Efficacy of an Enterotoxigenic Escherichia coli (ETEC) Vaccine on the Incidence and Severity of Traveler’s Diarrhea (TD): Evaluation of Alternative Endpoints and a TD Severity Score
Abstract
1. Introduction
2. Materials and Methods
2.1. OEV-118 Phase 3 Study
2.2. Study Population
2.3. Health Data and Sample Collection
2.4. Alternative Endpoints and TD Score Calculation
2.5. Pathogen Determination
2.6. Serum IgA CTB Antibody Determinations
2.7. Data Analysis
3. Results
3.1. Primary OEV Trial Analysis Using Per-Protocol VPO-ETEC TD Case Definitions
3.2. Post Hoc Assessment of Vaccine Efficacy: Impact of Immunologic Take and Application of Alternative Endpoints
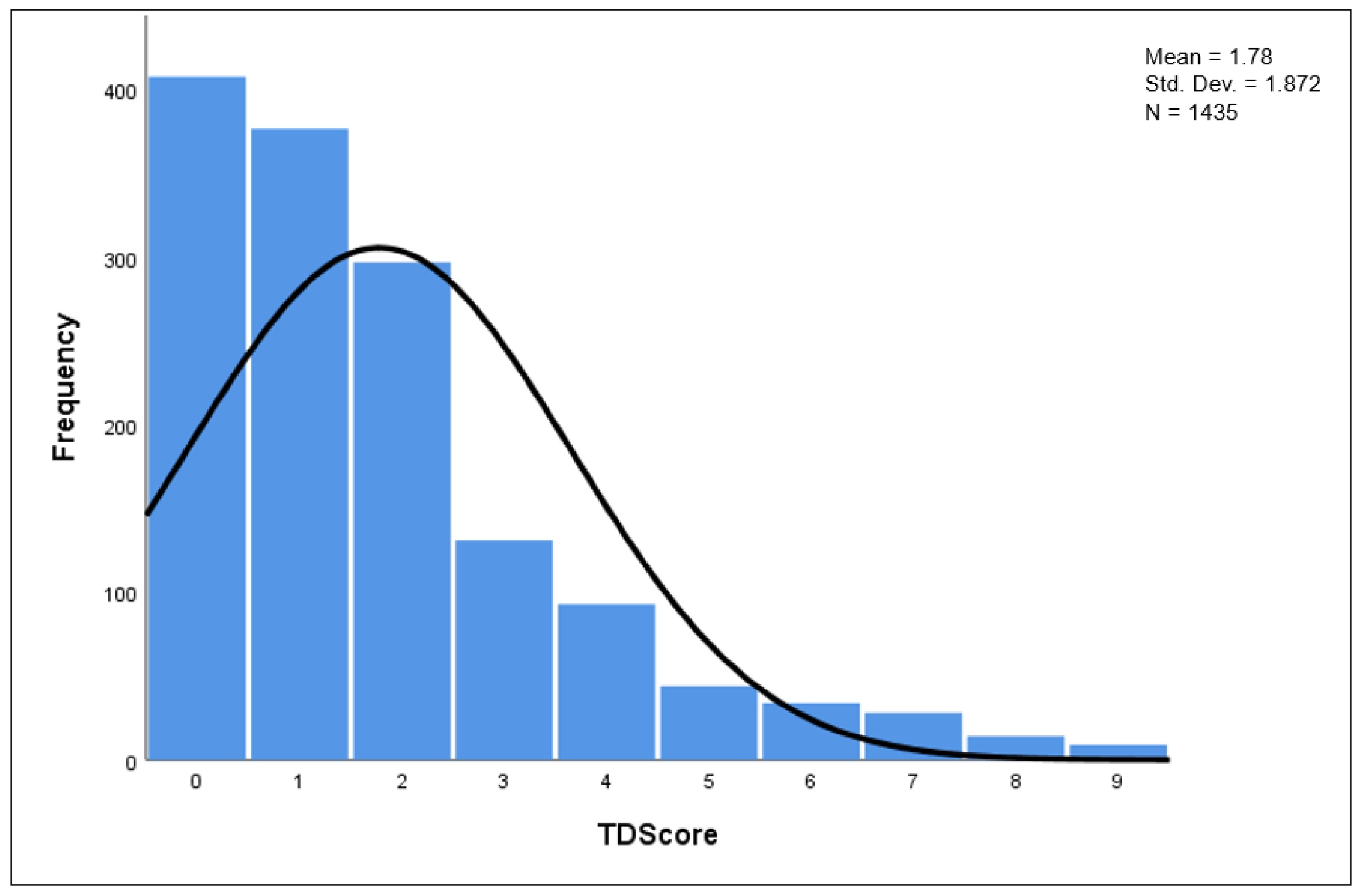
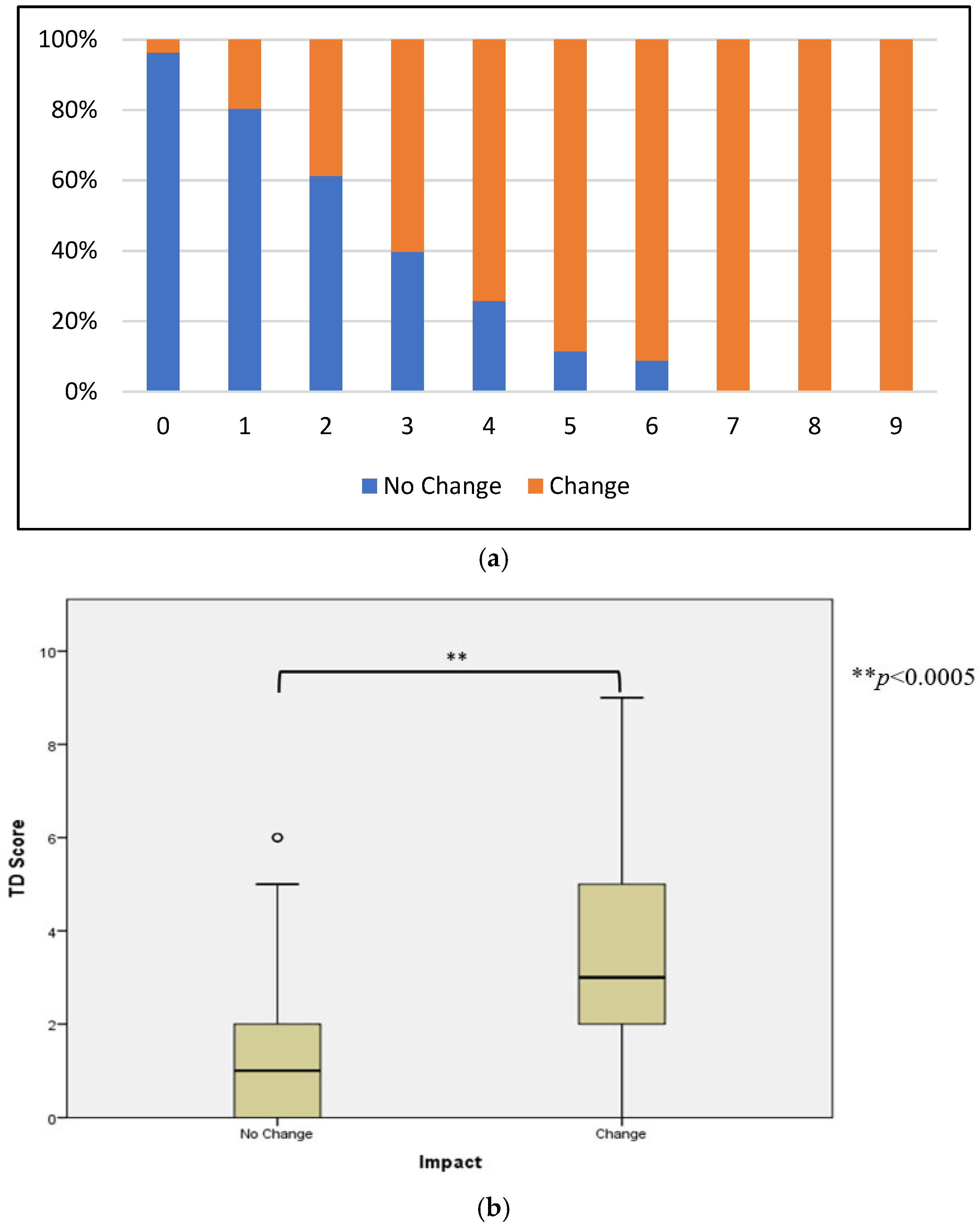
3.3. TD Score Analysis
3.4. Impact of Immune Status on Risk of Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Copyright Statement
References
- Centers for Disease Control and Prevention. The Pretravel Consultation: Self-Treatable Conditions: Traveler’s Diarrhea (Chapter 2: Perspectives: Antibiotics in Travelers’ Diarrhea–Balancing the Risks & Benefits). Available online: https://wwwnc.cdc.gov/travel/yellowbook/2018/the-pre-travel-consultation/travelers-diarrhea (accessed on 20 June 2023).
- Steffen, R. Epidemiology of travellers’ diarrhea. J. Travel Med. 2017, 24, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Steffen, R.; Hill, D.R.; DuPont, H.L. Traveler’s diarrhea: A clinical review. JAMA 2015, 313, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Walker, R.; Porter, C.K.; Muhib, F.; Chilengi, R.; Cravioto, A.; Guerrant, R.; Svennerholm, A.-M.; Qadri, F.; Baqar, S.; et al. Enterotoxigenic Escherichia coli (ETEC) vaccines: Priority activities to enable product development, licensure and global access. Vaccine 2021, 39, 4266–4277. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Anderson, J.D.; Bagamian, K.H.; Baqar, S.; Giersing, B. Vaccine value profile for enterotoxigenic Escherichia coli (ETEC). Vaccine 2023. [Google Scholar] [CrossRef]
- Wellcome Trust. Vaccines to Tackle Drug Resistant Infections: An Evaluation of R&D Opportunities. Available online: https://vaccinesforamr.org/wp-content/uploads/2018/09/Vaccines_for_AMR.pdf (accessed on 20 June 2023).
- The Case for Investment in Enterotoxigenic Escherichia coli Vaccines. Available online: https://www.path.org/resources/the-case-for-investment-inenterotoxigenic-Escherichia-coli-vaccines/ (accessed on 16 June 2023).
- PATH. Shigella Vaccines for Traveler and Military Populations. Available online: https://www.path.org/resources/shigella-vaccines-for-traveler-and-military-populations-a-market-assessment-report/ (accessed on 16 June 2023).
- Riddle, M.S.; Connor, B.A.; Beeching, N.J.; DuPont, H.L.; Hamer, D.H.; Kozarsky, P.; Libman, M.; Steffen, R.; Taylor, D.; Tribble, D.R.; et al. Guidelines for the prevention and treatment of travelers’ diarrhea: A graded expert panel report. J. Travel Med. 2017, 24, S57–S74. [Google Scholar] [CrossRef]
- Porter, C.K.; Gutierrez, R.L.; Kotloff, K.L. Clinical endpoints for efficacy studies. Vaccine 2019, 37, 4814–4822. [Google Scholar] [CrossRef]
- Peltola, H.; Siitonen, A.; Kyrönseppä, H.; Simula, I.; Mattila, L.; Oksanen, P.; Kataja, M.J.; Cadoz, M. Prevention of travellers’ diarrhoea by oral B-subunit/whole-cell cholera vaccine. Lancet 1991, 338, 1285–1289. [Google Scholar] [CrossRef]
- Scerpella, E.G.; Sanchez, J.L.; Mathewson, J.J., III; Torres-Cordero, J.V.; Sadoff, J.C.; Svennerholm, A.-M.; DuPont, H.L.; Taylor, D.N.; Ericsson, C.D. Safety, Immunogenicity, and Protective Efficacy of the Whole-Cell/Recombinant B Subunit (WC/rBS) Oral Cholera Vaccine Against Travelers’ Diarrhea. J. Travel Med. 1995, 2, 22–27. [Google Scholar] [CrossRef]
- Wiedermann, G.; Kollaritsch, H.; Kundi, M.; Svennerholm, A.-M.; Bjare, U. Double-blind, randomized, placebo-controlled pilot study evaluating efficacy and reactogencity of an oral ETEC B-Subunit-Inactivated whole cell vaccine against Travelers’ Diarrhea (Preliminary Report). J. Travel Med. 2000, 7, 27–29. [Google Scholar] [CrossRef][Green Version]
- Leyten, E.M.; Soonawala, D.; Schultsz, C.; Herzog, C.; Ligthelm, R.J.; Wijnands, S.; Visser, L.G. Analysis of efficacy of CVD 103-HgR live oral cholera vaccine against all-cause travellers’ diarrhoea in a randomised, double-blind, placebo-controlled study. Vaccine 2005, 23, 5120–5126. [Google Scholar] [CrossRef]
- Frech, S.A.; Dupont, H.L.; Bourgeois, A.L.; McKenzie, R.; Belkind-Gerson, J.; Figueroa, J.F.; Okhuysen, P.C.; Guerrero, N.H.; Martinez-Sandoval, F.G.; Meléndez-Romero, J.H.; et al. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: A phase II, randomised, double-blind, placebo-controlled field trial. Lancet 2008, 371, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Steffen, R.; Cramer, J.P.; Burchard, G.; Jelinek, T.; Schwarz, U.; Ramdas, P.; Chatterjee, S.; Jiang, Z.-D.; DuPont, H.L.; Dewasthaly, S.; et al. Efficacy of a Travelers’ Diarrhea Vaccine System in Travelers to India. J. Travel Med. 2013, 20, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Behrens, R.H.; Cramer, J.P.; Jeliek, T.; Shaw, H.; von Sonnenburg, F.; Wilbraham, D.; Weinke, T.; Bell, D.J.; Asturias, E.; Pauwells, H.L.; et al. Efficacy and safety of a patch vaccine containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: A phase 3, randomised, double-blind, placebo-controlled field trial in travellers from Europe to Mexico and Guatemala. Lancet Infect. Dis. 2014, 14, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Sack, D.A.; Shimko, J.; Torres, O.; Bourgeois, A.L.; Francia, D.S.; Gustafsson, B.; Kärnell, A.; Nyquist, I.; Svennerholm, A.M. Randomised, double-blind, safety and efficacy of a killed oral vaccine for enterotoxigenic E. Coli diarrhoea of travellers to Guatemala and Mexico. Vaccine 2007, 25, 4392–4400. [Google Scholar] [CrossRef] [PubMed]
- Maier, N.; Riddle, M.S.; Gutiérrez, R.; Fraser, J.A.; Connor, P.; Tribble, D.R.; Porter, C.K. A disease severity scale for the evaluation of vaccine and other preventive or therapeutic interventions for travellers’ diarrhoea. J. Travel Med. 2022, 29, taab139. [Google Scholar] [CrossRef]
- Walker, R.I.; Bourgeois, A.L. Oral inactivated whole cell vaccine for mucosal immunization: ETVAX case study. Front. Immunol. 2023, 14, 1125102. [Google Scholar] [CrossRef]
- Bourgeois, A.L.; Halpern, J.; Grahek, S.L.; Shimko, J.; Golub, S.; Kyle, J.; Torres, O.; Belkind-Gerson, J.; Sanchez, D.; Nyquist, I.; et al. Poster 32 entitled: Vaccination of travelers to Guatemala (GU) and Mexico (MX) with an oral killed vaccine(OKV) for enterotoxigenic Escherichia coli (ETEC): Impact of vaccine “take” on risk of ETEC disease and infection with enteric pathogens. In Proceedings of the 4th International Conference for Enteric Diseases, Lisbon, Portugal, 25–27 April 2007. [Google Scholar]
- Torres, O.R.; González, W.; Lemus, O.; Pratdesaba, R.A.; Matute, J.A.; Wiklund, G.; Sack, D.A.; Bourgeois, A.L.; Svennerholm, A.M. Toxins and virulence factors of enterotoxigenic Escherichia coli associated with strains isolated from indigenous children and international visitors to a rural community in Guatemala. Epidemiol. Infect. 2015, 143, 1662–1671. [Google Scholar] [CrossRef]
- Coyle, M.B.; Morello, J.A.; Smith, P.B. Section III: Aerobic Bacteria. In Manual of Clinical Microbiology, 4th ed.; Lennette, E.H., Balows, A., Hausler, W.J., Shadomy, H.J., Eds.; ASM Press: Herndon, VA, USA, 2003; Volume 2. [Google Scholar]
- Chapin, A.R.; Carpenter, C.M.; Dudley, W.C.; Gibson, L.C.; Pratdesaba, R.; Torres, O.; Sanchez, D.; Belkind-Gerson, J.; Nyquist, I.; Kärnell, A.; et al. Prevalence of norovirus among visitors from the United States to Mexico and Guatemala who experience traveler’s diarrhea. J. Clin. Microbiol. 2005, 43, 1112–1117. [Google Scholar] [CrossRef]
- Sjöling, A.; Wiklund, G.; Savarino, S.J.; Cohen, D.I.; Svennerholm, A.M. Comparative analyses of phenotypic and genotypic methods for detection of enterotoxigenic Escherichia coli toxins and colonization factors. J. Clin. Microbiol. 2007, 45, 3295–3301. [Google Scholar] [CrossRef]
- Glenn, G.M.; Francis, D.H.; Danielsen, E.M. Toxin-medicated effects on the innate mucosal defenses: Implications for enteric vaccines. Infect. Immun. 2009, 77, 5206–5215. [Google Scholar] [CrossRef]
- World Health Organization. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Relev. Epidemiol. Hebd. 2006, 81, 97–104. [Google Scholar]
- World Health Organization. 2020 Meetings: WHO Product Development for Vaccines Advisory Committee (PDVAC). 22 April–4 December 2020. Available online: https://www.who.int/news-room/events/detail/2020/04/22/default-calendar/pdvac-2020 (accessed on 30 June 2023).
- Bourgeois, A.L.; Wierzba, T.F.; Walker, R.I. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 2016, 34, 2880–2886. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, J.; Zhang, X.; Bourgeois, A.L.; Harro, C.; Sack, D.A.; Chakraborty, S. Intestinal and systemic inflammation induced by symptomatic and asymptomatic enterotoxigenic E. coli infection and impact on intestinal colonization and ETEC specific immune responses in an experimental human challenge model. Gut Microbes 2021, 13, 1891852. [Google Scholar] [CrossRef] [PubMed]
- Upfill-Brown, A.; Taniuchi, M.; Platts-Mills, J.A.; Kirkpatrick, B.; Burgess, S.L.; Oberste, M.S.; Weldon, W.; Houpt, E.; Haque, R.; Zaman, K.; et al. Nonspecific Effects of Oral Polio Vaccine on Diarrheal Burden and Etiology Among Bangladeshi Infants. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 65, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.J.; Haridas, S.; Steer, D.; Dhaunsi, G.S.; Smith, A.I.; Adler, B. Identification of a Campylobacter protein that cross-reacts with cholera toxin. Infect. Immun. 2007, 75, 3070–3073. [Google Scholar] [CrossRef]
- Mwape, K.; Mubanga, C.; Kennedy, C.; Chisenga, C.; Obvious, C.; Randall, A.; Liang, X. Proteomic microarray analysis reveals high exposure to diarrheagenic Escherichia coli among children in Zambia participating in a phase I clinical trial. Microorganisms 2023, in press. [Google Scholar]
| Endpoint | Abbreviation | Definition | Rationale for Inclusion |
|---|---|---|---|
| Vaccine Preventable ETEC TD | VPO-ETEC TD | ≥5 unformed stools in a 24-h period, accompanied by abdominal pain/cramps, nausea, and/or vomiting of any intensity; plus ETEC sharing antigens with OEV as the sole pathogen 2 and isolated in a window of 24 h before to 72 h after illness onset among subjects completing 2-dose regimen, traveling during the window of 7 to 28 days post 2nd dose, and completing 14 to 28 days surveillance | Original Study Endpoint |
| Classic TD | Classic TD | ≥3 unformed stools in a 24-h period, accompanied by ≥1 accompanying GI symptom (abdominal pain or cramps, nausea, vomiting) | Classic TD Endpoint |
| ETEC TD | ETEC TD | ≥3 unformed stools in a 24-h period, accompanied by abdominal pain or cramps, nausea, or vomiting of any intensity, plus ETEC as the sole pathogen 2 isolated | Recommended by DSMB |
| More Severe ETEC TD | MS-ETEC TD | ETEC TD, plus at least one moderate to severe GI symptom or ETEC TD, or changes in activity due to illness severity | Recommended by DSMB |
| Clinically Significant ETEC TD | CS ETEC TD | ≥4 unformed stools in a 24-h period, accompanied by ≥1 abdominal pain or cramps, nausea, or vomiting of any intensity, plus ETEC as the sole pathogen 2 isolated | Developed to align with efficacy endpoints associated with the evaluation of reformulated OKV plus dmLT adjuvant (OEV-123) (NCT03729219); also aligns with preferred product characteristics for ETEC candidate vaccines presented at WHO 2020 PDVAC meeting 1 |
| More Severe Clinically Significant ETEC TD | MS-CS ETEC TD | Clinically significant ETEC TD; plus at least one moderate to severe GI symptom or moderate-to-severe ETEC TD, or change in activity due to illness severity | Developed to align with efficacy endpoints associated with the evaluation of reformulated OKV plus dmLT adjuvant (OEV-123) |
| Duration of Diarrhea | Points |
|---|---|
| Less than 2 days | 1 |
| 2–3 days | 2 |
| 4 or more days | 3 |
| Maximum number of diarrhea stools in 24 h | |
| 3 | 1 |
| 4–5 | 2 |
| More than 5 | 3 |
| Duration of vomiting | |
| No vomiting | 0 |
| 1–2 days | 2 |
| 3 or more days | 3 |
| Maximum number of vomiting episodes in 24 h | |
| 1 | 1 |
| 2 | 2 |
| >2 | 3 |
| Dehydration documented by provider | |
| None | 0 |
| Clinically present | 2 |
| Fever (oral) documented by coordinator or provider | |
| Less than 38 °C | 0 |
| ≥38 °C | 2 |
| Hospitalization needed | |
| Yes | 3 |
| No | 0 |
| Endpoint Definition 1 | Vaccinees (N = 705) | Placebos (N = 701) | PE (%) (95% CI; p-Value) |
|---|---|---|---|
| VPO-ETEC TD (n = 13) | 8 | 5 | −63% (−91.1–79.0%; p = 0.29) |
| Modified Vesikari Diarrhea Score (median) 2 | 6.5 | 6 | NS |
| ETEC-TD (n = 28) | 11 | 17 | 35.7% (−36.6–69.0%; p = 0.17) |
| Modified Vesikari Diarrhea Score (median) 2 | 5 | 6 | NS |
| MS-ETEC TD 3 (n = 20) | 6 | 14 | 57.4% (−10.3–83.5%; p = 0.06) |
| Modified Vesikari Diarrhea Score (median) 2 | 5 | 6.5 | NS |
| Protective Efficacy (95% CI; p-Value) | ||||
|---|---|---|---|---|
| Endpoint 2 | ETEC TD | MS-ETEC TD † | CS TD | MS-CS TD † |
| Take (n = 251) | 46.7% (−19.8–76.3%; p = 0.09) | 76.3% (19.3–93.0%; p = 0.01) | 54.8% (−23.6–83.5%; p = 0.09) | 80.5% (19.4–98.6%; p = 0.01) |
| Protective Efficacy (95% CI; p-Value) | |||
|---|---|---|---|
| TD Severity Score ≥ 3 | TD Severity Score ≥ 4 | TD Severity Score ≥ 5 | |
| Take 1 (n = 251) | 63.8% (8.6–87.9%; p = 0.05) | 81.8% (21.1–95.8%; p = 0.01) | 85.9% (10.5–98.6%; p = 0.02) |
| Efficacy Estimate (95% CI) | Outcome | Outcome Description |
|---|---|---|
| Etiology Independent | Serology subset population, independent of etiology and symptom severity. | |
| 15.4% (−7.9–33.8%) | Etiology Independent + TD severity ≥ 3 | ETEC VPO (alt) and TD severity score ≥ 3 |
| 24.8% (−5.0–46.0%) | Etiology Independent + TD severity ≥ 4 | ETEC VPO (alt) and TD severity score ≥ 4 |
| 48.7% (12.8–69.8%) | Etiology Independent + TD severity ≥ 5 | ETEC VPO (alt) and TD severity score ≥ 5 |
| 57.6% (10.9–79.8%) | Etiology Independent + TD severity ≥ 6 | ETEC VPO (alt) and TD severity score ≥ 6 |
| Efficacy Estimate (95% CI) | Outcome | Outcome Description |
|---|---|---|
| −59% (−38.4–48%) | ETEC VPO (per protocol) | ≥5 unformed stools in 24 h, with abdominal pain/cramps, nausea, or vomiting, plus ETEC detected in limited time window |
| 35.7% (−36.6–69.0%) | ETEC VPO (per protocol) | ≥3 loose stools in 24 h with abdominal pain/cramps, nausea, or vomiting, plus ETEC detected at any time during disease |
| 45% (−30.0–76.0%) | ETEC VPO + TD severity ≥ 3 | ETEC VPO (alt) and TD severity score ≥ 3 |
| 72% (16.0–91.0%) | ETEC VPO + TD severity ≥ 4 | ETEC VPO (alt) and TD severity score ≥ 4 |
| 79% (1.1–95.0%) | ETEC VPO + TD severity ≥ 5 | ETEC VPO (alt) and TD severity score ≥ 5 |
| 57.7% (−9.5–83.6%) | MS-ETEC VPO | ≥3 unformed stools in 24 h, with moderate to severe abdominal pain/cramps, nausea, or vomiting; or change in activity due to illness plus ETEC detected at any time during disease |
| 54.4% (−19.2–82.1%) | MS-ETEC VPO + TD severity ≥ 3 | MS-ETEC VPO and TD severity score ≥ 3 |
| 83.5% (26.7–96.3) | MS-ETEC VPO + TD severity ≥ 4 | MS-ETEC VPO and TD severity score ≥ 4 |
| 87.7% (1.6–98.5%) | MS-ETEC VPO + TD severity ≥ 5 | MS-ETEC VPO and TD severity score ≥ 5 |
| ETEC Strain | Arrival Anti-CTB IgA Titer | Number of Participants | Total Infections (%) | TD 2 | MS-ETEC TD 3 |
|---|---|---|---|---|---|
| LT, ST or LT/ST 1 | ≤360 | 383 | 40 (10.4%) | 35 (8.6%) Median Duration = 6 days | 18 (4.7%) Median Duration = 6 days |
| >360 | 263 | 10 (3.8%) | 7 (2.6%) Median Duration = 2.5 days | 1 (0.4%) Median Duration = 1.5 days | |
| RR = 0.26 (0.14–0.35; p = 0.002) | RR = 0.24 (0.18–0.38; p = 0.001) | RR = 0.37 (0.29–0.44; p = 0.001) | |||
| ST 4 | ≤360 | 383 | 40 (10.4%) | 36 (9.4%) Median Duration = 4 days | 28 (7.3%) Median Duration = 4 days |
| >360 | 263 | 38 (14.4%) | 31 (11.8%) Median Duration = 6 days | 28 (10.6%) Median Duration = 5 days | |
| RR = NS | RR = NS | RR = NS | |||
| Enteric Pathogen | Arrival Anti-CTB IgA Titer | Number of Participants | Total Infections (%) | TD 1 | MS-ETEC TD 2 |
|---|---|---|---|---|---|
| Campylobacter jejuni/E. coli 3 | ≤360 | 383 | 17 (4.4%) | 9 (2.3%) Median Duration = 6 days | 9 (2.3%) Median Duration = 6 days |
| >360 | 263 | 4 (1.5%) | 3 (1.1%) Median Duration = 7 days | 3 (1.1%) Median Duration = 7 days | |
| RR = 0.34 (0.12–1.1; p = 0.03) | RR = 0.48 (0.13–1.8; p = NS) | RR = 0.48 (0.13–1.8; p = NS) | |||
| Salmonella spp. 4 | ≤360 | 383 | 9 (2.3%) | 3 (0.8%) Median Duration = 7 days | 3 (0.8%) Median Duration = 7 days |
| >360 | 263 | 2 (0.8%) | 1 (0.4%) Median Duration = 1 day | 1 (0.4%) Median Duration = 1 day | |
| RR = 0.32 (0.07–1.5; p = NS) | RR = 0.48 (0.05–4.6; p = NS) | RR = 0.48 (0.05–4.6; p = NS) | |||
| Shigella spp. | ≤360 | 383 | 5 (1.3%) | 5 (1.3%) Median Duration = 6 days | 5 (1.3%) Median Duration = 6 days |
| >360 | 263 | 6 (2.3%) | 6 (2.3%) Median Duration = 6 days | 5 (1.3%) Median Duration = 6 days | |
| RR = NS | RR = NS | RR = NS | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maier, N.; Grahek, S.L.; Halpern, J.; Restrepo, S.; Troncoso, F.; Shimko, J.; Torres, O.; Belkind-Gerson, J.; Sack, D.A.; Svennerholm, A.-M.; et al. Efficacy of an Enterotoxigenic Escherichia coli (ETEC) Vaccine on the Incidence and Severity of Traveler’s Diarrhea (TD): Evaluation of Alternative Endpoints and a TD Severity Score. Microorganisms 2023, 11, 2414. https://doi.org/10.3390/microorganisms11102414
Maier N, Grahek SL, Halpern J, Restrepo S, Troncoso F, Shimko J, Torres O, Belkind-Gerson J, Sack DA, Svennerholm A-M, et al. Efficacy of an Enterotoxigenic Escherichia coli (ETEC) Vaccine on the Incidence and Severity of Traveler’s Diarrhea (TD): Evaluation of Alternative Endpoints and a TD Severity Score. Microorganisms. 2023; 11(10):2414. https://doi.org/10.3390/microorganisms11102414
Chicago/Turabian StyleMaier, Nicole, Shannon L. Grahek, Jane Halpern, Suzanne Restrepo, Felipe Troncoso, Janet Shimko, Olga Torres, Jaime Belkind-Gerson, David A. Sack, Ann-Mari Svennerholm, and et al. 2023. "Efficacy of an Enterotoxigenic Escherichia coli (ETEC) Vaccine on the Incidence and Severity of Traveler’s Diarrhea (TD): Evaluation of Alternative Endpoints and a TD Severity Score" Microorganisms 11, no. 10: 2414. https://doi.org/10.3390/microorganisms11102414
APA StyleMaier, N., Grahek, S. L., Halpern, J., Restrepo, S., Troncoso, F., Shimko, J., Torres, O., Belkind-Gerson, J., Sack, D. A., Svennerholm, A.-M., Gustafsson, B., Sjöstrand, B., Carlin, N., Bourgeois, A. L., & Porter, C. K. (2023). Efficacy of an Enterotoxigenic Escherichia coli (ETEC) Vaccine on the Incidence and Severity of Traveler’s Diarrhea (TD): Evaluation of Alternative Endpoints and a TD Severity Score. Microorganisms, 11(10), 2414. https://doi.org/10.3390/microorganisms11102414






