Gluten Degradation by the Gut Microbiota of Ulcerative Colitis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Assessment of Gluten Intake in Study Participants:
2.3. Quantification of Fecal Gluten Content
2.4. Isolation of Gluten Degrading Bacteria
2.5. Identification of Gluten-Degrading Microorganisms
2.6. Epithelial Cell Assessment of Bacterial Products of Gluten Degradation
2.7. Statistical Analysis
3. Results
3.1. The Gut of UC Patients Retains Gluten Degrading Capability
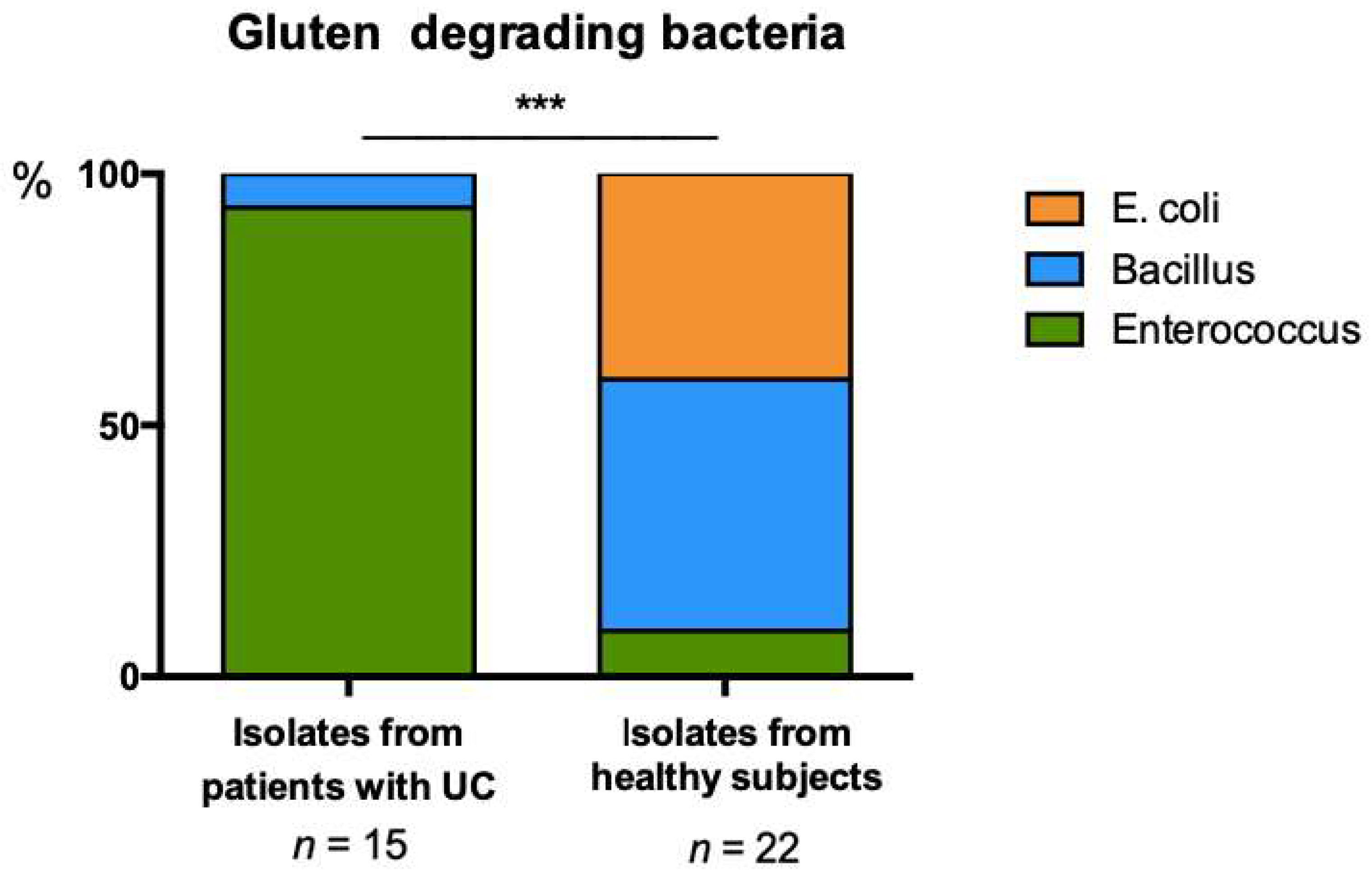
3.2. Distinct Profiles of Gluten Degrading Bacteria Are Evident in UC and Healthy Feces
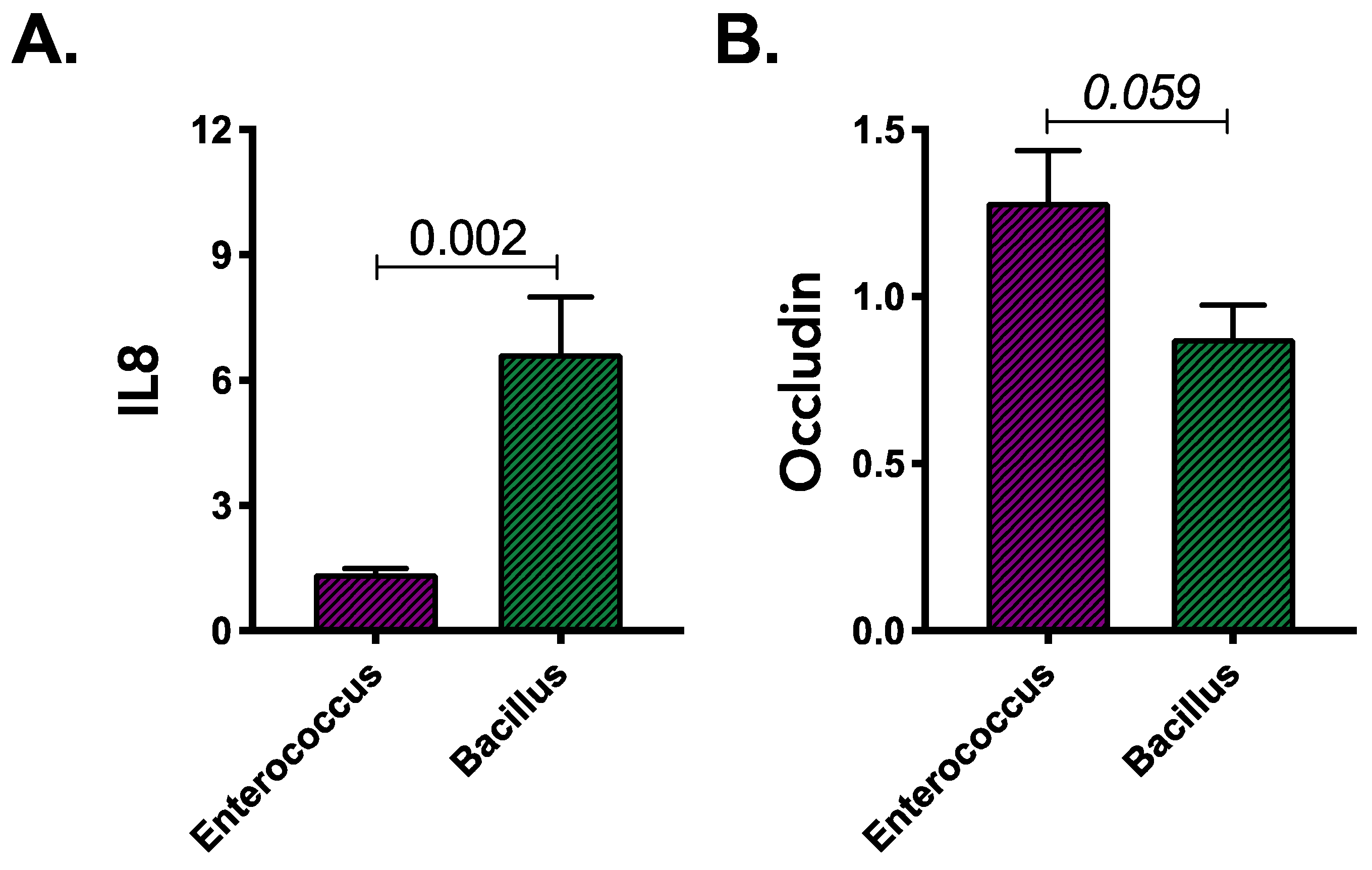
3.3. Products of Gluten Degradation by Enterococcus and Bacillus Isolates Induce Distinct Immune Response in Colonocytes Irrespective of Their Source
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Evolving strategies and goals of treatment in ulcerative colitis. Best Pr. Res. Clin. Gastroenterol. 2018, 32–33, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Sykora, J.; Pomahacova, R.; Kreslova, M.; Cvalinova, D.; Stych, P.; Schwarz, J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 2741–2763. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.; Chan, F.K.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [Green Version]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [Green Version]
- Mar, J.S.; LaMere, B.J.; Lin, D.L.; Levan, S.; Nazareth, M.; Mahadevan, U.; Lynch, S.V. Disease Severity and Immune Activity Relate to Distinct Interkingdom Gut Microbiome States in Ethnically Distinct Ulcerative Colitis Patients. MBio 2016, 7, e01072-16. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Dong, Y.; Huang, W.; Zhu, D.; Mao, H.; Su, P. Fecal Microbiota Transplantation for Ulcerative Colitis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0157259. [Google Scholar] [CrossRef] [Green Version]
- Heinken, A.; Hertel, J.; Thiele, I. Metabolic modelling reveals broad changes in gut microbial metabolism in inflammatory bowel disease patients with dysbiosis. NPJ Syst. Biol. Appl. 2021, 7, 19. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Lapointe, T.K.; Buret, A.G. Interleukin-18 facilitates neutrophil transmigration via myosin light chain kinase-dependent disruption of occludin, without altering epithelial permeability. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G343–G351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barmeyer, C.; Harren, M.; Schmitz, H.; Heinzel-Pleines, U.; Mankertz, J.; Seidler, U.; Horak, I.; Wiedenmann, B.; Fromm, M.; Schulzke, J.D. Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G244–G252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, A.; Knapp, M.; Lang, I.; Köhler, G. Interleukin 8 (IL-8)—A universal biomarker? Int. Arch. Med. 2010, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myles, I.A. Fast food fever: Reviewing the impacts of the Western diet on immunity. Nutr. J. 2014, 13, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valitutti, F.; Fasano, A. Breaking Down Barriers: How Understanding Celiac Disease Pathogenesis Informed the Development of Novel Treatments. Dig. Dis. Sci. 2019, 64, 1748758. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Sarantopoulos, C.; Ongchangco, D.; Sry, J.; Cesario, T. Rapid isolation of gluten-digesting bacteria from human stool and saliva by using gliadin-containing plates. Exp. Biol. Med. 2015, 240, 917–924. [Google Scholar] [CrossRef] [Green Version]
- Siegel, M.; Bethune, M.T.; Gass, J.; Ehren, J.; Xia, J.; Johannsen, A.; Stuge, T.B.; Gray, G.M.; Lee, P.P.; Khosla, C. Rational design of combination enzyme therapy for celiac sprue. Chem. Biol. 2006, 13, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Caminero, A.; Herrán, A.R.; Nistal, E.; Pérez-Andrés, J.; Vaquero, L.; Vivas, S.; Ruiz de Morales, J.M.; Albillos, S.M.; Casqueiro, J. Diversity of the cultivable human gut microbiome involved in gluten metabolism: Isolation of microorganisms with potential interest for coeliac disease. FEMS Microbiol. Ecol. 2014, 88, 309–319. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Rheumatoid arthritis-celiac disease relationship: Joints get that gut feeling. Autoimmun. Rev. 2015, 14, 1038–1047. [Google Scholar] [CrossRef]
- Kolchak, N.A.; Tetarnikova, M.K.; Theodoropoulou, M.S.; Michalopoulou, A.P.; Theodoropoulos, D.S. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J. Multidiscip. Healthc. 2018, 11, 13–19. [Google Scholar] [CrossRef]
- Aziz, I.; Branchi, F.; Pearson, K.; Priest, J.; Sanders, D.S. A study evaluating the bidirectional relationship between inflammatory bowel disease and self-reported non-celiac gluten sensitivity. Inflamm. Bowel Dis. 2015, 21, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Herfarth, H.H.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D.; Long, M.D. Prevalence of a gluten-free diet and improvement of clinical symptoms in patients with inflammatory bowel diseases. Inflamm. Bowel Dis. 2014, 20, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Bosca-Watts, M.M.; Minguez, M.; Planelles, D.; Navarro, S.; Rodriguez, A.; Santiago, J.; Tosca, J.; Mora, F. HLA-DQ: Celiac disease vs. inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 96–103. [Google Scholar] [CrossRef] [PubMed]
- DiGiacomo, D.; Santonicola, A.; Zingone, F.; Troncone, E.; Caria, M.C.; Borgheresi, P.; Parrilli, G.; Ciacci, C. Human leukocyte antigen DQ2/8 prevalence in non-celiac patients with gastrointestinal diseases. World J. Gastroenterol. 2013, 19, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Pascual, V.; Dieli-Crimi, R.; Lopez-Palacios, N.; Bodas, A.; Medrano, L.M.; Nunez, C. Inflammatory bowel disease and celiac disease: Overlaps and differences. World J. Gastroenterol. 2014, 20, 4846–4856. [Google Scholar] [CrossRef]
- Di Tola, M.; Sabbatella, L.; Anania, M.C.; Viscido, A.; Caprilli, R.; Pica, R.; Paoluzi, P.; Picarelli, A. Anti-tissue transglutaminase antibodies in inflammatory bowel disease: New evidence. Clin. Chem. Lab. Med. 2004, 42, 1092–1097. [Google Scholar]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Roesbuck, K.A. Regulation of Interleukin-8 Gene Expression. J. Interferon Cytokine Res. 1999, 19, 429–438. [Google Scholar] [CrossRef]
- Feldman, G.J.; Mullin, J.M.; Ryan, M.P. Occludin: Structure, function and regulation. Adv. Drug Deliv. Rev. 2005, 57, 883–917. [Google Scholar] [CrossRef]
- Herrán, A.R.; Pérez-Andrés, J.; Caminero, A.; Nistal, E.; Vivas, S.; de Morales, J.M.; Casqueiro, J. Gluten-degrading bacteria are present in the human small intestine of healthy volunteers and celiac patients. Res. Microbiol. 2017, 168, 673–684. [Google Scholar] [CrossRef]
- Rashmi, B.S.; Gayathri, D. Molecular characterization of gluten hydrolysing Bacillus sp. and their efficacy and biotherapeutic potential as probiotics using Caco-2 cell line. J. Appl. Microbiol. 2017, 123, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Cong, Y. Bacillus coagulans and its applications in medicine. Benef. Microbes 2019, 10, 679–688. [Google Scholar] [CrossRef]
- Jensen, G.S.; Cash, H.A.; Farmer, S.; Keller, D. Inactivated probiotic Bacillus coagulans GBI-30 induces complex immune activating, anti-inflammatory, and regenerative markers in vitro. J. Inflamm. Res. 2017, 10, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steck, N.; Hoffmann, M.; Sava, I.G.; Kim, S.C.; Hahne, H.; Tonkonogy, S.L.; Mair, K.; Krueger, D.; Pruteanu, M.; Shanahan, F.; et al. Enterococcus faecalis Metalloprotease Compromises Epithelial Barrier and Contributes to Intestinal Inflammation. Gastroenterology 2011, 141, 959–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balish, E.; Warner, T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am. J. Pathol. 2002, 160, 2253–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wang, W.; Zhou, R.; Ng, S.C.; Li, J.; Huang, M.; Zhou, F.; Wang, X.; Shen, B.; Kamm, M.A.; et al. Characteristics of fecal and mucosa-associated microbiota in Chinese patients with inflammatory bowel disease. Medicine 2014, 93, e51. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harringer, E.O.S.; Durack, J.; Piceno, Y.; Andersen, V.; Lynch, S.V. Gluten Degradation by the Gut Microbiota of Ulcerative Colitis Patients. Microorganisms 2023, 11, 12. https://doi.org/10.3390/microorganisms11010012
Harringer EOS, Durack J, Piceno Y, Andersen V, Lynch SV. Gluten Degradation by the Gut Microbiota of Ulcerative Colitis Patients. Microorganisms. 2023; 11(1):12. https://doi.org/10.3390/microorganisms11010012
Chicago/Turabian StyleHarringer, Emma Olivia Schultz, Juliana Durack, Yvette Piceno, Vibeke Andersen, and Susan V. Lynch. 2023. "Gluten Degradation by the Gut Microbiota of Ulcerative Colitis Patients" Microorganisms 11, no. 1: 12. https://doi.org/10.3390/microorganisms11010012
APA StyleHarringer, E. O. S., Durack, J., Piceno, Y., Andersen, V., & Lynch, S. V. (2023). Gluten Degradation by the Gut Microbiota of Ulcerative Colitis Patients. Microorganisms, 11(1), 12. https://doi.org/10.3390/microorganisms11010012






