Carbapenem-Resistant Acinetobacter baumannii: Biofilm-Associated Genes, Biofilm-Eradication Potential of Disinfectants, and Biofilm-Inhibitory Effects of Selenium Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates and Species Identification
2.2. Antimicrobial Susceptibility Testing
Detection of Carbapenemase-Encoding Genes
2.3. Biofilm Production Assay
2.4. Genes Encoding Virulence Factors Associated with Biofilms
2.5. Antibiofilm Effect of 70% Ethanol and 0.5% NaOCl on Mature Biofilm
2.6. Selenium Nanoparticles Synthesis
2.7. Antimicrobial Testing of Selenium Nanoparticles against Planktonic and Biofilm-Embedded Carbapenem-Resistant Acinetobacter baumannii Isolates
2.8. Statistical Analysis
3. Results
3.1. Bacterial Isolates and Antimicrobial Susceptibilities
Carbapenemase-Encoding Genes
3.2. Biofilm Production Assay
3.3. Genes Encoding Virulence Factors Associated with Biofilms
3.4. Antibiofilm Effect of 70% Ethanol and 0.5% NaOCl on Mature Biofilm
3.5. Selenium Nanoparticles and Their Antimicrobial Effects on Carbapenem-Resistant Acinetobacter baumannii Isolates
3.5.1. Selenium Nanoparticles Characterisation
3.5.2. Antimicrobial Effects of Selenium Nanoparticles against Planktonic and Biofilm-Embedded Carbapenem-Resistant Acinetobacter baumannii Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primers and Sequences 5′ to 3′ | Annealing Temperature (°C) | Amplicon Size (bp) | Reference |
|---|---|---|---|
| csuEF: 5′-CATCTTCTATTTCGGTCCC-3′ csuER: 5′-CGGTCTGAGCATTGGTAA-3′ | 59 | 168 | [39] |
| bapF: 5′-AGTTAAAGAAGGGCAAGAAG-3′ bapR: 5′-GGAGCACCACCTAACTGA-3′ | 45 | 850 | [63] |
| ompAF: 5′-GGAATGGTATAACTGACATAATC-3′ ompAR: 5′-GAATCAGGAGATTTACAAATGACC-3′ | 47 | 1600 | [64] |
| blaOXA-51-likeF: 5′-TAATGCTTTGATCGGCCTTG-3′ blaOXA-51-like R: 5′-TGGATTGCACTTCATCTTGG-3′ | 60 | 353 | [16] |
| blaOXA-23-like F: 5′-GATCGGATTGGAGAACCAGA-3′ blaOXA-23-like R: 5′-ATTTCTGACCGCATTTCCAT-3′ | 52 | 501 | [21] |
| blaOXA-24-like F: 5′-GGTTAGTTGGCCCCCTTAAA-3′ blaOXA-24-like R: 5′-AGTTGAGCGAAAAGGGGATT-3′ | 52 | 246 | [21] |
| blaOXA-58-like F: 5′-AAGTATTGGGGCTTGTGCTG-3′ blaOXA-58-like R: 5′-CCCCTCTGCGCTCTACATAC-3′ | 52 | 599 | [21] |
| blaOXA-143-likeF: 5′-TGGCACTTTCAGCAGTTCCT-3′ blaOXA-143-likeR: 5′-TAATCTTGAGGGGGCCAACC-3′ | 52 | 149 | [22] |
References
- Roy, S.; Chowdhury, G.; Mukhopadhyay, A.K.; Dutta, S.; Basu, S. Convergence of Biofilm Formation and Antibiotic Resistance in Acinetobacter baumannii Infection. Front. Med. 2022, 9, 793615. [Google Scholar] [CrossRef] [PubMed]
- Visca, P.; Seifert, H.; Towner, K.J. Acinetobacter infection—An emerging threat to human health. IUBMB Life 2011, 63, 1048–1054. [Google Scholar] [CrossRef]
- Nguyen, M.; Joshi, S.G. Carbapenem resistance in Acinetobacter baumannii, and their importance in hospital-acquired infections: A scientific review. J. Appl. Microbiol. 2021, 131, 2715–2738. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, F.G.; Corcione, S.; Pagani, N.; Di Perri, G. From ESKAPE to ESCAPE, From KPC to CCC. Clin. Infect. Dis. 2015, 60, 1289–1290. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef]
- Gajic, I.; Jovicevic, M.; Milic, M.; Kekic, D.; Opavski, N.; Zrnic, Z.; Dacic, S.; Pavlovic, L.; Mijac, V. Clinical and molecular characteristics of OXA-72-producing Acinetobacter baumannii ST636 outbreak at a neonatal intensive care unit in Serbia. J. Hosp. Infect. 2021, 112, 54–60. [Google Scholar] [CrossRef]
- Lukovic, B.; Gajic, I.; Dimkic, I.; Kekic, D.; Zornic, S.; Pozder, T.; Radisavljevic, S.; Opavski, N.; Kojic, M.; Ranin, L. The first nationwide multicenter study of Acinetobacter baumannii recovered in Serbia: Emergence of OXA-72, OXA-23 and NDM-1-producing isolates. Antimicrob. Resist. Infect. Control 2020, 9, 101. [Google Scholar] [CrossRef]
- Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J. Biomed. Sci. 2020, 27, 26. [Google Scholar] [CrossRef]
- Zarrilli, R. Acinetobacter baumannii virulence determinants involved in biofilm growth and adherence to host epithelial cells. Virulence 2016, 7, 367–368. [Google Scholar] [CrossRef]
- Pompilio, A.; Scribano, D.; Sarshar, M.; Di Bonaventura, G.; Palamara, A.T.; Ambrosi, C. Gram-Negative Bacteria Holding Together in a Biofilm: The Acinetobacter baumannii Way. Microorganisms 2021, 9, 1353. [Google Scholar] [CrossRef] [PubMed]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative Study of the Antimicrobial Activity of Selenium Nanoparticles With Different Surface Chemistry and Structure. Front. Bioeng. Biotechnol. 2021, 8, 624621. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Chen, Q.; Yu, Q.; Sun, D.; Liu, J. Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater. 2016, 30, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Ušjak, D.; Novović, K.; Filipić, B.; Kojić, M.; Filipović, N.; Stevanović, M.M.; Milenković, M.T. In vitro colistin susceptibility of pandrug-resistant Ac. baumannii is restored in the presence of selenium nanoparticles. J. Appl. Microbiol. 2022, 133, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Gajic, I.; Ranin, L.; Kekic, D.; Opavski, N.; Smitran, A.; Mijac, V.; Jovanovic, S.; Hadnadjev, M.; Travar, M.; Mijovic, G. Tigecycline susceptibility of multidrug-resistant Acinetobacter baumannii from intensive care units in the western Balkans. Acta Microbiol. Immunol. Hung. 2020, 67, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.F.; Woodford, N.; Glover, J.; Yarde, S.; Kaufmann, M.E.; Pitt, T.L. Identification of Acinetobacter baumannii by Detection of the blaOXA-51-like Carbapenemase Gene Intrinsic to This Species. J. Clin. Microbiol. 2006, 44, 2974. [Google Scholar] [CrossRef]
- La Scola, B.; Gundi, V.A.K.B.; Khamis, A.; Raoult, D. Sequencing of the rpoB Gene and Flanking Spacers for Molecular Identification of Acinetobacter Species. J. Clin. Microbiol. 2006, 44, 827. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M100: Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 12.0. Available online: http://www.eucast.org (accessed on 23 February 2022).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance Background Emergence of resistance to multiple antimicrobial agents in pathogenic bac. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Woodford, N.; Ellington, M.; Coelho, J.; Turton, J.; Ward, M.; Brown, S.; Amyes, S.; Livermore, D. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 2006, 27, 351–353. [Google Scholar] [CrossRef]
- Higgins, P.G.; Prior, K.; Harmsen, D.; Seifert, H. Development and evaluation of a core genome multilocus typing scheme for whole-genome sequence-based typing of Acinetobacter baumannii. PLoS ONE 2017, 12, e179228. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Narayanan, A.; Nair, M.S.; Karumathil, D.P.; Baskaran, S.A.; Venkitanarayanan, K.; Amalaradjou, M.A.R. Inactivation of Acinetobacter baumannii Biofilms on Polystyrene, Stainless Steel, and Urinary Catheters by Octenidine Dihydrochloride. Front. Microbiol. 2016, 7, 847. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.I.; Djukić, S.; ĆIRKOVIĆ, I.; RUZICKA, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Josheghani, S.; Bakhshi, B. Investigation of the Antibacterial and Antibiofilm Activity of Selenium Nanoparticles against Vibrio cholerae as a Potent Therapeutics. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3432235. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Wang, L.; Zhang, D.; Xiang, D.; Liu, Q.; Xing, X. Prognosis of patients with Acinetobacter baumannii infection in the intensive care unit: A retrospective analysis. Exp. Ther. Med. 2017, 13, 1630–1633. [Google Scholar] [CrossRef]
- Franolić-Kukina, I.; Bedenić, B.; Budimir, A.; Herljević, Z.; Vraneš, J.; Higgins, P.G. Clonal spread of carbapenem-resistant OXA-72-positive Acinetobacter baumannii in a Croatian university hospital. Int. J. Infect. Dis. 2011, 15, e706–e709. [Google Scholar] [CrossRef]
- Mugnier, P.D.; Poirel, L.; Naas, T.; Nordmann, P. Worldwide Dissemination of the bla OXA-23 Carbapenemase Gene of Acinetobacter baumannii 1. Emerg. Infect. Dis. 2009, 16, 35–40. [Google Scholar] [CrossRef]
- Pournaras, S.; Dafopoulou, K.; Del Franco, M.; Zarkotou, O.; Dimitroulia, E.; Protonotariou, E.; Poulou, A.; Zarrilli, R.; Tsakris, A.; Skoura, L.; et al. Predominance of international clone 2 OXA-23-producing- Acinetobacter baumannii clinical isolates in Greece, 2015: Results of a nationwide study. Int. J. Antimicrob. Agents 2017, 49, 749–753. [Google Scholar] [CrossRef]
- Strateva, T.; Sirakov, I.; Stoeva, T.; Stratev, A.; Dimov, S.; Savov, E.; Mitov, I. Carbapenem-resistant Acinetobacter baumannii: Current status of the problem in four Bulgarian university hospitals (2014–2016). J. Glob. Antimicrob. Resist. 2019, 16, 266–273. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Hittle, L.E.; O’Hara, J.A.; Rivera, J.I.; Syed, A.; Shields, R.K.; Pasculle, A.W.; Ernst, R.K.; Doi, Y. Colistin-Resistant Acinetobacter baumannii: Beyond Carbapenem Resistance. Clin. Infect. Dis. 2015, 60, 1295–1303. [Google Scholar] [CrossRef]
- Snyman, Y.; Reuter, S.; Whitelaw, A.C.; Stein, L.; Maloba, M.R.B.; Newton-Foot, M. Characterisation of mcr-4.3 in a colistin-resistant Acinetobacter nosocomialis clinical isolate from Cape Town, South Africa. J. Glob. Antimicrob. Resist. 2021, 25, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.C.; Oliveira, M.S.; Perdigão-Neto, L.V.; Rocha, C.K.D.; Guimarães, T.; Rizek, C.; Levin, A.S.; Costa, S.F. Antimicrobial Combinations against Pan-Resistant Acinetobacter baumannii Isolates with Different Resistance Mechanisms. PLoS ONE 2016, 11, e0151270. [Google Scholar] [CrossRef] [PubMed]
- Mea, H.J.; Yong, P.V.C.; Wong, E.H. An overview of Acinetobacter baumannii pathogenesis: Motility, adherence and biofilm formation. Microbiol. Res. 2021, 247, 126722. [Google Scholar] [CrossRef]
- Wroblewska, M.M.; Sawicka-Grzelak, A.; Marchel, H.; Luczak, M.; Sivan, A. Biofilm production by clinical strains of Acinetobacter baumannii isolated from patients hospitalized in two tertiary care hospitals. FEMS Immunol. Med. Microbiol. 2008, 53, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A.M.; Alsultan, A.A.; Ansari, M.A.; Alnimr, A.M. Biofilm-Formation in Clonally Unrelated Multidrug-Resistant Acinetobacter baumannii Isolates. Pathogens 2020, 9, 630. [Google Scholar] [CrossRef]
- Šmitran, A.; Vuković, D.; Opavski, N.; Gajić, I.; Marinković, J.; Božić, L.; Živanović, I.; Kekić, D.; Popović, S.; Ranin, L. Influence of subinhibitory antibiotic concentration on Streptococcus pyogenes adherence and biofilm production. Acta Microbiol. Immunol. Hung. 2018, 65, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y. Molecular Characterization and Antimicrobial Susceptibility of Biofilm-forming Acinetobacter baumannii Clinical Isolates from Daejeon, Korea. Korean J. Clin. Lab. Sci. 2018, 50, 100–109. [Google Scholar] [CrossRef]
- Silva, A.M.C.M.D.; Costa Júnior, S.D.; Lima, J.L.C.; Farias Filho, J.L.B.; Cavalcanti, I.M.F.; Maciel, M.A.V. Investigation of the association of virulence genes and biofilm production with infection and bacterial colonization processes in multidrug-resistant Acinetobacter spp. An. Acad. Bras. Cienc. 2021, 93, e20210245. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.H.; Weber, B.S.; Feldman, M.F. Subinhibitory concentrations of trimethoprim and sulfamethoxazole prevent biofilm formation by Acinetobacter baumannii through inhibition of Csu pilus expression. Antimicrob. Agents Chemoth. 2017, 61, e00778-17. [Google Scholar] [CrossRef]
- Khoshnood, S.; Savari, M.; Abbasi Montazeri, E.; Farajzadeh Sheikh, A.; Khoshnood, S.; Savari, M.; Abbasi Montazeri, E.; Farajzadeh Sheikh, A. Survey on Genetic Diversity, Biofilm Formation, and Detection of Colistin Resistance Genes in Clinical Isolates of Acinetobacter baumannii. Infect.Drug. Resist. 2020, 13, 1547–1558. [Google Scholar] [CrossRef]
- Amin, M.; Navidifar, T.; Shooshtari, F.S.; Rashno, M.; Savari, M.; Jahangirmehr, F.; Arshadi, M. Association Between Biofilm Formation, Structure, and the Expression Levels of Genes Related to biofilm formation and Biofilm-Specific Resistance of Acinetobacter baumannii Strains Isolated from Burn Infection in Ahvaz, Iran. Infect. Drug. Resist. 2019, 12, 3867–3881. [Google Scholar] [CrossRef]
- Romero, M.; Mayer, C.; Heeb, S.; Wattanavaekin, K.; Cámara, M.; Otero, A.; Williams, P. Mushroom-shaped structures formed in Acinetobacter baumannii biofilms grown in a roller bioreactor are associated with quorum sensing–dependent Csu-pilus assembly. Environ. Microbiol. 2022, 24, 4329–4339. [Google Scholar] [CrossRef]
- Thummeepak, R.; Kongthai, P.; Leungtongkam, U.; Sitthisak, S. Distribution of virulence genes involved in biofilm formation in multi-drug resistant Acinetobacter baumannii clinical isolates. Int. Microbiol. 2016, 19, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Upmanyu, K.; Haq, Q.M.R.; Singh, R. Factors mediating Acinetobacter baumannii biofilm formation: Opportunities for developing therapeutics. Curr. Res. Microb. Sci. 2022, 3, 100131. [Google Scholar] [CrossRef]
- Zeighami, H.; Valadkhani, F.; Shapouri, R.; Samadi, E.; Haghi, F. Virulence characteristics of multidrug resistant biofilm forming Acinetobacter baumannii isolated from intensive care unit patients. BMC Infect. Dis. 2019, 19, 629. [Google Scholar] [CrossRef]
- Drugeon, B.; Pichon, M.; Marjanovic, N.; Mousse, S.; Seguin, S.; Raynaud, C.; Rahoui, A.; Frasca, D.; Mimoz, O.; Guenezan, J. Peripheral venous catheter colonization after skin disinfection with 0.5% aqueous sodium hypochlorite, preceded or not by one application of 70% ethanol (DACLEAN): A single-centre, randomized, open-label, pilot study. J. Hosp. Infect. 2022, 120, 123–126. [Google Scholar] [CrossRef]
- Bakht, M.; Alizadeh, S.A.; Rahimi, S.; Kazemzadeh Anari, R.; Rostamani, M.; Javadi, A.; Peymani, A.; Marashi, S.M.A.; Nikkhahi, F. Phenotype and genetic determination of resistance to common disinfectants among biofilm-producing and non-producing Pseudomonas aeruginosa strains from clinical specimens in Iran. BMC Microbiol. 2022, 22, 124. [Google Scholar] [CrossRef]
- Kampf, G.; Kramer, A. Epidemiologic Background of Hand Hygiene and Evaluation of the Most Important Agents for Scrubs and Rubs. Clin. Microbiol. Rev. 2004, 17, 863–893. [Google Scholar] [CrossRef]
- US EPA Office of Pesticide Programs, U.E. Pesticide Product Label, PDI SANI-CLOTH BLEACH WIPES. Available online: https://www3.epa.gov/pesticides/chem_search/ppls/009480-00008-20201222.pdf (accessed on 13 December 2022).
- Betchen, M.; Giovinco, H.M.; Curry, M.; Luu, J.; Fraimow, H.; Carabetta, V.J.; Nahra, R. Evaluating the Effectiveness of Hospital Antiseptics on Multidrug-Resistant Acinetobacter baumannii: Understanding the Relationship between Microbicide and Antibiotic Resistance. Antibiotics 2022, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Vickery, K.; Walker, J.T.; deLancey Pulcini, E.; Stoodley, P.; Goldenberg, S.D.; Salkeld, J.A.G.; Chewins, J.; Yezli, S.; Edgeworth, J.D. Surface-attached cells, biofilms and biocide susceptibility: Implications for hospital cleaning and disinfection. J. Hosp. Infect. 2015, 89, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, S.; Tahan, M.; Sadeghian, H.; Nazari, R.; Behmadi, M.; Hosseini Bafghi, M. Effect of biosynthesized selenium nanoparticles using Nepeta extract against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. J. Basic Microbiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Zaboli, F.; Fattahi, E.; Babavalian, H. Biosynthesis of Selenium Nanoparticles and Evaluation of Its Antibacterial Activity against Pseudomonas aeruginosa. Adv. Mater. Sci. Eng. 2022, 2022, 4118048. [Google Scholar] [CrossRef]
- Nair, M.S.; Upadhyay, A.; Fancher, S.; Upadhyaya, I.; Dey, S.; Kollanoor-Johny, A.; Zhao, J.; Venkitanarayanan, K. Inhibition and Inactivation of Escherichia coli O157:H7 Biofilms by Selenium. J. Food Prot. 2018, 81, 926–933. [Google Scholar] [CrossRef]
- Ramya, S.; Shanmugasundaram, T.; Balagurunathan, R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 2015, 32, 30–39. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Hachicho, N.; Hoffmann, P.; Ahlert, K.; Heipieper, H.J. Effect of silver nanoparticles and silver ions on growth and adaptive response mechanisms of Pseudomonas putida mt-2. FEMS Microbiol. Lett. 2014, 355, 71–77. [Google Scholar] [CrossRef]
- Tan, V.L.C.; Hinchman, A.; Williams, R.; Tran, P.A.; Fox, K. Nanostructured biomedical selenium at the biological interface (Review). Biointerphases 2018, 13, 06D301. [Google Scholar] [CrossRef]
- Zhang, R.; Carlsson, F.; Edman, M.; Hummelgård, M.; Jonsson, B.G.; Bylund, D.; Olin, H. Escherichia coli Bacteria Develop Adaptive Resistance to Antibacterial ZnO Nanoparticles. Adv. Biosyst. 2018, 2, 1800019. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef]
- Luo, T.L.; Rickard, A.H.; Srinivasan, U.; Kaye, K.S.; Foxman, B. Association of blaOXA-23 and bap with the persistence of Acinetobacter baumannii within a major healthcare system. Front. Microbiol. 2015, 12, 182. [Google Scholar] [CrossRef]
- Smani, Y.; Fàbrega, A.; Roca, I.; Sánchez-Encinales, V.; Vila, J.; Pachón, J. Role of OmpA in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014, 58, 1806–1808. [Google Scholar] [CrossRef] [PubMed]
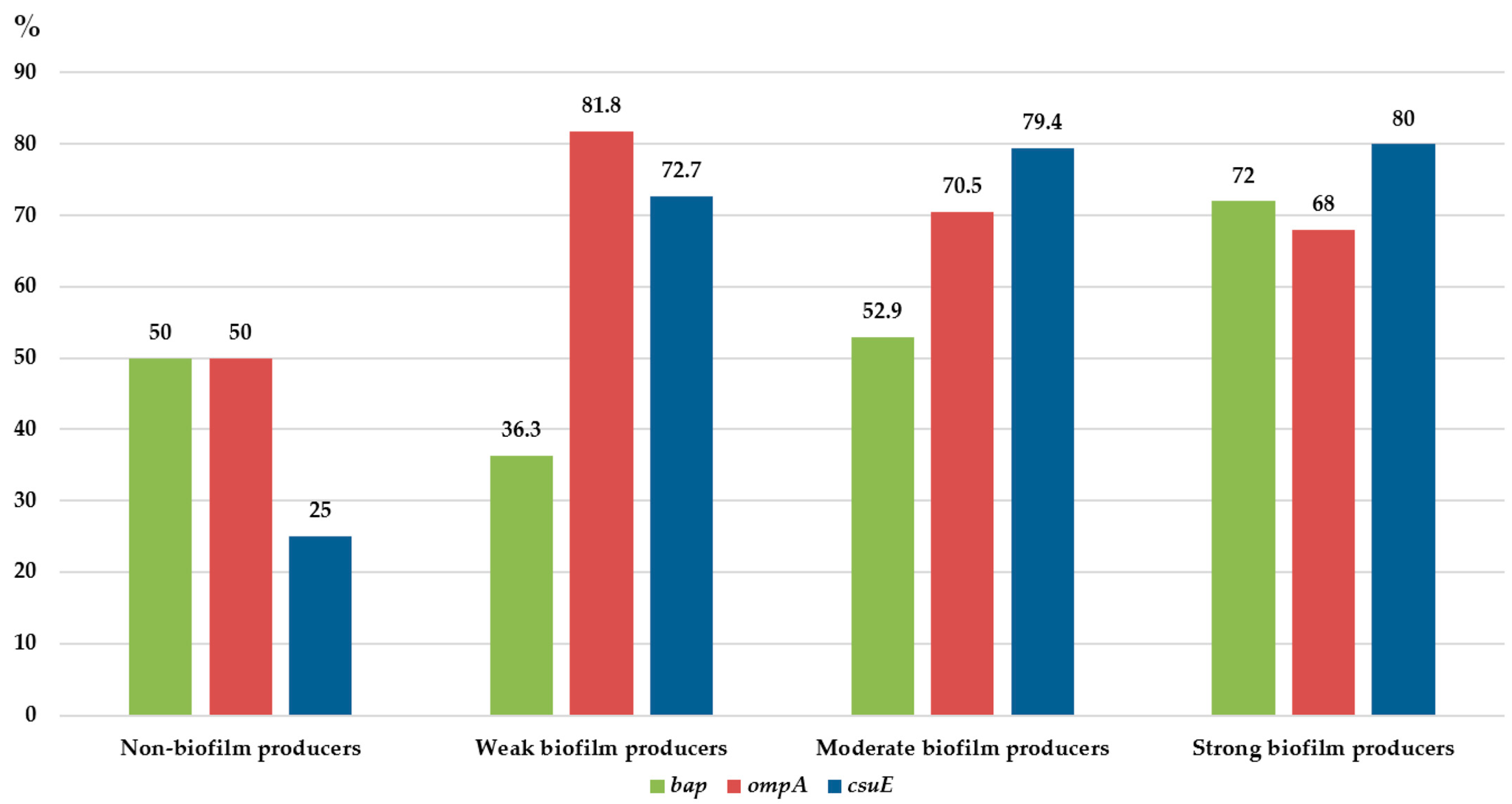
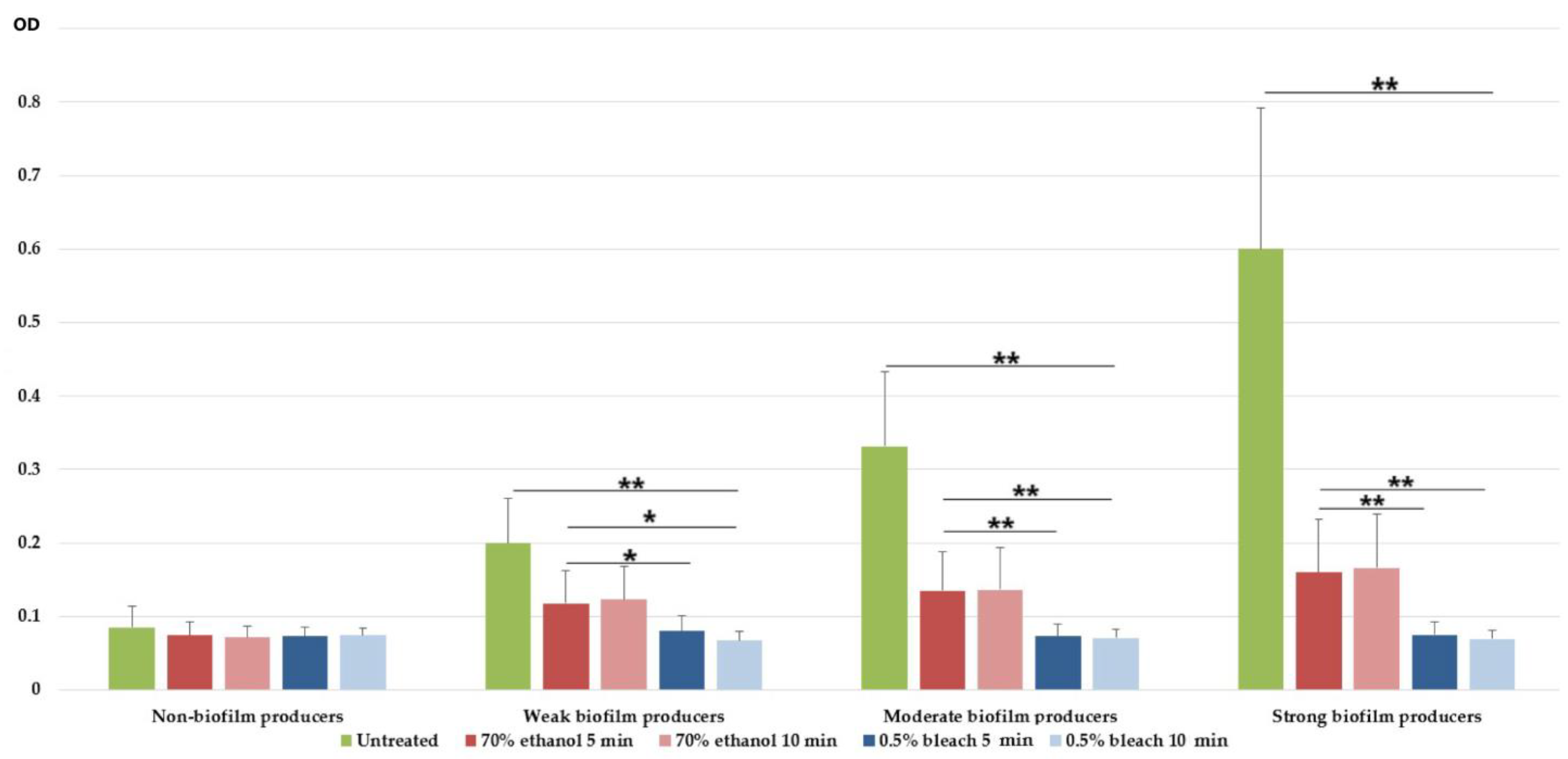

| Biofilm-Producing Capacity | Isolates N (%) | |||||
|---|---|---|---|---|---|---|
| Non-Invasive CRAB (n = 67) | Invasive CRAB (n = 44) | |||||
| Skin and Soft Tissue (n = 26) | Urine (n = 3) | Lower Respiratory Tract Samples (n = 38) | Peritoneal Fluid (n = 8) | CSF (n = 1) | Blood (n = 35) | |
| Non-producers | 4 (15.4%) | 0 (0%) | 5 (13.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Total | 9 (13.4%) | 0 (0%) | ||||
| Weak producers | 4 (15.4%) | 0 (0%) | 1 (2.6%) | 0 (0%) | 0 (0%) | 8 (22.9%) |
| Moderate producers | 7 (26.9%) | 2 (66.7%) | 22 (57.8%) | 0 (0%) | 0 (0%) | 20 (57.1%) |
| Strong producers | 11 (42.3%) | 1 (33.3%) | 10 (26.4%) | 8 (100%) | 1 (100%) | 7 (20%) |
| Total | 58 (86.6%) | 44 (100%) | ||||
| Non-Producers (n = 9) | Weak Producers (n = 13) | Moderate Producers (n = 51) | Strong Producers (n = 38) | |
|---|---|---|---|---|
| median MIC (mg/mL) [range] | 0.03 [0.01–1.25] | 0.03 [0.001–1.25] | 0.03 [0.01–0.15] | 0.07 [0.001–1.25] |
| median MBIC (mg/mL) [range] | 0.00 [0.00–0.30] | 0.03 [0.01–1.25] ** | 0.07 [0.01–0.60] ** | 0.15 [0.01–1.25] ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smitran, A.; Lukovic, B.; Bozic, L.; Jelic, D.; Jovicevic, M.; Kabic, J.; Kekic, D.; Ranin, J.; Opavski, N.; Gajic, I. Carbapenem-Resistant Acinetobacter baumannii: Biofilm-Associated Genes, Biofilm-Eradication Potential of Disinfectants, and Biofilm-Inhibitory Effects of Selenium Nanoparticles. Microorganisms 2023, 11, 171. https://doi.org/10.3390/microorganisms11010171
Smitran A, Lukovic B, Bozic L, Jelic D, Jovicevic M, Kabic J, Kekic D, Ranin J, Opavski N, Gajic I. Carbapenem-Resistant Acinetobacter baumannii: Biofilm-Associated Genes, Biofilm-Eradication Potential of Disinfectants, and Biofilm-Inhibitory Effects of Selenium Nanoparticles. Microorganisms. 2023; 11(1):171. https://doi.org/10.3390/microorganisms11010171
Chicago/Turabian StyleSmitran, Aleksandra, Bojana Lukovic, LJiljana Bozic, Dijana Jelic, Milos Jovicevic, Jovana Kabic, Dusan Kekic, Jovana Ranin, Natasa Opavski, and Ina Gajic. 2023. "Carbapenem-Resistant Acinetobacter baumannii: Biofilm-Associated Genes, Biofilm-Eradication Potential of Disinfectants, and Biofilm-Inhibitory Effects of Selenium Nanoparticles" Microorganisms 11, no. 1: 171. https://doi.org/10.3390/microorganisms11010171
APA StyleSmitran, A., Lukovic, B., Bozic, L., Jelic, D., Jovicevic, M., Kabic, J., Kekic, D., Ranin, J., Opavski, N., & Gajic, I. (2023). Carbapenem-Resistant Acinetobacter baumannii: Biofilm-Associated Genes, Biofilm-Eradication Potential of Disinfectants, and Biofilm-Inhibitory Effects of Selenium Nanoparticles. Microorganisms, 11(1), 171. https://doi.org/10.3390/microorganisms11010171






