R.; Lewis, E. In vivo production of entomopathogenic nematodes. Int. J. Nematol. 2004, 14, 13–18. [Google Scholar]
- Sáenz, A. Importancia de los nemátodos entomopatógenos para el control biológico de plagas en palma de aceite. Palmas 2005, 26, 41–57. [Google Scholar]
- Nguyen, K.; Buss, E. Steinernema phyllophagae n. sp (Rhabditida: Steinernematidae), a new entomopathogenic nematode from Florida, USA. Nematology 2011, 13, 425–442. [Google Scholar] [CrossRef]
- Pilz, C.; Wegensteiner, R.; Keller, S. Natural occurrence of insect pathogenic fungi and insect parasitic nematodes in Diabrotica virgifera virgifera populations. Biocontrol 2008, 53, 353–359. [Google Scholar] [CrossRef]
- Kaya, H.; Stock, S. Techniques in insect nematology. In Manual of Techniques in Insect Pathology; Academic Press: Cambridge, MA, USA, 1997; pp. 281–324. [Google Scholar] [CrossRef]
- Campos-Herrera, R.; Escuer, M.; Labrador, S.; Robertson, L.; Barrios, L.; Gutiérrez, C. Distribution of the entomopathogenic nematodes from La Rioja (Northern Spain). J. Invertebr. Pathol. 2007, 95, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Koppenhöfer, A.; Kaya, H. Ecological characterization of Steinernema rarum. J. Invertebr. Pathol. 1999, 73, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, M.; Clausi, M. Biological control of chestnut insect pests by means of entomopathogenic nematodes. Adv. Hortic. Sci. 2006, 20, 40–44. [Google Scholar]
- Castillo, C.; Gallegos, P.; Oña, M. Manejo de Nemátodos Entomopatógenos en Laboratorio; Instituto Nacional Autónomo de Investigaciones Agropecuarias (INIAP): Quito, Ecuador; Estación Experimental Santa Catalina (EESC): Quito, Ecuador; Departamento Nacional de Protección Vegetal (DNPV): Quito, Ecuador; Panamericana Sur de Quito: Quito, Ecuador, 2011; pp. 48–49. [Google Scholar]
- Woodring, J.; Kaya, H. Steinernematid and Heterorhabditid Nematodes: A Handbook of Techniques; Agricultural Experiment Station Fayetteville: Fayetteville, AR, USA, 1988; Volume 31, pp. 1—130, 249. [Google Scholar]
- Maldonado, G. Manual de Prácticas de Parasitología Con Énfasis en Helmintos Parásitos de Peces de Agua Dulce y Otros Animales Silvestres de México; Universidad Nacional Autónoma de México: México City, Mexico, 2009; pp. 15–18. Available online: http://www.ibiologia.unam.mx (accessed on 9 November 2021).
- Caspeta-Mandujano, J. Nemátodos Parásitos de Peces de Agua Dulce de México: Clave de Identificación, Descripción y Distribución de Las Especies; AGT Editor: Los Angeles, CA, USA, 2010; pp. 3–6. [Google Scholar]
- Acevedo, J.; Cavalcanti, R.; Andaló, V.; Mendonça, L. Efecto de temperatura, concentración y tiempo de almacenamiento en la supervivencia de nemátodos entomopatógenos. Rev. Colomb. Entomol. 2006, 32, 24–30. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.; Gaugler, R.; Tedders, L.; Brown, I.; Lewis, E. Optimization of inoculation for in vivo production of entomopathogenic nematodes. J. Nematol. 2002, 34, 343–350. [Google Scholar]
- Saenz, A.; Luque, J. Cultivation in vivo and method of storage for infective juveniles of Steinernema feltiae (Rhabdita: Stenernematidae). Agron. Colomb. 2000, 17, 37–42. [Google Scholar]
- Realpe, A.; Bustillo, P.; López, N. Optimización de la cría de Galleria mellonella (L.) para la producción de nematodos entomopatógenos parásitos de la broca del café. Cenicafé 2007, 58, 142–157. [Google Scholar]
- Lindegreen, J.; Valero, K.; Mackey, B. Simple in vivo production and storage methods for Steinernema carpocapsae infective juveniles. J. Nematol. 1993, 25, 193–197. [Google Scholar]
- Boff, M.; Wiegers, G.; Smits, P. Effect of storage time and temperature on infectivity, reproduction and development of Heterorhabditis megidis in Galleria mellonella. Nematology 2000, 2, 635–644. [Google Scholar] [CrossRef]
- Sáenz, A.; Mejia-Torres, M.C. Ecological characterisation of the Colombian entomopathogenic nematode Heterorhabditis sp. SL0708. Braz. J. Biol. 2013, 73, 239–243. [Google Scholar] [CrossRef]
- Çimen, H.; Lee, M.; Hatting, J.; Hazir, S.; Stock, S. Steinernema innovationi n. sp. (Panagrolaimomorpha: Steinernematidae), a new entomopathogenic nematode species from South Africa. J. Helminthol. 2015, 89, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H. Soil Ecology. In Entomopathogenic Nematodes in Biological Control; CRC Press: Boca Raton, FL, USA, 1990; Volume 93. [Google Scholar]
- Gaugler, R.; McGuire, T.; Campbell, J. Genetic variability among strains of the entomopathogenic nematode Steinernema feltiae. J. Nematol. 1989, 21, 247–253. [Google Scholar]
- Somasekhar, N.; Grewal, P.; Klein, M. Genetic variability in stress tolerance and fitness among natural populations of Steinernema carpocapsae. Biol. Control 2002, 23, 303–310. [Google Scholar] [CrossRef]
- Hominick, W. Entomopathogenic rhabditid nematodes and pest-control. Parasitol. Today 1990, 6, 148–152. [Google Scholar] [CrossRef]
- Rosa, J.; Simoes, N. Evaluation of twenty-eight strains of Heterorhabditis bacteriophora isolated in Azores for biocontrol of the armyworm, Pseudaletia unipuncta (Lepidoptera: Noctuidae). Biol. Control 2004, 29, 409–417. [Google Scholar] [CrossRef]
- Morton, A.; Garcia-del-Pino, F. Ecological characterization of entomopathogenic nematodes isolated in stone fruit orchard soils of Mediterranean areas. J. Invertebr. Pathol. 2009, 102, 203–213. [Google Scholar] [CrossRef]
- Glazer, I. Survival biology. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI: Wallingford, UK, 2002; pp. 169–187. [Google Scholar] [CrossRef]
- Rolston, A.; Meade, C.; Boyle, S.; Kakouli-Duarte, T.; Downes, M. Intraspecific variation among isolates of the entomopathogenic nematode Steinernema feltiae from Bull Island, Ireland. Nematology 2009, 11, 439–451. [Google Scholar]
- Hazir, S.; Stock, S.; Kaya, H.; Koppenhofer, A.; Keskin, N. Developmental temperature effects on five geographic isolates of the entomopathogenic nematode Steinernema feltiae (Nematoda: Steinernematidae). J. Invertebr. Pathol. 2001, 77, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Grewal, P.; Selvan, S.; Gaugler, R. Thermal adaptation of entomopathogenic nematodes -niche breadth for infection, establishment, and reproduction. J. Therm. Biol. 1994, 19, 245–253. [Google Scholar] [CrossRef]

| Temperature | Inoculum of IJs | IJs/Larvae Mean ± SD |
|---|---|---|
| [50] | 13,100 ± 4319 A | |
| 27 °C | [100] | 10,849 ± 2716 A |
| [500] | 19,166 ± 7462 A |
| Hours | Stage | Mean ± SD | n | |
|---|---|---|---|---|
| Length | Width | |||
| 8 | J3 | 526.66 ± 20.54 | 38.33 ± 2.35 | 3 |
| 12 | J3 | 535 ± 11.18 | 35.75 ± 3.76 | 4 |
| 24 | N/A | N/A | N/A | N/A |
| 48 | J3 | 525 ± 20.61 | 33.83 ± 3.43 | 6 |
| 72 | J3 | 510 ± 28.86 | 37 ± 3.16 | 7 |
| 96 | J4 | 608 ± 30.22 | 42.6 ± 2.24 | 5 |
| 120 | J4 | 610 ± 26.07 | 43.6 ± 1.95 | 5 |
| Female | 1090 ± 48.98 | 78 ± 6.78 | 5 | |
| 144 | Female | 1320 ± 52.38 | 90 ±3.16 | 5 |
| J1 | 256 ± 25.76 | 22 ± 1.89 | 5 | |
| 168 | J1 | 250 ± 18.97 | 22 ± 2.44 | 5 |
| J2 | 386 ± 64.37 | 28 ± 6.78 | 5 | |
| J3 | 582 ± 38.67 | 43 ± 5.09 | 5 | |
| 192 | J4 | 615 ± 35.93 | 40.8 ± 3.43 | 6 |
| Female | 850 ± 44.72 | 68 ± 2.44 | 5 | |
| 216 | Female | 906 ± 33.82 | 65 ± 3.16 | 5 |
| 240 | Female | 990 ± 66.33 | 75 ± 4.47 | 5 |
| J1 | 240 ± 27.56 | 22 ± 2.44 | 5 | |
| 264 | J3 | 516.66 ± 23.57 | 35 ± 2.88 | 6 |
| J4 | 576 ± 22.44 | 56 ± 5.09 | 5 | |
| Female | 980 ± 24.49 | 80 ± 3.16 | 5 | |
| Male | 850 ± 54.77 | 56 ± 8.60 | 5 | |
| 288 | J4 | 620 ± 40 | 55 ± 3.16 | 5 |
| Female | 1008.33 ± 53.35 | 83.33 ± 2.35 | 6 | |
| O. myriophila | |||
|---|---|---|---|
| Days | 12 °C | 20 °C | 27 °C |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| 30 | 97.5 ± 4.33 A | 97.5 ± 4.33 A | 95 ± 8.66 A |
| 60 | 92.5 ± 8.29 A | 85 ± 5 A | 77.5 ± 4.33 A |
| 90 | 87.5 ± 4.33 A | 72.5 ± 8.29 AB | 62.5 ± 8.29 B |
| 120 | 80 ± 7.07 A | 55 ± 5 B | 45 ± 8.66 B |
| 150 | 72.5 ± 8.29 A | N/A | N/A |
| 180 | 67.5 ± 9.57 A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guadarrama-Avila, T.M.; Ramírez-Trujillo, J.A.; Rodríguez-Ocampo, T.G.; Peña-Chora, G.; Arenas-Sosa, I.; Hernández-Velázquez, V.M. In Vivo Production, Development and Storage of Oscheius myriophila (Nematoda: Rhabditida) in Galleria mellonella (Lepidoptera: Pyralidae). Microorganisms 2023, 11, 2571. https://doi.org/10.3390/microorganisms11102571
Guadarrama-Avila TM, Ramírez-Trujillo JA, Rodríguez-Ocampo TG, Peña-Chora G, Arenas-Sosa I, Hernández-Velázquez VM. In Vivo Production, Development and Storage of Oscheius myriophila (Nematoda: Rhabditida) in Galleria mellonella (Lepidoptera: Pyralidae). Microorganisms. 2023; 11(10):2571. https://doi.org/10.3390/microorganisms11102571
Chicago/Turabian StyleGuadarrama-Avila, Tania Marel, José Augusto Ramírez-Trujillo, Thania Gisel Rodríguez-Ocampo, Guadalupe Peña-Chora, Iván Arenas-Sosa, and Víctor Manuel Hernández-Velázquez. 2023. "In Vivo Production, Development and Storage of Oscheius myriophila (Nematoda: Rhabditida) in Galleria mellonella (Lepidoptera: Pyralidae)" Microorganisms 11, no. 10: 2571. https://doi.org/10.3390/microorganisms11102571
APA StyleGuadarrama-Avila, T. M., Ramírez-Trujillo, J. A., Rodríguez-Ocampo, T. G., Peña-Chora, G., Arenas-Sosa, I., & Hernández-Velázquez, V. M. (2023). In Vivo Production, Development and Storage of Oscheius myriophila (Nematoda: Rhabditida) in Galleria mellonella (Lepidoptera: Pyralidae). Microorganisms, 11(10), 2571. https://doi.org/10.3390/microorganisms11102571








