Effects of Anthropogenic Disturbance and Seasonal Variation on Aerobiota in Highly Visited Show Caves in Slovenia
Abstract
:1. Introduction
2. Materials and Methods
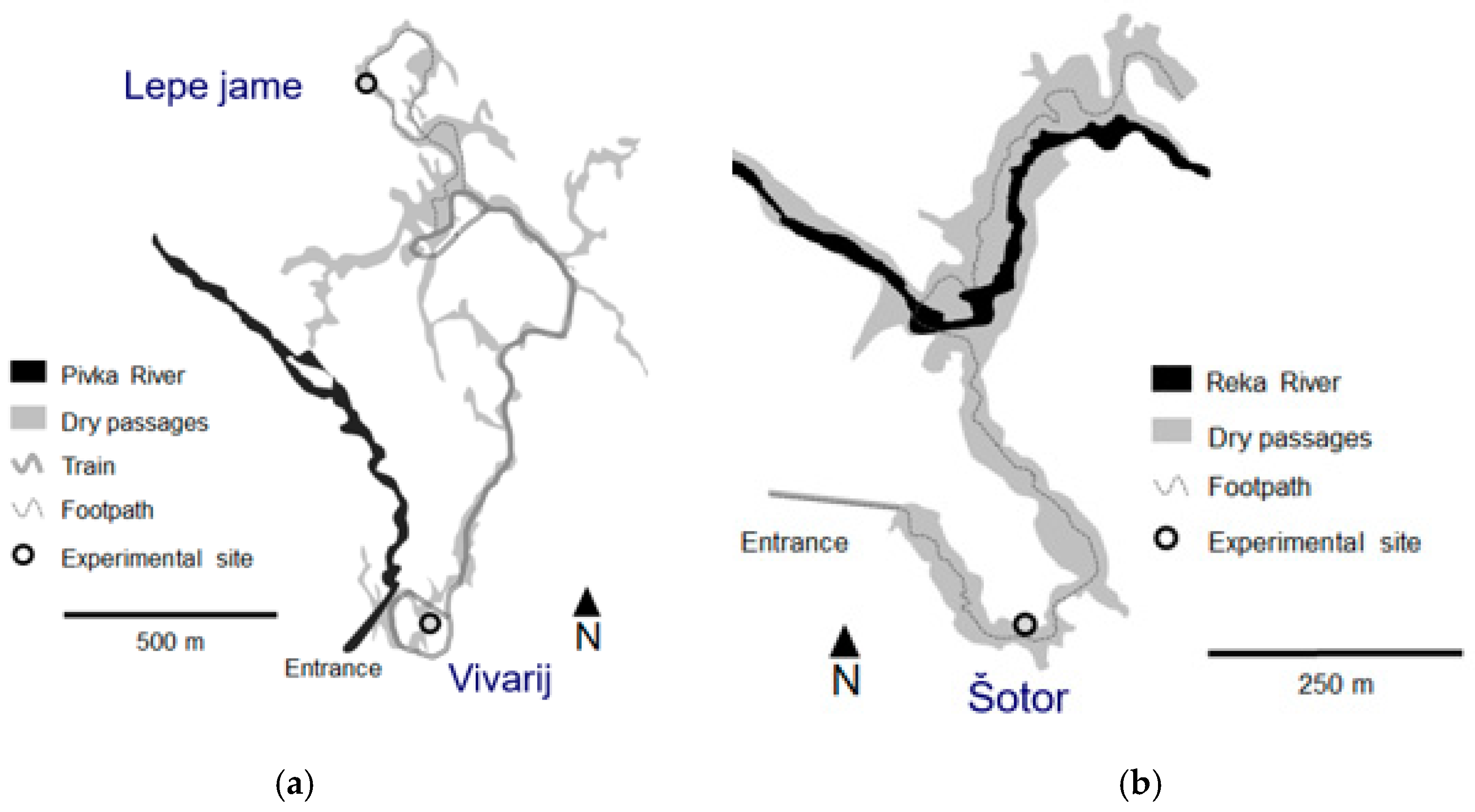
2.1. Sampling Sites
2.2. Air Sampling and Measurement of Environmental Parameters
2.3. Biomass Estimators and Microbial Identification
2.3.1. Total Cell Counts and Cell Viability
2.3.2. Detection of (1→3)-β-D-Glucan (BG)
2.3.3. Detection of Lipopolysaccharide (LPS)
2.3.4. Microbial Cultivation
2.3.5. Microbial Identification
2.4. Statistical Analysis
3. Results
3.1. Environmental Parameters, Quantification of Airborne Microorganisms and Biomass Estimators
3.2. Identification of Airborne Microorganisms
3.3. Microbial Indicators of Cave Anthropisation
4. Discussion
4.1. Microclimatic Parameters and β-(1,3)-D-Glucan
4.2. Airborne Microorganisms and Tourist Visits
4.3. Human-Associated Microbiota and Ecological Disturbance
4.4. MALDI-TOF MS Identification Success Rate
4.5. Microorganisms and Potential Indicators of Human Impact
4.6. Opportunistic Pathogens in Cave Aerobiota
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calò, F.; Parise, M. Evaluating the human disturbance to karst environments in southern Italy. Acta Carsologica 2006, 35. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats; Oxford University Press: Oxford, UK, 2019; ISBN 0-19-255276-7. [Google Scholar]
- Mulec, J.; Oarga-Mulec, A.; Šturm, S.; Tomazin, R.; Matos, T. Spacio-temporal distribution and tourist impact on airborne bacteria in a cave (Škocjan Caves, Slovenia). Diversity 2017, 9, 28. [Google Scholar] [CrossRef]
- Mulec, J.; Skok, S.; Tomazin, R.; Letić, J.; Pliberšek, T.; Stopinšek, S.; Simčič, S. Long-Term Monitoring of Bioaerosols in an Environment without UV and Desiccation Stress, an Example from the Cave Postojnska Jama, Slovenia. Microorganisms 2023, 11, 809. [Google Scholar] [CrossRef] [PubMed]
- Bercea, S.; Năstase-Bucur, R.; Mirea, I.C.; Măntoiu, D.Ş.; Kenesz, M.; Petculescu, A.; Baricz, A.; Andrei, A.-Ş.; Banciu, H.L.; Papp, B. Novel approach to microbiological air monitoring in show caves. Aerobiologia 2018, 34, 445–468. [Google Scholar] [CrossRef]
- Cigna, A.A. Show caves. In Encyclopedia of Caves; Elsevier: Amsterdam, The Netherlands, 2019; pp. 909–921. [Google Scholar]
- Kowalczk, A.J.; Froelich, P.N. Cave air ventilation and CO2 outgassing by radon-222 modeling: How fast do caves breathe? Earth Planet. Sci. Lett. 2010, 289, 209–219. [Google Scholar] [CrossRef]
- Mammola, S.; Di Piazza, S.; Ziotti, M.; Badino, G.; Marco, I. Human-induced alterations of the mycobiota in an alpine show cave (Italy, SW-Alps). Acta Carsologica 2017, 46. [Google Scholar] [CrossRef]
- Šebela, S. Chapter 6—Sustainable use of show caves. In Natural and Anthropogenic Impacts on Cave Climates; Šebela, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 231–239. Available online: https://www.sciencedirect.com/science/article/pii/B9780128229545000299 (accessed on 13 June 2023).
- Šebela, S. Chapter 5—Microclimatic monitoring in show caves. In Natural and Anthropogenic Impacts on Cave Climates; Šebela, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 33–43. Available online: https://www.sciencedirect.com/science/article/pii/B9780128229545000056 (accessed on 13 June 2023).
- Šebela, S.; Prelovšek, M.; Turk, J. Impact of peak period visits on the Postojna Cave (Slovenia) microclimate. Appl. Clim. 2013, 111, 51–64. [Google Scholar] [CrossRef]
- Dominguez-Moñino, I.; Jurado, V.; Rogerio-Candelera, M.A.; Hermosin, B.; Saiz-Jimenez, C. Airborne fungi in show caves from Southern Spain. Appl. Sci. 2021, 11, 5027. [Google Scholar] [CrossRef]
- Jurado, V.; Laiz, L.; Rodriguez-Nava, V.; Boiron, P.; Hermosin, B.; Sanchez-Moral, S.; Saiz-Jimenez, C. Pathogenic and opportunistic microorganisms in caves. Int. J. Speleol. 2010, 39, 2. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. 2018, 67, 23–35. [Google Scholar] [CrossRef]
- Barton, H.A. Introduction to cave microbiology: A review for the non-specialist. J. Cave Karst Stud. 2006, 68, 43–54. [Google Scholar]
- Biagioli, F.; Coleine, C.; Piano, E.; Nicolosi, G.; Poli, A.; Prigione, V.; Zanellati, A.; Varese, C.; Isaia, M.; Selbmann, L. Microbial diversity and proxy species for human impact in Italian karst caves. Sci. Rep. 2023, 13, 689. [Google Scholar] [CrossRef] [PubMed]
- Piano, E.; Biagioli, F.; Nicolosi, G.; Coleine, C.; Poli, A.; Prigione, V.; Zanellati, A.; Addesso, R.; Varese, C.; Selbmann, L. Human disturbance drives differential diversity patterns of microbial communities in hypogean habitats. Authorea Prepr. 2022, 16. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C. The Conservation of Subterranean Cultural Heritage; CRC Press: Boca Raton, FL, USA, 2014; ISBN 1-315-73997-6. [Google Scholar]
- Taş, N.; de Jong, A.E.; Li, Y.; Trubl, G.; Xue, Y.; Dove, N.C. Metagenomic tools in microbial ecology research. Curr. Opin. Biotechnol. 2021, 67, 184–191. [Google Scholar] [CrossRef]
- Wiseschart, A.; Pootanakit, K. Metagenomic-based approach to a comprehensive understanding of cave microbial diversity. Recent Adv. Microb. Divers. 2020, 561–586. [Google Scholar] [CrossRef]
- González-Riancho, C. Development of Protocols for Microbiological Control in Altamira Cave; Development of Protocols for Microbiological Control in Altamira Cave|DIGITAL.CSIC, 1 September 2023. 2015. Available online: http://hdl.handle.net/10261/164966 (accessed on 13 June 2023).
- Miller, A.Z.; Caldeira, A.T.; De Waele, J.; D’Angeli, I.M.; Payler, S.J.; Gabrovšek, F.; Bessone, L.; Sauro, F. Searching for subterranean-adapted microorganisms as part of the ESA CAVES and PANGAEA Astronaut training programs for planetary exploration. In Proceedings of the 19th European Astrobiology Network Association (EANA) Conference, Orléans, France, 3–6 September 2019. [Google Scholar]
- Mulec, J.; Oarga-Mulec, A. ATP luminescence assay as a bioburden estimator of biomass accumulation in caves. Int. J. Speleol. 2016, 45, 2. [Google Scholar] [CrossRef]
- Borderie, F.; Denis, M.; Barani, A.; Alaoui-Sossé, B.; Aleya, L. Microbial composition and ecological features of phototrophic biofilms proliferating in the Moidons Caves (France): Investigation at the single-cell level. Environ. Sci. Pollut. Res. 2016, 23, 12039–12049. [Google Scholar] [CrossRef]
- Borderie, F.; Tête, N.; Cailhol, D.; Alaoui-Sehmer, L.; Bousta, F.; Rieffel, D.; Aleya, L.; Alaoui-Sossé, B. Factors driving epilithic algal colonization in show caves and new insights into combating biofilm development with UV-C treatments. Sci. Total Environ. 2014, 484, 43–52. [Google Scholar] [CrossRef]
- Pfendler, S.; Alaoui-Sossé, B.; Alaoui-Sossé, L.; Bousta, F.; Aleya, L. Effects of UV-C radiation on Chlorella vulgaris, a biofilm-forming alga. J. Appl. Phycol. 2018, 30, 1607–1616. [Google Scholar] [CrossRef]
- Mayeux, P.R. Pathobiology of lipopolysaccharide. J. Toxicol. Environ. Health 1997, 51, 415–435. [Google Scholar] [CrossRef]
- Virzì, G.M.; Mattiotti, M.; de Cal, M.; Ronco, C.; Zanella, M.; De Rosa, S. Endotoxin in Sepsis: Methods for LPS Detection and the Use of Omics Techniques. Diagnostics 2023, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quinn, P.J. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog. Lipid Res. 2010, 49, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.J.; Tamma, P.D.; Zhang, S.X. Challenges with Utilizing the 1, 3-Beta-d-Glucan and galactomannan assays to diagnose invasive mold infections in immunocompromised children. J. Clin. Microbiol. 2021, 59, e03276-20. [Google Scholar] [CrossRef] [PubMed]
- Kritikos, A.; Poissy, J.; Croxatto, A.; Bochud, P.-Y.; Pagani, J.-L.; Lamoth, F. Impact of the beta-glucan test on management of intensive care unit patients at risk for invasive candidiasis. J. Clin. Microbiol. 2020, 58, e01996-19. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M.; Verweij, P.E.; Arendrup, M.C.; Arikan-Akdagli, S.; Bille, J.; Donnelly, J.P.; Jensen, H.E.; Lass-Flörl, C.; Richardson, M.D.; Akova, M. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Diagnostic procedures. Clin. Microbiol. Infect. 2012, 18, 9–18. [Google Scholar] [CrossRef]
- Šebela, S.; Stemberk, J.; Briestenský, M. Micro-displacement monitoring in caves at the Southern Alps–Dinarides–Southwestern Pannonian Basin junction. Bull. Eng. Geol. Environ. 2021, 80, 7591–7611. [Google Scholar] [CrossRef]
- Šebela, S.; Turk, J. Local characteristics of Postojna Cave climate, air temperature, and pressure monitoring. Theor. Appl. Climatol. 2011, 105, 371–386. [Google Scholar] [CrossRef]
- Gospodaric, R.O. Geologiji in Speleogenezi Škocjanskih Jam. Geološki Zbornik 1983, 4, 163–172. [Google Scholar]
- Habič, P.; Knez, M.; Kogovŝek, J.; Kranjc, A.; Mihevc, A.; Slabe, T.; Šebela, S.; Zupan, N. Škocjanske Jame speleological revue. Int. J. Speleol. 1989, 18, 1. [Google Scholar] [CrossRef]
- Gams, I. Concentration of CO2 in the caves in relation to the air circulation (in the case of the Postojna Cave). Acta Carsol. VI 1974, 184–192. [Google Scholar]
- Prelovšek, M.; Šebela, S.; Turk, J. Carbon dioxide in Postojna Cave (Slovenia): Spatial distribution, seasonal dynamics and evaluation of plausible sources and sinks. Environ. Earth Sci. 2018, 77, 289. [Google Scholar] [CrossRef]
- Smetanová, I.; Holý, K.; Luhová, Ľ.; Csicsay, K.; Haviarová, D.; Kunáková, L. Seasonal variation of radon and CO2 in the Važecká Cave, Slovakia. Nukleonika 2020, 65. [Google Scholar] [CrossRef]
- Antón, S.F.; de la Cruz, D.R.; Sánchez, J.S.; Sánchez Reyes, E. Analysis of the airborne fungal spores present in the atmosphere of Salamanca (MW Spain): A preliminary survey. Aerobiologia 2019, 35, 447–462. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Qin, W.; Zhao, C.; Li, J.; Hou, Y.; Xiong, J.; Li, A.; Gao, R. Seasonal structural characteristics of indoor airborne fungi in library rooms by culturing and high-throughput sequencing. Build. Environ. 2021, 206, 108368. [Google Scholar] [CrossRef]
- Porca, E.; Jurado, V.; Martin-Sanchez, P.M.; Hermosin, B.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Aerobiology: An ecological indicator for early detection and control of fungal outbreaks in caves. Ecol. Indic. 2011, 11, 1594–1598. [Google Scholar] [CrossRef]
- Dominguez-Moñino, I.; Jurado, V.; Rogerio-Candelera, M.A.; Hermosin, B.; Saiz-Jimenez, C. Airborne bacteria in show caves from Southern Spain. Microb. Cell 2021, 8, 247. [Google Scholar] [CrossRef]
- Stupar, M.; Savković, Ž.; Popović, S.; Simić, G.S.; Grbić, M.L. Airborne Mycobiota in a Subterranean Habitat–A Case of Show Cave in Serbia. Res. Sq. 2022; preprint. [Google Scholar]
- Del Rosal, Y.; Liñán, C.; Hernández-Mariné, M. The conservation of the Nerja Cave: Preserving anthropogenic impact in a tourist cave. In The Conservation of Subterranean Cultural Heritage; CRC Press/Balkema: Leiden, Holland, 2014; pp. 193–206. [Google Scholar]
- Kuzmina, L.Y.; Galimzianova, N.F.; Abdullin, S.R.; Ryabova, A.S. Microbiota of the kinderlinskaya cave (South Urals, Russia). Microbiology 2012, 81, 251–258. [Google Scholar] [CrossRef]
- Liñán, C.; Del Rosal, Y.; Carrasco, F.; Vadillo, I.; Benavente, J.; Ojeda, L. Highlighting the importance of transitional ventilation regimes in the management of Mediterranean show caves (Nerja-Pintada system, southern Spain). Sci. Total Environ. 2018, 631, 1268–1278. [Google Scholar] [CrossRef]
- Devender, M.; Paul, D.; Baskar, S.; Baskar, R.; Shouche, Y.S. Cultivable Microbial Diversity in Speleothems Using MALDI-TOF Spectrometry and DNA Sequencing from Krem Soitan, Krem Lawbah, Krem Mawpun; Khasi Hills: Meghalaya, India, 2021. [Google Scholar]
- Mudgil, D.; Paul, D.; Baskar, S.; Baskar, R.; Shouche, Y.S. Cultivable microbial diversity in speleothems using MALDI-TOF spectrometry and DNA sequencing from Krem Soitan, Krem Lawbah, Krem Mawpun, Khasi Hills, Meghalaya, India. Arch. Microbiol. 2022, 204, 495. [Google Scholar] [CrossRef]
- Adetutu, E.M.; Thorpe, K.; Shahsavari, E.; Bourne, S.; Cao, X.; Mazaheri Nezhad Fard, R.; Kirby, G.; Ball, A.S. Bacterial community survey of sediments at Naracoorte Caves, Australia. Int. J. Speleol. 2012, 41, 2. [Google Scholar] [CrossRef]
- Ikner, L.A.; Toomey, R.S.; Nolan, G.; Neilson, J.W.; Pryor, B.M.; Maier, R.M. Culturable microbial diversity and the impact of tourism in Kartchner Caverns, Arizona. Microb. Ecol. 2007, 53, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M. Analysis of bacterial communities and characterization of antimicrobial strains from cave microbiota. Braz. J. Microbiol. 2018, 49, 248–257. [Google Scholar] [CrossRef]
- Lavoie, K.H.; Northup, D.E. Bacteria as indicators of human impact in caves. In Proceedings of the 17th National Cave and Karst Management Symposium; The NCKMS Steering Committee: Albany, NY, USA, 2006; pp. 40–47. [Google Scholar]
- Jurado, V.; Laiz, L.; Sanchez-Moral, S.; Sáiz-Jiménez, C. Pathogenic microorganisms related to human visits in Altamira Cave, Spain. In The Conservation of Subterranean Cultural Heritage; CRC Press: Boca Raton, FL, USA, 2014; pp. 229–238. [Google Scholar]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The human microbiota in health and disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Mulec, J. Human impact on underground cultural and natural heritage sites, biological parameters of monitoring and remediation actions for insensitive surfaces: Case of Slovenian show caves. J. Nat. Conserv. 2014, 22, 132–141. [Google Scholar] [CrossRef]
- Borda, D.R.; Nåstase-Bucur, R.M.; Spinu, M.; Uricariu, R.; Mulec, J. Aerosolized microbes from organic rich materials: Case study of bat guano from caves in Romania. J. Cave Karst Stud. 2014, 76. [Google Scholar] [CrossRef]
- Ihara, Y.; Takeshita, T.; Kageyama, S.; Matsumi, R.; Asakawa, M.; Shibata, Y.; Sugiura, Y.; Ishikawa, K.; Takahashi, I.; Yamashita, Y. Identification of initial colonizing bacteria in dental plaques from young adults using full-length 16S rRNA gene sequencing. Msystems 2019, 4, e00360-19. [Google Scholar] [CrossRef]
- Karlsson, R.; Gonzales-Siles, L.; Gomila, M.; Busquets, A.; Salvà-Serra, F.; Jaén-Luchoro, D.; Jakobsson, H.E.; Karlsson, A.; Boulund, F.; Kristiansson, E. Proteotyping bacteria: Characterization, differentiation and identification of pneumococcus and other species within the Mitis Group of the genus Streptococcus by tandem mass spectrometry proteomics. PLoS ONE 2018, 13, e0208804. [Google Scholar] [CrossRef]
- Wilson, M. The Human Microbiota in Health and Disease: An Ecological and Community-Based Approach; Garland Science: New York, NY, USA, 2018. [Google Scholar]
- Khawcharoenporn, T.; Apisarnthanarak, A.; Mundy, L.M. Non-neoformans cryptococcal infections: A systematic review. Infection 2007, 35, 51–58. [Google Scholar] [CrossRef]
- Morales-López, S.E.; Garcia-Effron, G. Infections due to rare Cryptococcus species. A literature review. J. Fungi 2021, 7, 279. [Google Scholar] [CrossRef]
- Murray, P.R.; Rosenthal, K.S.; Pfaller, M.A. Medical Microbiology E-book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020; ISBN 0-323-67450-X. [Google Scholar]
- Dlauchy, D.; Tornai-Lehoczki, J.; Sedláček, I.; Audy, M.; Péter, G. Debaryomyces psychrosporus sp. nov., a yeast species from a Venezuelan cave. Antonie Van Leeuwenhoek 2011, 99, 619–628. [Google Scholar] [CrossRef]
- Ogórek, R.; Kozak, B.; Višňovská, Z.; Tančinová, D. Phenotypic and genotypic diversity of airborne fungal spores in Demänovská Ice Cave (Low Tatras, Slovakia). Aerobiologia 2018, 34, 13–28. [Google Scholar] [CrossRef]
- Ogórek, R.; Lejman, A.; Matkowski, K. Fungi isolated from niedźwiedzia cave in kletno (Lower Silesia, Poland). Int. J. Speleol. 2013, 42, 9. [Google Scholar] [CrossRef]
- Takashima, M.; Kurakado, S.; Cho, O.; Kikuchi, K.; Sugiyama, J.; Sugita, T. Description of four Apiotrichum and two Cutaneotrichosporon species isolated from guano samples from bat-inhabited caves in Japan. Int. J. Syst. Evol. Microbiol. 2020, 70, 4458–4469. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.-P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Heseltine, E.; Rosen, J. (Eds.) WHO Guidelines for Indoor Air Quality: Dampness and Mould; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Rao, C.Y.; Burge, H.A.; Chang, J.C. Review of quantitative standards and guidelines for fungi in indoor air. J. Air Waste Manag. Assoc. 1996, 46, 899–908. [Google Scholar] [CrossRef]
- Wanner, H.; Verhoeff, A.; Colombi, A.; Flannigan, B.; Gravesen, S.; Mouilleseaux, A.; Nevalainen, A.; Papadakis, J.; Seidel, K. Indoor air quality and its impact on man: Report no. 12: Biological particles in indoor Environments. In Brussels-Luxembourg: ECSC-EEC-EAEC; Publications Office of the European Union: Luxembourg, 1993. [Google Scholar]
| Before Tourist Visitation | During/After Tourist Visitation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Date | T | CO2 | Microbes (Culture) | BDG | Total Microbes (Flow Cytometry) | % Live Microbes (Flow Cytometry) | T | CO2 | Microbes (Culture) | BDG | Total Microbes (Flow Cytometry) | % Live Microbes (Flow Cytometry) | No. of Tourists |
| (°C) | (ppm) | (CFU/m3) | (pg/m3) | (Microbes/m3) | (°C) | (ppm) | (CFU/m3) | (pg/m3) | (Microbes/m3) | |||||
| Lepe jame | 31 January2017 | 10.7 | 560 | 51 | <7.8 | 6556 | 88.1 | 10.6 | 540 | 188 | <7.8 | 6622 | 86.2 | 91 |
| 21 March 2017 | 10.7 | 660 | 93 | <7.8 | 30,600 | 85.2 | 10.6 | 690 | 169 | <7.8 | 39,289 | 84.1 | 136 | |
| 25 May 2017 | 10.8 | 1190 | 67 | 10 | 10,356 | 90.8 | 10.7 | 1230 | 51 | 19.1 | 12,800 | 88.5 | 135 | |
| 22 August 2017 | 10.8 | 1410 | 400 | 26.5 | 41,863 | 88.4 | 10.9 | 1550 | 787 | 30.6 | 41,919 | 89.3 | 154 | |
| 3 October 2017 | 10.9 | 2040 | 113 | 90.9 | 22,768 | 85.2 | 11 | 2000 | 68 | 151.2 | 8145 | 77.9 | 358 | |
| 5 December 2017 | 10.7 | 790 | 421 | 409.3 | 9111 | 81.9 | 12.3 | 800 | 327 | 584.7 | 6346 | 70.1 | 144 | |
| 28 August 2018 | 10.8 | 1580 | 17 | 85.2 | 10,616 | 76.1 | 11 | 1330 | 173 | 513.9 | 7976 | 71.3 | 602 | |
| 28 November 2018 | 10.4 | 1630 | 40 | 114.9 | 8321 | 70.9 | 10.5 | 1430 | 100 | 252.5 | 74,396 | 80.4 | 109 | |
| Vivarium | 21 March 2017 | 12.5 | 660 | 143 | <7.8 | 49,622 | 83.2 | - | - | 326 | <7.8 | 69,822 | 63.5 | 0 |
| 25 May 2017 | 12.7 | 1150 | 152 | 16.2 | 8888 | 86 | 10.7 | 1240 | 1287 | 18.5 | 13,426 | 80.7 | 25 | |
| 22 August 2017 | 13.9 | 1410 | 74 | 32.7 | 41,756 | 87.9 | 10.9 | 1650 | 333 | 26.8 | 46,451 | 83.7 | 246 | |
| 3 October 2017 | 13.1 | 760 | 643 | <7.8 | 5348 | 74.2 | 11 | 850 | 786 | 30.1 | 9145 | 79.4 | 63 | |
| 5 December 2017 | 13.6 | 600 | 322 | 191.9 | 28,567 | 80.8 | 12.3 | 640 | 59 | 348.3 | 14,868 | 79.3 | 0 | |
| 28 August 2018 | 12.7 | 1350 | 11 | 62.5 | 6183 | 78.1 | 12.1 | 1410 | 81 | 157.9 | 6926 | 67.3 | 18 | |
| 28 November 2018 | 12.2 | 640 | 229 | 141.6 | 9157 | 81.5 | 11.9 | 750 | 920 | <7.8 | 15,088 | 75.8 | 2 | |
| Šotor | 1 February 2017 | 12.5 | 540 | 215 | 40.8 | 21,378 | 95.5 | 12.3 | 540 | 327 | <7.8 | 24,311 | 96.5 | 7 |
| 22 March 2017 | 12.6 | 810 | 65 | 8.4 | 25,911 | 84.4 | 12.4 | 820 | 60 | <7.8 | 23,667 | 86.6 | 97 | |
| 24 May 2017 | 12.7 | 1300 | 51 | 8.9 | 10,454 | 90.4 | 12.5 | 1330 | 335 | <7.8 | 10,319 | 84.9 | 119 | |
| 23 August 2017 | 12.6 | 1910 | 251 | 59 | 45,272 | 88.2 | 12.7 | 1950 | 459 | 17 | 44,728 | 86.2 | 182 | |
| 4 October 2017 | 12.7 | 1880 | 393 | 1326 | 14,800 | 78 | 12.5 | 1920 | 539 | 1437.7 | 6873 | 70.6 | 79 | |
| 6 December .2017 | 12.4 | 520 | 20 | 592.2 | 4882 | 77.1 | 12.4 | 510 | 122 | 296.5 | 7170 | 71.5 | 12 | |
| 29 August 2018 | 12.9 | 2390 | 77 | 185.4 | 25,010 | 30.2 | 12.6 | 2110 | 218 | 631.5 | 15,245 | 57.6 | 278 | |
| 29 November 2018 | 12.7 | 630 | 51 | 87 | 12,503 | 79.7 | 12.5 | 610 | 100 | 39.1 | 105,733 | 84.1 | 9 | |
| Location | CO2 [ppm] | p | Microbes (Culture) [CFU/m3] | p | BDG [pg/m3] | p | |
|---|---|---|---|---|---|---|---|
| Lepe jame | Before tourists | 990 | 0.400 | 103 | 0.463 | 18.25 | 0.068 |
| After tourists | 1015 | 178.5 | 24.85 | ||||
| Vivarium | Before tourists | 955 | 0.344 | 152 | 0.345 | 24.45 | 0.138 |
| After tourists | 1045 | 333 | 28.45 | ||||
| Šotor | Before tourists | 1055 | 0.102 | 140 | 0.046 | 49.90 | 0.249 |
| After tourists | 1075 | 331 | 12.40 |
| Lepe Jame | Vivarium | Šotor | Locations Combined | ||
|---|---|---|---|---|---|
| Non-identified microorganisms (CFU/m3) | Before tourists | 692 | 911 | 571 | 2174 |
| After tourists | 566 | 1152 | 865 | 2583 | |
| All isolated microorganisms (CFU/m3) | Before tourists | 1202 | 1571 | 1123 | 3896 |
| After tourists | 1863 | 3792 | 2160 | 7815 | |
| % of identified microorganisms | Before tourists | 42.4 | 42.0 | 49.2 | 44.2 |
| After tourists | 69.6 | 69.6 | 60.0 | 66.9 |
| Microorganisms | Sampling Sites * | Risk Group (Country) | Human Microbiota |
|---|---|---|---|
| Bacteria | |||
| Dermacoccus spp. | 2 | - | skin, scalp |
| Micrococcus luteus | 1, 2, 3 | 2 (BE, CH, DE, NIH) | skin |
| Moraxella osloensis | 1 | - | skin, mucosae |
| Staphylococcus capitis | 1 | - | skin, scalp |
| Staphylococcus epidermidis | 1, 2, 3 | 2 (BE, CH, DE) | skin, nasopharynx |
| Staphylococcus haemolyticus | 1, 2 | 2 (CH, DE) | skin |
| Staphylococcus hominis | 3 | - | skin |
| Staphylococcus lugdunensis | 1, 2 | 2 (BE, CH, DE) | skin |
| Staphylococcus saprophyticus | 2, 3 | 2 (BE, CA, CH, DE) | skin |
| Staphylococcus warneri | 1, 2, 3 | - | skin |
| Streptococcus mitis | 1, 2 | 2 (BE, CH, DE, NIH) | oropharynx |
| Fungi | |||
| Naganishia diffluens | 1, 2 | - | skin |
| Naganishia liquefaciens | 2, 3 | - | skin |
| Rhodotorula mucilaginosa | 2, 3 | 1 (DE) | skin, oropharynx |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomazin, R.; Simčič, S.; Stopinšek, S.; Kopitar, A.N.; Kukec, A.; Matos, T.; Mulec, J. Effects of Anthropogenic Disturbance and Seasonal Variation on Aerobiota in Highly Visited Show Caves in Slovenia. Microorganisms 2023, 11, 2381. https://doi.org/10.3390/microorganisms11102381
Tomazin R, Simčič S, Stopinšek S, Kopitar AN, Kukec A, Matos T, Mulec J. Effects of Anthropogenic Disturbance and Seasonal Variation on Aerobiota in Highly Visited Show Caves in Slovenia. Microorganisms. 2023; 11(10):2381. https://doi.org/10.3390/microorganisms11102381
Chicago/Turabian StyleTomazin, Rok, Saša Simčič, Sanja Stopinšek, Andreja Nataša Kopitar, Andreja Kukec, Tadeja Matos, and Janez Mulec. 2023. "Effects of Anthropogenic Disturbance and Seasonal Variation on Aerobiota in Highly Visited Show Caves in Slovenia" Microorganisms 11, no. 10: 2381. https://doi.org/10.3390/microorganisms11102381
APA StyleTomazin, R., Simčič, S., Stopinšek, S., Kopitar, A. N., Kukec, A., Matos, T., & Mulec, J. (2023). Effects of Anthropogenic Disturbance and Seasonal Variation on Aerobiota in Highly Visited Show Caves in Slovenia. Microorganisms, 11(10), 2381. https://doi.org/10.3390/microorganisms11102381






