Abstract
The Kosakonia cowanii Cp1 strain was isolated from seeds of Capsicum pubescens R. & P. cultivated in Michoacan, Mexico. Genetic and ecological role analyses were conducted for better characterization. The results show that genome has a length of 4.7 Mbp with 56.22% G + C and an IncF plasmid of 128 Kbp with 52.51% G + C. Furthermore, pathogenicity test revealed nonpathogenic traits confirmed by the absence of specific virulence-related genes. Interestingly, when fungal inhibitory essays were carried out, the bacterial synthesis of volatile organic compounds (VOCs) with antifungal activity showed that Sclerotinia sp. and Rhizoctonia solani were inhibited by 87.45% and 77.24%, respectively. Meanwhile, Sclerotium rolfsii, Alternaria alternata, and Colletotrichum gloeosporioides demonstrated a mean radial growth inhibition of 52.79%, 40.82%, and 55.40%, respectively. The lowest inhibition was by Fusarium oxysporum, with 10.64%. The VOCs’ characterization by headspace solid–phase microextraction combined with gas chromatography–mass spectrometry (HS-SPME-GC–MS) revealed 65 potential compounds. Some of the compounds identified with high relative abundance were ketones (22.47%), represented by 2-butanone, 3-hydroxy (13.52%), and alcohols (23.5%), represented by ethanol (5.56%) and 1-butanol-3-methyl (4.83%). Our findings revealed, for the first time, that K. cowanii Cp1 associated with C. pubescens seeds possesses potential traits indicating that it could serve as an effective biocontrol.
1. Introduction
Pepper (Capsicum spp.) has an exciting history of domestication and has seen more than 6000 years of use in diverse foodstuffs in Mexico, such as in fresh, dried, or processed products. It is believed that this crop originated in South America [1,2,3]. Therefore, Mexico and Central America are considered genetic diversity hotspots that have generated important domesticated varieties of pepper. These peppers have relevance in the agriculture and food industry, specifically those industries that are linked to the use of pigments, shape, size, appearance, flavor, pungency, and nutritive components [4,5]. In addition, historical records indicate that Capsicum was introduced to Spain by Christopher Columbus after discovering America in 1493, and then to the rest of Europe and—eventually—to India, Asia, and Africa [6]. Geographical displacement and domestication processes such as artificial and natural selection in agricultural environments have led to the existence of a great number of species in the Capsicum genus. However, there are five important cultivated species of economical relevance that are considered here: C. annuum L., C. frutescens L., C. chinense Jacq., C. baccatum L., and C. pubescens R. & P. [1,2,3,4,5,6].
Therefore, all agronomic management approaches for pepper cultivation during historical domestication are important to consider in terms of obtaining fruits and seeds with high quality for diverse purposes [3]. From these significant traits, genetic lines with resistances to diverse pathogens, such as viruses, bacteria, and fungi, are of ecological and economical relevance during plant and seed development stages [7,8,9,10]. In addition to modern breeding programs, recently, diverse methodologies, such as metagenomics, transcriptomics, proteomics, or metabolomics approaches, have been relevant to understanding microorganism–plant interactions or plant development during the lifecycle of Capsicum [11,12,13,14,15].
Throughout the last decade, the study of microbiomes on the seeds from diverse plant species, including Capsicum, has increased due to its ecological importance [16,17,18]. These studies highlight the metabolic activities of microorganism endophytes or epiphytes on seed germination, seedling establishment, plant growth, and plant development stages and as a biocontrol [16,17,18]. Moreover, it is of special interest that the variations in the diversity of seed-associated microorganisms (SAMs) could be determined by their plant genotype as well as by environmental and soil management practices [16]. Therefore, the characterization of the SAMs on Capsicum with beneficial or detrimental traits is necessary during agricultural practices for an assessment of seed quality and plant development.
Although the origin or transmission route of SAMs is under study, it is interesting to understand the recruitment of diverse communities of microorganisms during plant seed development and to evaluate their metabolic potential through molecule production and characterization, which may indicate ecological significance and agricultural applications [16]. Thus, for instance (and of particular interest for our research work), the diverse members of the family Enterobacteriaceae were isolated from seeds of a xerophytic plant, Lactuca serriola. Among them is Kosakonia cowanii, which possesses the ability to produce a high concentration of exopolysaccharides with effects on plant drought tolerance [19]. In addition, K. cowanii has been isolated from diverse crops with plant-promoting activities through auxin (IAA) and siderophore production [20,21]. Also, the great metabolic ability of the Kosakonia genus has been characterized by the diverse strains isolated from insects, plants, human, or other sources [22,23,24,25,26,27,28,29,30]. Recently, our research group reported the presence of K. cowanii in chili powder via 16s rRNA library sequencing [31], and the isolation of a K. cowanii Ch1 strain with the ability to produce active VOCs against certain fungal pathogens and colonize the seeds of C. annuum L. [32] was also achieved. These results allow us to hypothesize that K. cowanii could be associated with fruits or seeds of C. annuum L.
Although systematic research of SAMs in Capsicum is limited, this plant is cultivated around the world, and its production is close to 34.5 million tons annually. Furthermore, in Mexico, it is one of the most important economic and agricultural crops, cultivated on a total of 150 million hectares [33]. Therefore, the high diversity of genetic varieties of Capsicum plants that are grown in diverse soils and environmental conditions make it necessary to explore SAMs as a source of beneficial microorganisms. In the present report, we hypothesize that K. cowanii could be associated with Capsicum seeds. Therefore, our results show, for the first time, that the presence of K. cowanii, specifically, the strain associated with Capsicum pubescens seeds, has an important ecological role in terms of controlling pathogenic fungi. In addition, this opens the possibility of a further exploration of the SAMs in the Capsicum that are cultivated under different environmental conditions in Mexico.
2. Materials and Methods
2.1. Bacterial Strain Isolation
Serrano pepper (Capsicum annuum L.) and manzano pepper (Capsicum pubescens) fruits without visual damage or infections were obtained from cultivated plants in Querétaro and Michoacán, México. Then, they were surface disinfected with sodium hypochlorite (10% v/v) for 10 min; this was followed by disinfection with ethanol (50% v/v) for 10 min. Then, they were air dried at room temperature in a biosafety cabinet. The seeds were placed on tryptic soy agar (TSA) medium (Difco Laboratories, Detroit, MI, USA) with 100 μg/mL of ampicillin and incubated at 37 °C for 24 h. Bacterial colonies were isolated in the same culture medium. For taxonomy identification, the 16s rDNA gene was amplified by PCR, and amplicon was sequenced at Macrogen Inc. (Seoul, Republic of Korea). The sequences obtained were analyzed by neighbor-joining method in MEGA X software and compared with the sequences of K. cowanii obtained from NCBI database [34,35,36,37].
2.2. Pathogenicity Test
A pathogenicity test was conducted using five bacterial strains isolated from C. pubescens seeds (which were identified as K. cowanii), following the methodology in our previous report [32]. In general, seeds of Capsicum annuum L. var. serrano were disinfected in sodium hypochlorite (10%, v/v) for 5 min and rinsed with sterile distilled water. Germination was carried out in seedling trays under greenhouse conditions. After 45 to 50 days post-germination, seedlings were transplanted into pots. Plants with 13–14 true leaves were used for pathogenicity test. On the other hand, fruits without damage or infection were disinfected with sodium hypochlorite (10%, v/v) for 10 min, ethanol (50%, v/v) for 10 min, and, finally, rinsed with sterile distilled water. Bacterial suspension (10 μL) with approximately 1 × 108 CFU/mL was inoculated on six leaves of each plant in triplicate and on three pepper fruits; both were damaged with a sterile needle and inoculated at that zone. Negative controls were inoculated with sterile distilled water. Inoculated plants were kept in greenhouse conditions while inoculated fruits were placed inside of sealed container at room temperature. The plants were observed for 5–7 days.
2.3. Genome Sequencing and Assembly
We randomly selected the K. cowanii strain Cp1 based on pathogenicity test and fungal inhibitory essays to isolate its genomic DNA for sequencing. DNA was extracted with the ZymoBIOMICSTM DNA Miniprep Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s instructions. We used the Genomic Sequencing Service at Zymo Research, Irvine, CA, USA, which used the NovaSeq® (Illumina, San Diego, CA, USA) platform. Sequence reads were processed at the Bacterial and Viral Bioinformatics Resource Center (BV-BRC). First, a Fastq was used to filtrate the sequences [38]. Second, for genome annotation, the RAST tool kit (RASTtk) was used [39]. Lastly, the circular genome map was modeled in CIRCOS software [40]. The genome statistics were as follows: coarse consistency (98.7%), fine consistency (98%), CheckM completeness (99.7%), and CheckM contamination (0.1%). The bacterial phylogenetic tree was constructed by using the pipelines at BV-BRC, where Mash/MinHash [41], PGFams [38], MUSCLE v5 [37], RaxML v8.2.11 [37], and fast bootstrapping [38] were included in order to conduct the phylogenetic analysis. PlasmidSPAdes version v3.13.0, with default settings, was used for the plasmid assembly [42]. DNA sequences were deposited in NCBI as BioProject ID PRJNA1003013. The genome accession number is JAUZWC000000000.
2.4. Antibiotic Susceptibility Testing
The antimicrobial phenotype of K. cowanii Cp1 and Escherichia coli XL1 blue as a control were evaluated using the guidelines of CLSI [43]. In general, bacterial strains were inoculated in tryptic soy broth (TSB) medium and growth at 37 °C and shaken until an optical density (OD) between 0.4 and 0.5 at 600 nm was achieved. Then, 100 μL of bacterial culture was spread on Mueller–Hinton agar (Bioxon). The antimicrobial test discs (Oxoid) were as follows: penicillin (10 U), ampicillin (10 μg), carbenicillin (100 μg), dicloxacillin (1 μg), and amoxicillin/clavulanic acid (20/10 μg); cephalosporins were cephalothin (30 μg) and cefotaxime (30 μg); fluoroquinolones were ciprofloxacin (5 μg) and norfloxacin (10 μg); aminoglycosides were amikacin (30 μg), gentamicin (10 μg), and netilmicin (30 μg); macrolides were clindamycin (30 μg) and erythromycin (15 μg); carbapenems were imipenem (10 μg) and meropenem (10 μg); chloramphenicol (30 μg); nitrofurantoin (300 μg), tetracycline (30 μg); trimethoprim-sulfamethoxazole (25 μg) and the glycopeptide vancomycin (30 μg). Culture plates were incubated overnight at 37 °C and the inhibitory or clear zones around the disc were recorded in mm, according to guidelines.
2.5. Inhibitory Effects of VOCs
The following fungal strains were provided by the Laboratory of Plants and Agriculture Biotechnology at Queretaro University, México: Alternaria alternata, Colletotrichum gloeosporioides, Fusarium oxysporum, Rhizoctonia solani, Sclerotium rolfsii, and Sclerotinia sp. The growing conditions were as follows: potato dextrose agar (PDA) medium (Difco Laboratories, Detroit, MI, USA) kept at 28 °C over a 5- to 7-day period, conducted according to the growth rate of each fungal strain.
The inhibitory assays were evaluated by radial mycelial growth according to the methodology described previously in [32]. In general, the two-compartment plastic plate device was sealed with parafilm to avoid VOC loss. On the lower side of the device, which was used with TSA medium (Difco Laboratories, Detroit, MI, USA), a bacterial suspension (1 × 108 CFU/mL) was inoculated. On the upper side of the device, which was used with PDA medium (Difco Laboratories, Detroit, MI, USA), a fungal growth disk was inoculated. Each control was tested for fungi and growth on PDA medium (Difco Laboratories, Detroit, MI, USA). Each device was an experimental unit and was incubated at 28 °C to obtain the radial mycelial growth according to the following equation: mycelial growth inhibition (%) = [(dc−dt)/dc] × 100, where dc and dt represent the mycelial growth diameters (in mm) of the control and treatment groups, respectively. The experiments were conducted in triplicate for a statistical analysis of variance and for Duncan’s multiple range test (p = 0.05), which were performed using DPS V12.01 software. Alternatively, bacterial antagonism toward S. rolfsii was tested by dual-culture assay on PDA to prove additional mechanism of inhibition. Bacterial strain (10 μL, 1 × 108 CFU/mL) was inoculated close to and around fungal disk. The essay was kept at 28 °C over a 2- to 3-day period.
2.6. HS-SPME-GC–MS Analysis of VOCs
As the first step in obtaining a bacterial culture with volatile organic compounds (which have inhibitory effects on Sclerotium rolfsii), a cell-free filtrate was obtained of K. cowanii Cp1 grown on Tryptic soy broth (TSB) medium, with continuous shaking (100 rpm) at 37 °C, which was sampled every 6 h up to a total of 48 h. TSB medium without K. cowanii Cp1 strain was used as the control. The cell-free filtrate was obtained via centrifugation at 14,000 rpm for 5 min and after being filtered through a 0.2 μm membrane (Sigma-Aldrich). Then, 500 μL of the sample was placed on PDA medium (Difco Laboratories, Detroit, MI, USA), and a 7 mm mycelial disk of Sclerotium rolfsii was inoculated. The radial mycelial that was grown was evaluated according to the equation mentioned above. Furthermore, the sample that was obtained after 18 h of bacterial growth displayed the highest inhibitory effect; thus, it was used for VOC characterization. To recover the VOCs from the bacterial culture that was grown over 18 h on TSB medium, the sample was processed according to the methodology, and using the device, that was previously reported in [32].
Among the bacterial volatiles obtained, and based on the commercial availability (J.T., Baker, Fermont, Meyer, Sigma-Aldrich, Toluca, Mexico), seven standard compounds were evaluated individually on the inhibition of radial mycelial growth. Using the previous two-compartment plastic plate device, different amounts of individual VOCs were added to sterile filter papers on one side and on the upper side of the device, and an S. rolfsii growth disk of 8 mm was inoculated on PDA medium. The amounts VOCs assayed were: 50, 100, and 150 μL/plate of 2-Butanone; 50, 100, and 150 μL/plate of 2-Butanone, 3-hydroxy; 10, 20, and 40 μL/plate of 2-Nonanone; 50, 100, and 150 μL/plate of acetone; 5, 10, and 20 μL/plate of acetic acid (unidentified compound as VOC component in K. cowanii Cp1); 50, 100, and 150 μL/plate of benzyl alcohol; and 50, 100, and 200 μL/plate of ethanol. Sterile distilled water was used as control. Each device was incubated at 28 °C for a 3-day period to obtain the radial mycelial growth, according to equation previously described. The experiments were conducted in triplicate.
3. Results
3.1. Isolation of K. cowanii from Capsicum pubescens Seeds
Seeds from C. annuum and C. pubescens were obtained and processed for bacterial isolation on TSA medium. In a previous work [32], we isolated a K. cowanii strain that was resistant to β-lactam antibiotics; as such, in this case, we decided to supplement the TSA medium with 100 μg/mL of ampicillin in order to determine if this could be a similar case. From diverse seeds that were obtained from the fruits of Capsicum spp., a bacterial growth was noted on the seeds of manzano peppers (C. pubescens) that were obtained from Michoacan, Mexico. In 100% of the seeds of one fruit, there was a similar phenotype to K. cowanii (Figure 1). The bacterial isolates from the seeds were streaked on the same culture medium so as to obtain isolated colonies. Five bacterial colonies were selected and identified by using the 16s rDNA gene as a molecular marker. Sequence analysis confirmed them as K. cowanii. Then, we conducted a pathogenicity test for the K. cowanii strains to evaluate the phytopathogenic traits, as previously reported [32]. The infection assays on the fruits and leaves of Capsicum annuum L. showed no infection symptoms (Figure 2).
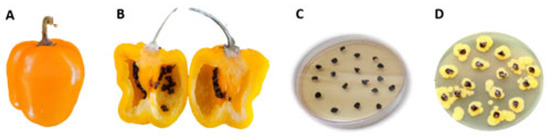
Figure 1.
Isolation of Kosakonia cowanii Cp1. (A) Capsicum pubescens fruits with no infection symptoms; (B) interior of Capsicum pubescens fruits displaying seeds with no infection symptoms; (C) Capsicum pubescens seeds on TSA medium that was supplemented with 100 μg/mL of ampicillin; (D) a growth of Kosakonia cowanii after 24 h of incubation.

Figure 2.
Pathogenicity test of K. cowanii Cp1 in a serrano pepper (Capsicum annuum L.). (A) Assay of K. cowanii Cp1 that grew on serrano pepper leaves; (B) assay of K. cowanii Cp1 on the external part of pepper fruits; (C) assay of K. cowanii Cp1 on the internal part of pepper fruits. No lesions were observed in any case.
3.2. General Genome and the Plasmid Features of K. cowanii Strain Cp1
From these bacterial isolates, based on fungal inhibitory essays (explained below), one of them was selected randomly for genomic characterization. The genome of K. cowanii Cp1 has a total length of 4,765,484 bp, 56.22% of G + C, and a plasmid (pCp1) of 128,063 bp with 52.51% of G + C(Figure 3). The annotation results indicated 4509 protein coding sequences (CDS) and 89 RNA genes (84 tRNA and 5 rRNA). The genome functional subsystem categories are shown in Figure 3, where the metabolism category is represented at 37.88%; protein processing at 9.57%; the stress response, defense, and virulence category at 8.43%; energy at 13.43%; membrane transport at 6.76%; cellular processes at 9.21%; DNA processing at 4.49%; RNA processing at 3.44%; cell envelope at 4.31%; and regulation and cell signaling at 1.31%. On the pCp1 plasmid, 105 hypothetical proteins and 59 proteins with subsystem functional assignments were detected. The BLASTN analysis of plasmid pCp1 showed a 49% alignment and 94.22% similarity with the plasmid 888-76-1 identified in the K. cowanii JCM 10956 strain. The plasmid Wem22 identified in the K. cowanii SMBL-WEM22 strain showed a 46% alignment and 94.22% similarity. The plasmid unnamed1 that was identified in the phytopathogenic K. cowanii strain Pa82 showed a 45% alignment and 95.0% similarity. When the pCh1 plasmid identified in the K. cowanii Ch1 strain that was isolated from chili powder was compared with the pCp1 plasmid, the sequence coverage and similarity was 100%. The pCp1 and pCh1 are IncF plasmids that have VapBC toxin–antitoxin systems; therefore, the presence or association of IncF plasmids with the K. cowanii isolates obtained from chili powder and Capsicum pubescens could be important. As such, additional research is necessary to understand IncF plasmid’s functionality.
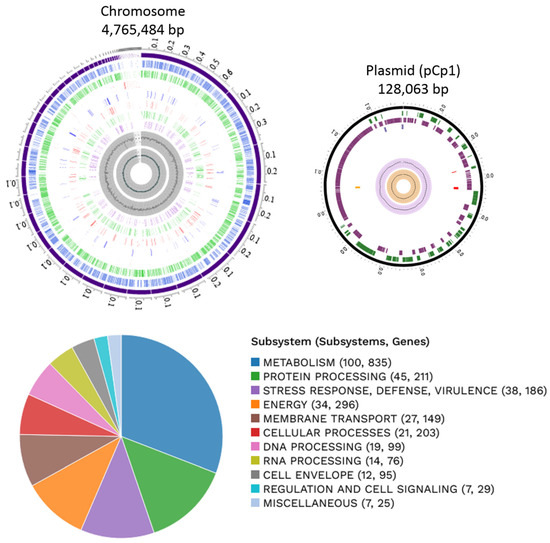
Figure 3.
Graphical circular map of the chromosome and plasmid of K. cowanii Cp1. From the outside to the center rings are the ensembled contigs, CDS on forward, CDS on reverse, non-CDS, AMR genes, VF genes, transporters, drug targets, GC content, and GC skew. The subsystem functional assignments are represented in the figure below.
Additionally, according to the reference database for bacterial virulence factors (VDFB), virulence-related genes are principally classified as flagellar, iron uptake, and siderophore and vir/avr genes were not identified (Table 1).

Table 1.
Virulence-related genes as predicted by VFDB source.
On the other hand, the antibiotic resistance (AMR) genes predicted in the genome were classified into eight categories (Figure 4). However, the genotype must be proven with an AMR phenotype. In this regard, as a first approximation, the AMR phenotype analysis showed that K. cowanii Cp1 has a resistance to the penicillin antibiotic class, such as ampicillin, penicillin G, and carbenicillin, probably due to the TEM β-lactamase detected. In addition, the bacterial strain showed resistance to erythromycin, clindamycin, and vancomycin. We suspect that this is most likely due to the genes being classified as efflux pumps (Figure 4). Interestingly, the rest of the antibiotics caused growth inhibition in K. cowanii Cp1, supported by a clear zone of inhibition (Supplementary Figure S2), which demonstrated the importance of the phenotype versus the genotype in the analysis. Therefore, additional AMR analysis is necessary to understand the complete AMR of K cowanii Cp1.
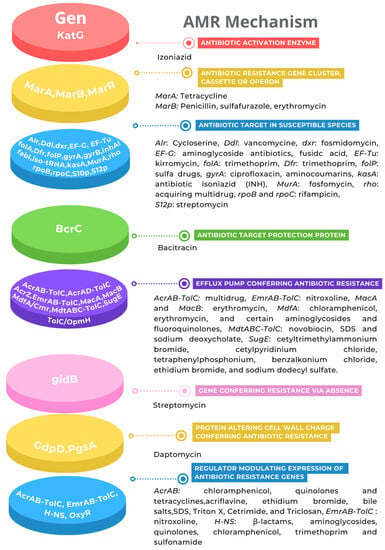
Figure 4.
Antibiotic resistance genes. The AMR mechanism and its related genes are indicated with a color diagram.
Based on the previous genome sequences of the Kosakonia genus isolates from diverse sources and geographical regions, we decided to perform a phylogenomic analysis that was based on 100 core genes for K. cowanii Cp1 and on the related strains of the Kosakonia genus (Figure 5). The results showed that K. cowanii Cp1 is clustered with K. cowanii strain 888-76, K. cowanii JCM 10956, K. cowanii Ch1, K. cowanii strain PF-104, K. cowanii Pa82, and K. cowanii strain Esp Z. Although it is closely related to K. cowanii Ch1—an isolate from Mexican chili powder—the high genetic similarity between both strains means that there is strong evidence to suspect that the K cowanii detected in chili powder is due to its spread through Capsicum seeds.
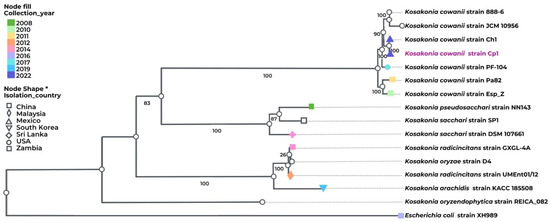
Figure 5.
Phylogenomic analysis of K. cowanii Cp1. Phylogenetic analysis was performed in BV-BRC. The following strains were included: K. cowanii strain 888-76, K. cowanii JCM 10956, K. cowanii Ch1, K. cowanii strain PF-104, K. cowanii Pa82, K. cowanii strain Esp Z, K. pseudosacchari strain NN143, K. sacchari SP1, K. sacchari strain DSM 107661, K. radicincitans strain GXGL-4A, K. oryzae strain D4, K. radicincitans UMEnt01/12, K. arachidis strain KACC 18508, K. oryzendophytica strain REICA 082, and—as an outgroup—Escherichia coli XH989. The label color represents the collection year. The node fill and shape represent the country from which the isolates were gathered, including Mexico, China, Malaysia, Republic of Korea, Sri Lanka, USA, and Zambia.
3.3. Effects of VOCs on Mycelial Growth
To investigate the effect of the VOCs on mycelial growth, each one of the fungi was exposed to K. cowanii Cp1 in a dual-plate culture. The results in Figure 6 show that the VOCs produced by K. cowanii Cp1 during growth on TSA medium had a significant inhibitory effect on the mycelial growth of the tested fungi. The colony diameter of the six-fungus plates that were treated with K. cowanii Cp1 VOCs were significantly lower than the diameter of those grown on the control plates (Figure 6). Thus, for instance, the highest inhibition rate caused by the VOCs of all the tested fungi was observed in Sclerotinia sp., which exhibited an 87.45% inhibition, followed by R. solani, with a 77.24% inhibition. S. rolfsii, A. alternata, and C. gloeosporioides demonstrated a mean radial growth inhibition of 52.79%, 40.82%, and 55.40%, respectively. The lowest inhibition percentage demonstrated was by F. oxysporum, with a 10.64% inhibition. Alternatively, to prove additional mechanisms on fungal inhibition, a dual-culture assay on PDA was tested. At first approximation, S. rolfsii demonstrated a bacterial strain; however, mycelial growth was not affected (Supplementary Figure S1), suggesting that VOCs produced during fermentation could be the principal mechanism of fungal inhibition. Based on this result, we decided to analyze the VOCs’ composition.
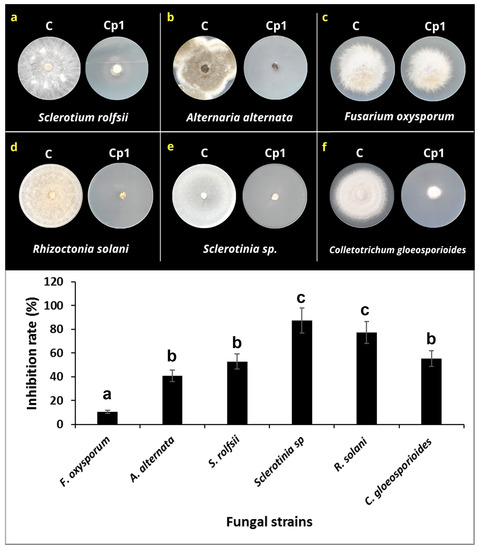
Figure 6.
Effect of the VOCs produced by K. cowanii Cp1 on mycelial growth. (a–f) show the radial mycelial growth for the untreated controls (indicated by C) and those that were VOC-treated (indicated by Cp1). The lower graph shows the inhibition mycelial rate (%). Statistical differences are represented by letters.
3.4. Characterization of VOCs
Characterization of the VOCs produced by K. cowanii Cp1 can clarify the diverse routes of metabolism that are used by this bacterial strain. With this in mind, we decided to analyze the VOCs by HS-SPME-GC–MS, which was achieved by using the bacterial culture that had 18 h of growth and was based on a 50% inhibition rate in a cell-free filtrate against Sclerotium rolfsii. The volatile organic compounds produced by K. cowanii Cp1 were compared with the control, and the results showed 65 VOCs (Table 2). They were then classified in the following functional groups (according to their total relative peak area (Figure 7)): alcohols (23.5%), ketones (22.97%), pyrazines (10.43%), esters (10.32%), hydrocarbons (9.5%), aldehydes (2.31%), acids (1.81%), and aromatics (0.11%).

Table 2.
The VOCs produced by K. cowanii Cp1 and detected by HS-SPME-GC–MS.
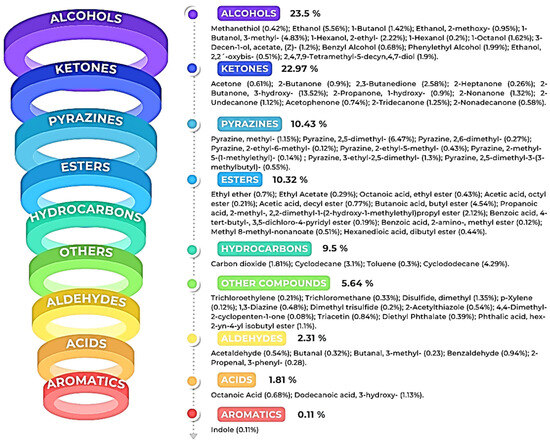
Figure 7.
The VOCs detected in K. cowanii Cp1. Each figure color shows the class family of the compounds and their percentage according to their relative peak-area profile.
As is indicated in Figure 8, the six compounds with the highest relative peak-area percentages were 2-butanone, 3-hydroxy (13.52%); pyrazine-2,5-dimethyl (6.47%); ethanol (5.56%); 1-butanol-3-methyl (4.83%); butanoic acid and butyl ester (4.54%); and cyclododecane (4.29%). All of the results show that, during the growth of K. cowanii Cp1 on TSA medium, there was a fermentation process that produced a mixture of VOCs—some of them, most likely, exerted effects on mycelial growth inhibition. However, further analysis is necessary to understand the potential mechanisms leading to the production of each VOC and its effects on cellular processes.
Figure 8.
The VOC profiles of K. cowanii Cp1. The relative peak abundance and retention time are shown.
3.5. Evaluation of Standard VOCs
According to the identified VOCs, we decided to analyze some compounds. The mycelial inhibition rate of the seven compounds were as follows: the strongest antifungal effect against S. rolfsii was benzyl alcohol, with 82.5% using 50 μL/plate and 100% using 100 and 150 μL/plate. Then, acetic acid (unidentified compound as VOC component in K. cowanii Cp1 and used as control positive) showed 85 and 100% inhibition using 10 and 20 μL/plate, respectively, and only 21% inhibition using 5 μL/plate. For 2-nonanone, a low inhibition of 25% was observed at 10 μL/plate, while an inhibition of 72.5 and 90% was observed with 20 and 40 μL/plate, respectively. With 2-butanone, 3-hydroxy, an inhibition of 70 and 82.5% was observed with 100 and 150 μL/plate, respectively, while the inhibition observed with 50 μL/plate was only 37.5%. With 2-butanone, inhibition was 15, 56.2, and 72.5% using 50, 100, and 150 μL/plate, respectively. Acetone only showed 25% inhibition using the highest volume (150 μL/plate). Ethanol was the compound that showed the lowest inhibition rate; only at the highest volume of 200 μL/plate was 21.25% inhibition observed (Figure 9).
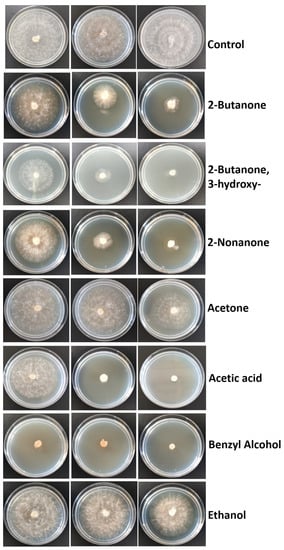
Figure 9.
Inhibitory effect of standard VOCs on the mycelia growth of S. rolfsii. The amount for each compound assayed were, from left to right: 50, 100, and 150 μL/plate of 2-Butanone; 50, 100, and 150 μL/plate of 2-Butanone, 3-hydroxy; 10, 20, and 40 μL/plate of 2-Nonanone; 50, 100, and 150 μL/plate of acetone; 5, 10, and 20 μL/plate of acetic acid; 50, 100, and 150 μL/plate of benzyl alcohol; and 50, 100, and 200 μL/plate of ethanol. Sterile distilled water was used in the control.
4. Discussion
According to the hypothesis established in this research work, the findings reported here present, for the first time, the association of the K. cowanii strain Cp1 with seeds of C. pubescens, a plant that is cultivated in different agroecological zones in Michoacan, Mexico [44]. Although C. annuum L. seeds from Queretaro, Mexico, were analyzed, bacterial strains related to the Kosakonia genus were not detected; however, several other isolated bacterial genera are under study in our laboratory. Although we analyzed a relatively limited number of samples of Capsicum seeds, it is necessary to continue exploring in order to understand the seed association of K. cowanii with the diverse species of the Capsicum genus in the diverse geographical regions of Mexico so as to determine if the plant genotype, plant species, environment, and soil management practices have an effect on the presence of this bacterial species. This should be performed because previous results have detected the presence of K. cowanii in diverse samples of chili powder [16,17,18,31].
Throughout the last decade, K. cowanii has been isolated and associated with the microbiome of diverse organisms and processed products. Its huge metabolic versatility has shown an extensive resistance to diverse environmental factors, such as pH, salinity, temperature, and, most likely, host biomolecules, which could promote diverse bacterial interactions with diverse organisms such as insects, plants, and humans [20,21,22,23,24,25,26,27,28,29,30,31,32]. In this sense, it is widely recognized that the fruits and seeds of C. pubescens are a source of capsaicinoids and phenolic compounds [45]. Research works have demonstrated their antimicrobial properties as a consequence of structural or functional disruptions to the bacterial cell membrane [46]. The fact that K. cowanii has been isolated from C. pubescens seeds demonstrates that it possesses mechanisms of resistance to these compounds. In this sense, the multidrug efflux pumps of MdtABC-TolC that were detected in K. cowanii Cp1 have been characterized as important components of Salmonella sp. during colonization in the intestine and of virulence in mice [47]. In addition, this multidrug efflux pump in the phytopathogenic Erwinia amylovora plays an important role during colonization and resistance to plant metabolites [48] and during insect infection by Photorhabdus luminescens [49]. Also, the expression regulation of mdtABC by the primary regulator BaeR has been observed in vitro in E. coli and Salmonella as a response to a wide range of stress conditions, including plant metabolites such as tannins and flavonoids [49,50,51]. With this in mind, it would be interesting, in the future, to understand these regulatory molecular mechanisms as well as the chemiotaxis pathways that could serve as a route of dispersion or interaction of the K. cowanii Cp1 strain with these biological Capsicum seed compounds.
The genome sequencing results of the K. cowanii Cp1 strain showed a close phylogenetic relationship with K. cowanii Ch1, including the IncF plasmid. Both strains presented virulence genes that are principally related with iron uptake, siderophore, invasion, and motility (Table 1). Interestingly, secretion system component genes were not detected, which is especially relevant because the K. cowanii Pa82 strain encodes the type VI secretion system as a virulence factor that causes plant disease [25]. Based on the results observed in the pathogenic test of K. cowanii Cp1 in C. annuum L. plants and fruits (Figure 2), we can conclude that K. cowanii Cp1 is not able to act as a phytopathogen despite the virulence related genes detected. Similar results were observed in K. cowanii Ch1 [32], which suggests that both bacterial strains most likely do not represent a potential risk of affecting Capsicum plants. However, the fact that the spread of the Kosakonia genus could be associated with Capsicum seeds is important to consider if the phytopathogenic strains of the Kosakonia genus associated with other cultivated plants eventually might cause damage in the Capsicum genus through different spread routes. Thus, for instance, K. cowanii has been reported as the causal agent of bacterial wilt in tomatoes and patchouli plants and, recently, as an emergent phytopathogen affecting soybean (Glycine max Willd) [25,26]. In Eucalyptus trees, symptoms of bacterial blight [27] are presented, and in Mabea fistulifera Mart. (Euphorbiaceae), necrotic spots can occur on leaves [28]. Therefore, the monitoring of the microorganism-associated seeds of the Capsicum genus in either fresh, dried, or processed products could be relevant in terms of avoiding the bacterial spread that comes with a potential risk of causing infection in susceptible Capsicum plants.
On the other hand, it is recognized that the antibiotic resistance in bacteria is part of their natural genetic characteristics and evolution processes, which play important roles during environmental competition [52]. However, it is also one of the greatest emergent problems in human or animal health due to the increase in the prevalence of multidrug-resistant (MDR) bacteria, which have arisen through natural selection due to the abusive overuse of antibiotics in various human activities [52,53]. Therefore, the presence of antibiotic-resistant mechanisms in K. cowanii Cp1, such as TEM β-lactamase (which allows for phenotypical resistance to penicillin G, ampicillin, and carbenicillin) is a pressing concern. In addition, the antibiotic efflux pump system that was detected and represented by acrAB-tolC and acrAD-tolC, respectively, is an intrinsic mechanism of multidrug resistance in the diverse members of Gram-negative bacteria. The antibiotic profile of the pump system includes tetracycline, novobiocin, chloramphenicol, fluoroquinolone, fusidic acid, nalidixic acid, lipophilic antibiotics, and β-lactam antibiotics [54]. Another tripartite efflux system identified was emrAB-tolC, which has been demonstrated to induce overexpression in Escherichia coli, which causes a resistance to nalidixic acid, thiolactomycin, and nitroxoline [55]. Also, the macrolide transporter macAB-tolC has been identified as the most likely cause of erythromycin resistance [56]. The transporter mdfA/cmr that has also been identified contributes to multidrug resistance in Gram-negative bacteria [57]. Additionally, the other genes that are linked to the resistance mechanism that was identified—such as transcription factors marAB, transcriptional repressor marR, target modification, and the protein-altering cell wall charge conferring antibiotic resistance—are shown in Figure 4. All of these resistance mechanisms that were detected in K. cowanii Cp1 make it necessary to conduct further work so as to evaluate any potential environmental risks. This is required because of its presence in Capsicum seeds and chili powder that are used in human consumption.
The colonization ability of microorganisms depends on the metabolic strategies that are ligated to the environmental factors that promote genetic expression regulation so as to synthetize biomolecules with diverse functions during competition in an ecological niche with a community structure [58]. Thus, for instance, the competition for nutrients could promote a change in growth rate and could generate metabolites with certain effects on their competitors. One class of these compounds are the VOCs, which are a huge variety of molecules that are produced by the different metabolic pathways that have been studied in a diverse array of microorganisms [59]. In this sense, the experimental strategy used in K. cowanii Cp1 during a dual experiment with six tested phytopatogenic fungi suggested that the volatile organic compounds produced during the growth of this bacterial strain on TSA medium has inhibitory effects on the mycelial rate growth of Sclerotinia sp., R. solani, S. rolfsii, A. alternata, and C. gloeosporioides, and this occurs by different mechanisms when compared with F. oxysporum (which showed a lower degree of effect). Our evidence, obtained by HS-SPME-GC–MS, demonstrates that the diverse chemical classes of VOCs were produced by K. cowanii Cp1 during its growth fermentation on TSA medium (Figure 7). The compounds with a high relative abundance were alcohols (23.5%), which were represented by ethanol (at 5.56%) and 1-butanol-3-methyl (at 4.83%) as the most abundant examples, and ketones (22.97%), where the most abundant was 2-butanone, 3-hydroxy, at 13.52%. In addition, those with a lower relative abundance were the diverse compounds detected from the chemical family of pyrazines, esters, aromatics, acids, hydrocarbons, aldehydes, and aromatics. Interestingly, similar results of VOC production were found in the K. cowanii Ch1 strain, where 2-butanone, 3-hydroxy was the most abundant compound (at 10.60%), as well as ethanol (at 5.40%), and 1-butanol-3-methyl (4.88%) [32]. According to diverse evidence, some of these identified compounds may affect plants through a diverse array of mechanisms and through the functionality of fungi cells (thereby causing a disruption of membrane fluidity or wall integrity) as well as alterations in metabolism and redox balance [59]. Although 2-butanone, 3-hydroxy could be one of the principal compounds with antifungal properties in K. cowanii Cp1, it is too complicated to understand because it is part of a heterogeneous mix of VOCs that have fluctuating concentrations during the fermentation processes that were tested. This interpretation was confirmed using standard VOCs, where 2-butanone, 3-hydroxy showed an inhibition rate of 82.5% when the highest volume was used (150 μL/plate) compared with acetic acid, benzyl alcohol, or 2-nonanone, which demonstrated high inhibition activities when using a lower volume. In addition, relative peak areas identified were 0.68% for benzyl alcohol, 1.32% for 2-nonanone, and 0.90% for 2-butanone compared with 13.52% for 2-butanone, 3-hydroxy or 5.56% for ethanol (Table 2). Therefore, a mix of VOCs has more impact than individual compounds during biocontrol.
According to the genome sequencing analysis of K. cowanii Cp1, metabolic pathways were present that produce 2-butanone, 3-hydroxy through the enzymatic activities of acetolactate decarboxylase and 2,3-butanediol dehydrogenase via the diverse carbon sources that are used in fermentation [60]. Evidence suggests that this molecule, functionally, has significant effects in plants, e.g., during growth development [61], drought tolerance [62], and defense against pathogens [63]. On the one hand, the VOC results suggest that, during fermentation pathways, aldehyde dehydrogenase and alcohol dehydrogenase enzymes could be necessary for ethanol production as well as for the butanal (butyraldehyde) pathway that is required to obtain butanol (which were detected in relatively high abundance in the VOCs from K. cowanii Cp1). These alcohol classes are components of the VOCs that are present in a diverse array of microorganisms with antifungal activity [64]. Another interesting compound that was identified as a component of the VOCs was Pyrazine-2,5-dimethyl, with a high relative peak area (6.47%). This compound is produced in diverse bacterial species, and it engages in antifungal activity against Magnaporthe oryzae, Phytophthora capsici, and A. solani [59]. Although other VOCs were produced that had a lower relative-peak abundance (Table 2), these could, nevertheless, be more important, because their antifungal activity has been registered in the mVOC 2.0 Database [65]. Therefore, additional work is necessary to understand the impact of each VOC, on its own or as part of a mixture, on the growth of phytopathogenic fungi strains.
In conclusion, the finding of K. cowanii Cp1 as a microorganism that is associated with Capsicum pubescens seeds and which has antifungal properties through VOC production opens up the possibility of further exploration of important ecological roles as well as of the plant–bacteria interactions with diverse molecules such as capsaicinoids and phenolic compounds. Additionally, the Capsicum genus, cultivated throughout all Mexican territory under different environmental conditions, remains to be explored in order to understand the microbiomes associated with the seeds that are of particular interest in terms of finding potential bacterial strains, such as Kosakonia cowanii, for the development of biocontrol agents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11102491/s1, Figure S1. Dual-culture assay on PDA. K. cowanii Cp1 and S. rolfsii were confronted. Mycelial and bacterial growth was register during 2 days of growth at 28 °C. Figure S2. Antibiotic resistance profile of K. cowanii CP1 strain
Author Contributions
J.C.G. and J.A.R.M.; methodology and software, Y.J.M., M.M.A.R., M.J.C.P., M.F.M., J.R.R., G.R.Z., P.L.T.B., L.V.S. and K.V.R.; validation and formal analysis, J.C.G., J.L.H.F., M.Á.R.L. and J.A.R.M.; writing—review and editing, J.C.G., C.S.G., J.L.H.F. and A.A.R.; visualization, supervision, and project administration, J.C.G.; funding acquisition, J.C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Universidad Autónoma de Querétaro (FONDEC-UAQ-2022; FOPER-2022).
Data Availability Statement
The data presented in this work are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kraft, K.H.; Brown, C.H.; Nabhan, G.P.; Luedeling, E.; de Luna Ruiz, J.; Coppens d’Eeckenbrugge, G.; Hijmans, R.J.; Gepts, P. Multiple lines of evidence for the origin of domesticated chili pepper, Capsicum annuum, in Mexico. Proc. Natl. Acad. Sci. USA 2014, 111, 6165–6170. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, P.; Rabanus-Wallace, M.T.; Barchi, L.; Kale, S.; Esposito, S.; Acquadro, A.; Schafleitner, R.; van Zonneveld, M.; Prohens, J.; Diez, M.J.; et al. Global range expansion history of pepper (Capsicum spp.) revealed by over 10,000 genebank accessions. Proc. Natl. Acad. Sci. USA 2021, 118, e2104315118. [Google Scholar] [CrossRef]
- Swamy, K.R.M. Origin, distribution, taxonomy, botanical description, genetic diversity and breeding of capsicum (Capsicum annuum L.). Int. J. Dev. Res. 2023, 13, 61956–61977. [Google Scholar]
- McCoy, J.; Martínez-Ainsworth, N.; Bernau, V.; Scheppler, H.; Hedblom, G.; Adhikari, A.; McCormick, A.; Kantar, M.; McHale, L.; Jardón-Barbolla, L.; et al. Population structure in diverse pepper (Capsicum spp.) accessions. BMC Res. Notes 2023, 16, 20. [Google Scholar] [CrossRef]
- Pereira-Dias, L.; Vilanova, S.; Fita, A.; Prohens, J.; Rodríguez-Burruezo, A. Genetic diversity, population structure, and relationships in a collection of pepper (Capsicum spp.) landraces from the Spanish centre of diversity revealed by genotyping-by-sequencing (GBS). Hortic. Res. 2019, 6, 54. [Google Scholar] [CrossRef]
- Zhang, X.-m.; Zhang, Z.-h.; Gu, X.-z.; Mao, S.-l.; Li, X.; Chadœuf, J.; Palloix, A.; Wang, L.-h.; Zhang, B.-x. Genetic diversity of pepper (Capsicum spp.) germplasm resources in China reflects selection for cultivar types and spatial distribution. J. Integr. Agric. 2016, 15, 1991–2001. [Google Scholar] [CrossRef]
- Sarath Babu, B.; Pandravada, S.R.; Pasada Rao, R.D.V.J.; Anitha, K.; Chakrabarty, S.K.; Varaprasad, K.S. Global sources of pepper genetic resources against arthropods, nematodes and pathogens. Crop Prot. 2011, 30, 389–400. [Google Scholar] [CrossRef]
- Ros, C.; Lacasa, C.M.; Martínez, V.; Bielza, P.; Lacasa, A. Response of pepper rootstocks to co-infection of Meloidogyne incognita and Phytophthora spp. Eur. J. Hortic. Sci. 2014, 79, 22–28. [Google Scholar]
- Özkaynak, E.; Devran, Z.; Kahveci, E.; Doğanlar, S.; Başköylü, B.; Doğan, F.; Yüksel, M. Pyramiding multiple genes for resistance to PVY, TSWV and PMMoV in pepper using molecular markers. Eur. J. Hortic. Sci. 2014, 79, 233–239. [Google Scholar]
- Parisi, M.; Alioto, D.; Tripodi, P. Overview of Biotic Stresses in Pepper (Capsicum spp.): Sources of Genetic Resistance, Molecular Breeding and Genomics. Int. J. Mol. Sci. 2020, 21, 2587. [Google Scholar] [CrossRef]
- Lozada, D.N.; Bosland, P.W.; Barchenger, D.W.; Haghshenas-Jaryani, M.; Sanogo, S.; Walker, S. Chile Pepper (Capsicum) Breeding and Improvement in the “Multi-Omics” Era. Front. Plant Sci. 2022, 13, 879182. [Google Scholar] [CrossRef]
- Kim, T.J.; Hyeon, H.; Park, N.I.; Yi, T.G.; Lim, S.H.; Park, S.Y.; Ha, S.H.; Kim, J.K. A high-throughput platform for interpretation of metabolite profile data from pepper (Capsicum) fruits of 13 phenotypes associated with different fruit maturity states. Food Chem. 2020, 331, 127286. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Kumar, A.; Islam, K.; Momo, J.; Ramchiary, N. Functional genomics to understand the tolerance mechanism against biotic and abiotic stresses in Capsicum species. In Transcriptome Profiling; Academic Press: Cambridge, MA, USA, 2023; pp. 305–332. [Google Scholar]
- Zhang, L.; Qin, C.; Mei, J.; Chen, X.; Wu, Z.; Luo, X.; Cheng, J.; Tang, X.; Hu, K.; Li, S.C. Identification of microRNA targets of Capsicum spp. using miRTrans—A trans-omics approach. Front. Plant Sci. 2017, 8, 495. [Google Scholar] [CrossRef][Green Version]
- Reimer, J.J.; Shaaban, B.; Drummen, N.; Sanjeev Ambady, S.; Genzel, F.; Poschet, G.; Wiese-Klinkenberg, A.; Usadel, B.; Wormit, A. Capsicum leaves under stress: Using multi-omics analysis to detect abiotic stress network of secondary metabolism in two species. Antioxidants 2022, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, W.; Gundel, P.E.; Verma, S.K.; White, J.F. (Eds.) Seed Microbiome Research; Frontiers Media SA: Lausanne, Switzerland, 2022. [Google Scholar]
- Tkalec, V.; Mahnic, A.; Gselman, P.; Rupnik, M. Analysis of seed-associated bacteria and fungi on staple crops using the cultivation and metagenomic approaches. Folia Microbiol. 2022, 67, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Dowarah, B.; Agarwal, H.; Krishnatreya, D.B.; Sharma, P.L.; Kalita, N.; Agarwala, N. Evaluation of seed associated endophytic bacteria from tolerant chilli cv. Firingi Jolokia for their biocontrol potential against bacterial wilt disease. Microbiol. Res. 2021, 248, 126751. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, T.M.; Choi, B.; Kim, Y.; Kim, E. Invasive Lactuca serriola seeds contain endophytic bacteria that contribute to drought tolerance. Sci. Rep. 2021, 11, 13307. [Google Scholar] [CrossRef]
- Jan-Roblero, J.; Cruz-Maya, J.A.; Barajas, C.G. Chapter 12—Kosakonia. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Annapurna, K., Sankaranarayanan, A., Kumar, M.S., Kumar, K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 213–231. [Google Scholar]
- Romano, I.; Ventorino, V.; Ambrosino, P.; Testa, A.; Chouyia, F.E.; Pepe, O. Development and Application of Low-Cost and Eco-Sustainable Bio-Stimulant Containing a New Plant Growth-Promoting Strain Kosakonia pseudosacchari TL13. Front. Microbiol. 2020, 11, 2044. [Google Scholar] [CrossRef]
- Yang, X.-J.; Wang, S.; Cao, J.M.; Hou, J.H. Complete genome sequence of human pathogen Kosakonia cowanii type strain 888-76. Braz. J. Micro-Biol. 2018, 49, 16–17. [Google Scholar] [CrossRef]
- Bhatti, M.D.; Kalia, A.; Sahasrabhojane, P.; Kim, J.; Greenberg, D.E.; Shelburne, S.A. Identification and Whole Genome Sequencing of the First Case of Kosakonia radicincitans Causing a Human Blood-stream Infection. Front. Microbiol. 2017, 8, 62. [Google Scholar] [CrossRef]
- Berinson, B.; Bellon, E.; Christner, M.; Both, A.; Aepfelbacher, M.; Rohde, H. Identification of Kosakonia cowanii as a rare cause of acute cholecystitis: Case report and review of the literature. BMC Infect. Dis. 2020, 20, 366. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Li, Q.; Huang, D.; Zhang, Y.; Li, G.; He, H. Isolation and Complete Genome Sequence Analysis of Kosakonia cowanii Pa82, a Novel Pathogen Causing Bacterial Wilt on Patchouli. Front. Microbiol. 2022, 12, 818228. [Google Scholar] [CrossRef]
- Krawczyk, K.; Borodynko-Filas, N. Kosakoniacowanii as the New Bacterial Pathogen Affecting Soybean (Glycine max Willd.). Eur. J. Plant Pathol. 2020, 157, 173–183. [Google Scholar] [CrossRef]
- Brady, C.L.; Venter, S.N.; Cleenwerck, I.; Engelbeen, K.; de Vos, P.; Wingfield, M.J.; Telechea, N.; Coutinho, T.A. Isolation of Enterobacter cowanii from Eucalyptus showing symptoms of bacterial blight and dieback in Uruguay. Lett. Appl. Microbiol. 2009, 49, 461–465. [Google Scholar] [CrossRef]
- Furtado, G.Q.; Guimarães, L.M.S.; Lisboa, D.O.; Cavalcante, G.P.; Arriel, D.A.A.; Alfenas, A.C.; Oliveira, J.R. First Report of Enterobacter cowanii Causing Bacterial Spot on Mabea fistulifera, a Native Forest Species in Brazil. Plant Dis. 2012, 96, 1576. [Google Scholar] [CrossRef] [PubMed]
- Petrzik, K.; Brázdová, S.; Krawczyk, K. Novel Viruses That Lyse Plant and Human Strains of Kosakonia cowanii. Viruses 2021, 13, 1418. [Google Scholar] [CrossRef]
- Dennison, N.J.; Jupatanakul, N.; Dimopoulos, G. The mosquito microbiota influences vector competence for human pathogens. Curr. Opin. Insect Sci. 2014, 3, 6–13. [Google Scholar] [CrossRef]
- Hernández Gómez, Y.F.; González Espinosa, J.; Ramos López, M.Á.; Arvizu Gómez, J.L.; Saldaña, C.; Rodríguez Morales, J.A.; García Gutiérrez, M.C.; Pérez Moreno, V.; Álvarez Hidalgo, E.; Nuñez Ramírez, J.; et al. Insights into the Bacterial Diversity and Detection of Opportunistic Pathogens in Mexican Chili Pow-der. Microorganisms 2022, 10, 1677. [Google Scholar] [CrossRef] [PubMed]
- González Espinosa, J.; Hernández Gómez, Y.F.; Javier Martínez, Y.; Flores Gallardo, F.J.; Pacheco Aguilar, J.R.; Ramos López, M.Á.; Arvizu Gómez, J.L.; Saldaña Gutierrez, C.; Rodríguez Morales, J.A.; García Gutiérrez, M.C.; et al. Kosakonia cowanii Ch1 Isolated from Mexican Chili Powder Reveals Growth Inhibition of Phytopathogenic Fungi. Microorganisms 2023, 11, 1758. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Rincón, V.H.; Corona Torres, P.T.; López López, L.; Latournerie Moreno, M.; Ramírez Meraz, H.; Villalón Mendoza, Y.J.; Aguilar Castillo, A. Los Chiles de México y su Distribución; Rev Fitotecnia Mex SINAREFI, Colegio de Postgraduados; INIFAP, ITConkal, UANL, UAN; Sociedad Mexicana de Fitogenética A.C.: Montecillo, Texcoco, México, 2010; p. 114. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Jukes, T.H.; Cantor, C.R. Evolution of protein molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: Cambridge, MA, USA, 1969; pp. 21–132. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.D.; Assaf, R.; Brettin, T.; Conrad, N.; Cucinell, C.; Davis, J.J.; Dempsey, D.M.; Dickerman, A.; Dietrich, E.M.; Kenyon, R.W.; et al. Introducing the Bacterial Viral Bioinformatics Resource Center (BV-BRC): A resource combining PATRIC, IRD and ViPR. Nucleic Acids Res. 2022, 51, D678–D689. [Google Scholar] [CrossRef] [PubMed]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef] [PubMed]
- Naquin, D.; d’Aubenton-Carafa, Y.; Thermes, C.; Silvain, M. CIRCUS: A package for Circos display of structural genome variations from paired-end and mate-pair sequencing data. BMC Bioinform. 2014, 15, 198. [Google Scholar] [CrossRef]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef]
- Dmitry, A.; Mikhail, R.; Alla, L.; Pevzner, P.A. Plasmid detection and assembly in genomic and metagenomic data sets. Genome Res. 2019, 29, 961–968. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests, approved standard, 12th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Escalera-Ordaz, A.K.; Guillén-Andrade, H.; Lara-Chávez, M.B.N.; Lemus-Flores, C.; Rodríguez-Carpena, J.G.; Valdivia-Bernal, R. Characterization of cultivated varieties of Capsicum pubescens in Michoacán, Mexico. Rev. Mex. Cienc. Agríc. 2019, 10, 239–251. [Google Scholar]
- Meckelmann, S.W.; Jansen, C.; Riegel, D.W.; van Zonneveld, M.; Ríos, L.; Peña, K.; Mueller-Seitz, E.; Petz, M. Phytochemicals in Native Peruvian Capsicum pubescens (Rocoto). Eur. Food Res. Technol. 2015, 241, 817–825. [Google Scholar] [CrossRef]
- Romero-Luna, H.E.; Colina, J.; Guzmán-Rodríguez, L.; Sierra-Carmona, C.G.; Farías-Campomanes, Á.M.; García-Pinilla, S.; González-Tijera, M.M.; Malagón-Alvira, K.O.; Peredo-Lovillo, A. Capsicum fruits as functional ingredients with antimicrobial activity: An emphasis on mechanisms of action. J. Food Sci. Technol. 2022, 60, 1–11. [Google Scholar] [CrossRef]
- Nishino, K.; Latifi, T.; Groisman, E.A. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2006, 59, 126–141. [Google Scholar] [CrossRef]
- Pletzer, D.; Weingart, H. Characterization and regulation of the resistance-nodulation-cell division-type multidrug efflux pumps MdtABC and MdtUVW from the fire blight pathogen Erwinia amylovora. BMC Microbiol. 2014, 14, 185. [Google Scholar] [CrossRef]
- Abi Khattar, Z.; Lanois, A.; Hadchity, L.; Gaudriault, S.; Givaudan, A. Spatiotemporal expression of the putative MdtABC efflux pump of Phtotorhabdus luminescens occurs in a protease-dependent manner during insect infection. PLoS ONE 2019, 14, e0212077. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Inazumi, Y.; Masaki, T.; Hirata, T.; Yamaguchi, A. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol. Microbiol. 2005, 55, 1113–1126. [Google Scholar] [CrossRef]
- Nishino, K.; Nikaido, E.; Yamaguchi, A. Regulation of multidrug efflux systems involved in multidrug and metal resistance of Salmonella enterica serovar Typhimurium. J. Bacteriol. 2007, 189, 9066–9075. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Koch, N.; Islam, N.F.; Sonowal, S.; Prasad, R.; Sarma, H. Environmental antibiotics and resistance genes as emerging contaminants: Methods of detection and bioremediation. Curr. Res. Microb. Sci. 2021, 2, 100027. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.; Suhani, S.; Purkaystha, A.; Begum, M.K.; Raihan, T.; Alam, M.J.; Islam, K.; Azad, A.K. Identification of AcrAB-TolC Efflux Pump Genes and Detection of Mutation in Efflux Repressor AcrR from Omeprazole Responsive Multidrug-Resistant Escherichia coli Isolates Causing Urinary Tract Infections. Microbiol. Insights 2019, 12, 1178636119889629. [Google Scholar] [CrossRef]
- Yousefian, N.; Ornik-Cha, A.; Poussard, S.; Decossas, M.; Berbon, M.; Daury, L.; Taveau, J.C.; Dupuy, J.W.; Đorđević-Marquardt, S.; Lambert, O.; et al. Structural characterization of the EmrAB-TolC efflux complex from E. coli. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183488. [Google Scholar] [CrossRef]
- Lu, S.; Zgurskaya, H.I. MacA, a periplasmic membrane fusion protein of the macrolide transporter MacAB-TolC, binds lipopolysaccharide core specifically and with high affinity. J. Bacteriol. 2013, 195, 4865–4872. [Google Scholar] [CrossRef]
- Swick, M.C.; Morgan-Linnell, S.K.; Carlson, K.M.; Zechiedrich, L. Expression of multidrug efflux pump genes acrAB-tolC, mdfA, and norE in Escherichia coli clinical isolates as a function of fluoroquinolone and multidrug resistance. Antimicrob. Agents Chemother. 2011, 55, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; Rojo, F. The importance of understanding the regulation of bacterial metabolism. Environ. Microbiol. 2023, 25, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.A.C.; de Araujo, N.O.; Dias, B.H.S.; de Sant’Anna Freitas, C.; Coerini, L.F.; Ryu, C.-M.; de Castro Oliveira, J.V. The power of the smallest: The inhibitory activity of microbial volatile organic compounds against phytopathogens. Front. Microbiol. 2023, 13, 951130. [Google Scholar] [CrossRef]
- Marquez-Villavicencio, M.d.P.; Weber, B.; Witherell, R.A.; Willis, D.K.; Charkowski, A.O. The 3-Hydroxy-2-Butanone Pathway Is Required for Pectobacterium carotovorum Pathogenesis. PLoS ONE 2011, 6, e22974. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial Volatiles Induce Systemic Resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R,3R-Butanediol, a Bacterial Volatile Produced by Pseudomonas chlororaphis O6, Is Involved in Induction of Systemic Tolerance to Drought in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial volatile organic compounds: Antifungal mechanisms, applications, and challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Gohlke, B.-O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. mVOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2017, 46, D1261–D1265. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).