Abstract
Maintaining the homeostasis balance of trace elements is crucial for the health of organisms. Human health is threatened by diseases caused by a lack of trace elements. Saccharomyces cerevisiae has a wide and close relationship with human daily life and industrial applications. It can not only be used as fermentation products and single-cell proteins, but also as a trace elements supplement that is widely used in food, feed, and medicine. Trace-element-enriched yeast, viz., chromium-, iron-, zinc-, and selenium-enriched yeast, as an impactful microelements supplement, is more efficient, more environmentally friendly, and safer than its inorganic and organic counterparts. Over the last few decades, genetic engineering has been developing large-scaled genetic re-design and reconstruction in yeast. It is hoped that engineered yeast will include a higher concentration of trace elements. In this review, we compare the common supplement forms of several key trace elements. The mechanisms of detoxification and transport of trace elements in yeast are also reviewed thoroughly. Moreover, genes involved in the transport and detoxification of trace elements are summarized. A feasible way of metabolic engineering transformation of S. cerevisiae to produce trace-element-enriched yeast is examined. In addition, the economy, safety, and environmental protection of the engineered yeast are explored, and the future research direction of yeast enriched in trace elements is discussed.
1. Introduction to Trace Elements
Micronutrients play an important role in maintaining the health of living things. The regulation of micronutrients on body metabolism is an important research topic for nutrition science and also an important biological research area. An expert committee of the World Health Organization (WHO) classifies the essential trace elements into three categories [1] which include essential trace elements: iodine (I), iron (Fe), zinc (Zn), selenium (Se), copper (Cu), molybdenum (Mo), chromium (Cr), and cobalt (Co). The second class includes manganese (Mn), silicon (Si), nickel (Ni), boron (B), and vanadium (V). The third class is the trace elements which are potentially toxic but may have necessary functions for the human body at low doses, including fluorine (F), lead (Pb), cadmium (Cd), mercury (Hg), arsenic (As), aluminum (Al), lithium (Li), and tin (Sn).
These trace elements play significant roles in biological systems, which are involved in gene regulation, nucleic acid metabolism, anti-inflammatory and antioxidant functions, and other special physiological functions. Different trace elements play different physiological functions in the body. Chromium functions in maintaining the normal metabolism of glucose, lipid, and protein in the body. It can maintain the dynamic balance of blood glucose and improves levels of blood sugar and lipid to a certain extent [2,3]. Chromium is also an important active component of glucose tolerance factor (GTF), which increases the sensitivity of tissue receptors towards insulin [4]. GTF can regulate the metabolism of biological macromolecules, deposition of muscle tissue, and utilization of cholesterol via increasing the affinity between the tissue receptors and insulin [5]. Meanwhile, chromium is also considered to be a promising anti-heat stress element that can reduce the use of antibiotics because of its strong antioxidant effect, which prevents reactive oxygen species (ROS) from degrading lipid film structures, resulting in lipid peroxidation and cell damage [6].
A grain-based diet generally causes iron deficiency. Iron deficiency leads to anemia because iron is a component of hemoglobin (a blood protein) [7]. Iron is involved in transporting oxygen through red blood cells, so iron deficiency may exacerbate chronic inflammation [8]. Iron also participates in many essential biochemical processes, such as the synthesis of deoxyribonucleotides, amino acids, lipids, and sterols. As a component of cytochrome (CYP) enzymes, iron participates in the electron transport chain and is pivotal to oxidative phosphorylation and redox reactions involved in the respiratory chain [9].
The element zinc contributes to cell growth and apoptosis. Zinc is abundant in the brains of mammals, and insufficient intake of zinc can affect intellectual development and immunity among children [10]. Zinc is also important for the central nervous system [11]. Zinc deficiency activates inflammatory responses which trigger ROS-induced oxidative stress [12]. Copper stimulates the growth of livestock and poultry, so it is often added to animal feed [13]. The molybdenum cofactor, which is required for purine metabolism and sulfite detoxification, is synthesized with molybdenum [14].
Selenium is a component of selenocysteine and an essential component of selenoproteins and selenases such as glutathione peroxidase (GPx), phospholipid hydrogen glutathione peroxidase (PHGPx) and thioredoxin reductase (TrxR) [15]. Approximately 10 additional selenoproteins have been identified. Selenium in the form of selenocysteine is the active center of many antioxidant enzymes in the body. Selenium is related to human antioxidant activity and anti-inflammatory and anti-virus properties [16]. Selenium deficiency can induce or increase skeletal muscle and myocardial necrosis, immune function decline, and tumor diseases [17].These micronutrients are important for health, but excessive consumption is toxic [18]. A high level of metal trace elements may cause inflammation in the body, induce an oxidative stress response, and may interfere with the absorption of other trace elements [12]. A large amount of selenium will cause acute toxicity, leading to diarrhea, vomiting, and other symptoms. Serious overdoses can cause heart problems or even death [19]. Continuous intake of selenium slightly higher than the toxic dose may cause chronic poisoning. It will cause skin rash, hair loss, etc., and also have a negative impact on the digestive system and nervous system [20]. Specific, adequate, and standard intakes of essential micronutrients are required to minimize the risk of nutrient deficiency or their excess. At the same time, the toxicity of metal trace elements in different valence states is different. Additionally, there is antagonism between metals, i.e., the increase in the absorption of one metal could result in the decrease of the absorption of another metal. Therefore, an appropriate trace element supplement is very important to maintain the balance of trace elements in the body [21].
2. Trace Element Supplement Form
Humans are not able to meet the body’s nutritional demand for trace elements from food alone, which leads to the steady state of trace elements in the human body often tending to be insufficient. For example, the estimated safe and adequate daily dietary intake (ESADDI) of human chromium is 50–200 μg [22]. It is hard to obtain this amount through daily meals. Moreover, about 1 billion people in the world have insufficient selenium intake, covering both developed and developing countries [23]. If trace elements are consumed in excessively low amounts, they can adversely affect various organs, including the immune system, the nervous system, and the metabolic system. Micronutrient deficiencies cause severe diseases such as iron deficiency anemia, night blindness, and even cancer [24,25]. Micronutrients are also found to be deficient in varying degrees at different ages in the body, and specifically, children have higher micronutrient requirements in comparison to adults [26]. At present, more than 2 billion people suffer from micronutrient deficiency [27]. It can be seen that it is far from enough to supplement trace elements merely from food. It is not surprising that mineral element deficiency is a common problem in both developing and underdeveloped nations.
2.1. Inorganic Compounds
There are currently mainly two types of chelating supplements available on the market: inorganic and organic. Trace elements in inorganic form are mainly inorganic salts whose production methods are simple. They are easy to produce on a large scale, but the human body usually has a slow digestion and utilization rate of inorganic salt. The inorganic salt forms of many trace elements do not possess biological activity, so they need to be further transformed into biologically active chemical forms by various physiological and biochemical processes. In the meantime, inorganic trace elements are usually toxic, and their improper use may cause severe adverse effects. For example, inorganic forms of chromium supplements include chromium chloride, chromium picolinate, and other forms of trivalent chromium [28]. Trivalent chromium, though, is less toxic than other forms of chromium. The only adverse effects found in humans with improper consumption of chromium salts in very high doses have been linked to liver and kidney health [29]. This is due to the difficulty in absorbing and utilizing inorganic chromium in the gastrointestinal tract of mammals. Indigestible inorganic chromium can accumulate in the kidneys and liver. It was found that the chromium content in the kidney after consuming inorganic chromium for 24 h was 10 times that of taking inorganic chromium for 1 h, and it was 5 times that of taking inorganic chromium in the liver. This situation can last up to six months [30]. Parenteral supplementation of zinc is usually made with zinc chloride or zinc sulfate. Similar to inorganic chromium, inorganic zinc is also not conducive to absorption by the human body, and it exhibits the same characteristics as inorganic chromium of low absorption efficiency. In addition, inorganic zinc is unstable and tends to deteriorate during production, transportation, and storage. Inorganic forms of selenium include selenate (Na2SeO4) and sodium selenite (Na2SeO3) [31], but inorganic selenium may cause genotoxicity in the human body [17]. Due to the low digestibility of inorganic forms of trace elements, to meet the human body’s demand, it is necessary to increase the dose, which not only aggravates the toxicity and side effects on human health, but also causes an environmental burden. For example, about 80% of the supplemented inorganic copper is discharged through feces, adding pollution to the natural ecosystem [32].
2.2. Organic Chelates
The organic form of trace elements is usually salts of organic acids or organic chelates. Compared with inorganic forms, it is less toxic and better utilized by organisms. However, due to the different organic forms, it is impossible to obtain multiple organic trace elements at the same time. The safety of synthetic organic compounds is also controversial. As food and feed additives, iron chelates (ferrous diglycine) are less sensitive to iron absorption inhibitors than iron salts. Zinc supplements can be taken orally in the form of salts of organic acids, such as zinc gluconate, zinc acetate, or zinc propionate. The complementary form of organic copper is mainly the complex of copper in combination with gluconic acid. Organic chromium as a trace element supplement includes phenylalanine chromium, nicotinate chromium, GTF-like chromium pyridinate, and so on [33]. Organic chromium has better performance both in terms of rate of absorption and bioactivity. Chromium picolinate, in particular, is one of the most popular forms of chromium supplements and has been marketed as a promoter of fat loss, muscle gain, and treatment for metabolic disorders including type II diabetes by reducing blood glucose levels on the premise of increasing insulin [34]. Nevertheless, organic chromium, especially chromium picolinate, has been reported to be harmful to humans and may cause cancer [35]. Organic chromium compounds are indeed more easily absorbed than inorganic chromium compounds, but even so, the absorption rate is seen to be <1% [36]. The organic supplementary forms of selenium include selenomethionine (SeMet) and selenocysteine (SeCys) [37]. Selenium exists in humans in the form of SeMet. As a result, SeMet is easier to be absorbed by the body than the inorganic form [38]. In summary, inorganic compounds generally are more toxic and indigestible than organic ones.
2.3. Yeast Cells Enriched in Trace Elements
S. cerevisiae is generally recognized as a safe (GRAS) microbe. Humans have used yeast to make fermented products for thousands of years. Nowadays, as a common food additive and nutritional supplement, yeast has a closer relationship with human life. The biomass of S. cerevisiae is especially rich in protein and contains eight essential amino acids and is expected to become an important source of protein in the future [39]. The protein content of its biomass can reach to 54% [40]. Because of its rich protein content, it can better enrich iron, zinc, chromium, selenium and other trace elements. Yeast biomass is used as a feed or food supplement. Because of its high bioavailability, it is mainly used as a source of micronutrient supplements.
As compared to the microelement preparations being synthesized by a chemical method, the microelements from yeast have higher biological activities and can be absorbed easily in the body. Moreover, the fermentation process of yeast is a simple and short operation. This makes yeast an ideal carrier for the production of metal trace element preparations. At present, industrial trace-element-enriched yeast is mainly produced by adding mineral salts of trace elements to the fermentation medium to complete the enrichment of trace elements in the metabolic process of S. cerevisiae. Screening and mutagenesis of yeast with high enrichment [41] and optimization of fermentation conditions [42] are the traditional ways to improve the enrichment of trace elements in yeast. The selection of inorganic salts of trace elements in the culture medium possibly impacts the enrichment of S. cerevisiae. For example, trivalent chromium shows quite low toxicity in several valence states of chromium [43]. Choosing chromium trichloride as a substrate might reduce the detoxification burden of S. cerevisiae.
In recent years, microorganisms, especially yeast, have been utilized as carriers for trace elements to obtain organic trace elements that are easier to digest and absorb with enhanced biological activity. They are also inexpensive to manufacture. In addition to the low cost, trace-element-enriched yeast can effectively replace other inorganic and organic trace element nutritional supplements. Moreover, yeast can also be used for soil treatment, water purification, and in many other fields. Based on the above advantages and application prospects in multiple fields, yeast as a source of trace elements has attracted intensive attention from the research and market point of view.
2.3.1. Chromium Yeast
The active form of chromium in yeast is GTF, which is formed by the coordination of trivalent chromium ions with amino acids. Yeast containing chromium is considered an ideal microelement supplement [44]. A study by Krol et al. [45] found that compared with chromium chloride, the addition of chromium-enriched yeast during chicken feeding could reduce the content of crude fat and dry matter in chicken chest and leg muscles. Another study carried out by Liu et al. [28] compared the bioactivity of different chromium-based compounds by using insulin-resistant 3T3-L1 adipocytes and showed that GTF can improve glucose metabolism much more efficiently than other forms of chromium such as chromium pyridinate or chromium trichloride.
At present, chromium-enriched yeast is mainly produced by yeast fermentation in the presence of chromium chloride [46]. Previous studies were mainly focused on optimizing fermentation conditions for improving chromium content and yield of chromium-enriched yeast. Ali et al. [41] carried out fermentation research on wild beer yeast, using a compound medium in batch-fed fermentation and sodium chromate as the chromium source. After 50 h of fermentation when the sodium chromate supplemental level was 7.1 g/L, the process yielded about 4.2 g/100 mL of chrome-enriched yeast biomass with a total chromium content of 3113 μg/g, where the organic chromium content was 795 μg/g.
2.3.2. Zinc Yeast
Zinc-enriched yeast is a kind of common organic zinc source which is an ideal zinc additive. Zinc-enriched yeast can organically combine zinc with proteins and polysaccharide yeast through the absorption and transformation of zinc during yeast growth, thus eliminating toxic side effects and gastrointestinal irritation caused by inorganic zinc and organic zinc in the human body and making it possible for zinc to be absorbed and utilized by the human body more efficiently and safely. It is superior to other types of zinc in digestion and absorption as well as physiological transformation efficiency. Maares et al. [47] showed that zinc-enriched yeast is a promising nutritional supplement by comparing it with other zinc preparations such as zinc sulfate and zinc gluconate. Fan et al. [48] used siderophore isolated from an iron-rich environment to produce zinc–copper enriched yeast, and finally obtained S. cerevisiae with intracellular organic Cu and Zn contents of 60.76 and 44.22 mg/g, respectively.
2.3.3. Iron Yeast
Iron supplements are usually associated with certain side effects and risks. They may cause gastrointestinal problems such as vomiting in up to 40% of patients [49]. In addition to their low absorption, these preparations are also associated with a metallic taste. Iron-enriched yeast can provide a better form of iron supplementation by being stable in form, absorbing easily, producing foods in coordination with other ingredients, and also having a good flavor. In a study conducted by Sabatier et al. [50], it was found that iron-enriched yeast exists in the form of a low molecular weight organic complex through HPLC-ICP-MS preliminary analysis. Nowosad et al. [51] used iron-enriched yeast to yield 100 g dry weight flatbread containing almost 385.8 ± 4.12 mg iron. Furthermore, this product had no metallic taste and can be used as a daily iron supplement. The study of Raguzzi et al. [52] showed that the yeast cells can store iron in two forms: one is stored in the form of cytoplasmic molecules similar to ferritin and the second is in vacuoles by binding iron with polyphosphates. By using interspecific protoplast technology and fermentation under optimal conditions, Yuan et al. [53] obtained iron-enriched yeast containing Fe at a concentration of 25 mg per gram of stem cells.
2.3.4. Selenium Yeast
Selenium yeast is a successful case of S. cerevisiae enriching trace elements. Selenium-enriched yeast is widely used not only as a selenium nutritional supplement, but also in the food field to produce selenium-enriched milk, selenium-enriched chicken, and so on. Organisms can biotransform inorganic selenium and combine selenium with amino acids, proteins, polysaccharides and other substances to convert it into organic selenium. Selenium (SeMet, etc.) transformed by S. cerevisiae can be better absorbed by the human body [17]. At the same time, it shows less toxicity [54]. However, the research not only focuses on the selenium enrichment of yeast, but also derives a new form of selenium supplement (nano-selenium). Compared with selenium compounds, nano-selenium produced by S. cerevisiae has better biocompatibility, lower cytotoxicity, and better biological activity than inorganic selenium and organic selenium [55]. The production of nano-selenium depends on the reduction of glutathione in S. cerevisiae [56]. The selenium content of selenium-enriched yeast can reach 5.64 mg per gram [57]. Khoei et al. [58] treated sodium selenite with S. cerevisiae to rapidly reduce to nano-selenium. The analysis showed that the size of nano-selenium was 100–300 nm. In addition to common yeasts enriched in chromium, iron, zinc, and selenium, there is little chance that the human body will become deficient in other metal trace elements, and daily diets can meet the body’s nutritional requirements. Therefore, yeast nutritional supplement products with other metal trace elements are rare. Additionally, copper yeast and molybdenum yeast have been studied as yeast nutritional supplements. By screening high tolerance strains and optimizing the composition of the culture medium and fermentation conditions, Guo et al. [59] made it possible to increase the copper ion absorption rate of S. cerevisiae to 90%. In comparison with organic copper salt, copper-enriched yeast demonstrated a higher utilization rate [60].
3. Mechanism of Trace Element Transport in Yeast
The heavy metal binding in yeast can be achieved in two different ways: bio-adsorption and bio-enrichment. Bio-adsorption is a metabolically passive physiochemical process. Through electrostatic force, chemical bond, and other physical and chemical forces, metal and yeast extracellular polymers are combined, so that the metal particles adsorb to the surface of microorganisms. The anionic groups on the outer surface of yeast cells provide binding sites for positively charged heavy metals. Metal cations can bind to microbial surface components [61]. The composition of yeast cell walls determines its ability to adsorb metals. Chitin and glucan-mannoprotein complex are the main active substances in yeast adsorption [62].
Bio-enrichment is a metabolically dependent process that occurs in yeast cells. A number of basic metal transport components, including membrane transport proteins, organelle storage systems, and chelating agent molecules, ensure metal uptake and their storage by yeast. Heavy metals can be transported into yeast cells, which depend on the existence of phospholipid bilayers. In the outer phospholipid layer, heavy metals pass through pore proteins. Then they enter yeast cells through channels, secondary carriers, or primary active transporters. Numerous channels and transporters located on the cell membrane have specificity and can specifically recognize and transport different heavy metals [63]. It is an important way for yeast to transport heavy metals. Once inside the intracellular space, heavy metals can be bound by proteins and peptide ligands (glutathione, metallothionein, phytochelatin, etc.) to limit cytotoxicity and isolate the binding within the cell, thereby eliminating metals from sensitive metabolic functions [64,65]. To achieve metal enrichment, some metal cations bind to functional groups on the cell wall and are then internalized into the cell [66].
Yeast enriches metal trace elements by using both common and specific pathways. Several genes are involved in both pathways, including those involved in metal transporters, glutathione synthesis-related enzymes, and genes related to the synthesis of other metal-binding proteins. There are some genes that regulate the transport and enrichment of many metals, while others are only related to a single/specific metal. With current genetic engineering technologies, yeast can now be engineered on all levels, from specific proteins to complex metabolic pathways.
3.1. Chromium and Selenium Transport
Yeast transports chromium in two ways, one by diffusion or phagocytosis [67], which transports chromium ions directly into cells. The other method involves the transport of chromium metal via transporters located on cell membranes. Studies have shown that the transporter transports chromium that adsorbed on the cell wall into yeast cells [68]. Two high-affinity sulfate transporters, Sul1 and Sul2, exist in S. cerevisiae, and their expression is thought to be regulated by at least two transcriptional activators, MET4 and MSN1 [69]. This observation suggests that chromates enter the cells through the sulfate assimilation pathway [70].
The uptake of selenium by cells is mainly through the transport system of sulfate and phosphate. Selenite resistance is closely related to the expression of high affinity orthophosphate vector pho84p [71]. The molecular structure and spatial arrangement are similar for sulfate (SO42−), chromate (CrO42−), and selenate(SeO42−) ions [72]. Therefore, Sul1 and Sul2 can also act as selenate transport channels.
3.2. Iron Transport
Yeast cells absorb iron mainly through enzymes and proteins on their cell membranes. The cellular systems involved in iron uptake and utilization are precisely regulated according to iron availability and the cellular requirement of iron. Iron depletion induces the expression of a family of cell wallproteins known as Fit1p, Fit2p, and Fit3p [73]. The yeast cells have evolved two iron transport systems to uptake iron at different concentrations. This is performed without causing iron toxicity in the yeast cells nor causing deficiency. Furthermore, yeast cells have evolved two iron transport mechanisms with a low-affinity [74] and a high-affinity system [75], which coordinate with the identification and absorption of iron.
These systems are responsible for iron transport in iron-sufficient and iron-deficient cells, respectively. Based on the analysis of yeast mutants, some genes related to iron transport have been cloned by specific mutations and effective screening. For example, plasma membrane metal reductases encoded by FRE1 and FRE2 genes can reduce ferric (Fe3+) iron [76]. Fe2+ transporter genes FTR1 and FET4 are located on the plasma membrane of yeast [77]. In S. cerevisiae, transcription factors Aft1/Aft2 and Yap5 regulate the metabolism of iron in response to low and high iron levels, respectively [78]. Lucia et al. [79] express constitutively active Aft1 alleles to increase the accumulation of iron in S. cerevisiae.
3.3. Zinc Transport
Similar to the transport of iron through yeast, concentration-dependent analysis of zinc uptake by yeast cells showed that there were at least two uptake systems which are involved: a high-affinity system in zinc-deficient cells [80] and a low-affinity transport system mainly expressed in zinc-abundant cells [81]. Zrt1 is a zinc transporter in the high-affinity transport system. When zinc is depleted in the cells, due to their high-affinity transport system and specificity for zinc, they can improve the absorption of zinc through absorption mediated by yeast [82]. The gene responsible for low-affinity zinc transport is Zrt2 [81]. Zap1 is a major regulator of zinc deficiency in S. cerevisiae, which regulates the expression of Zrt1 and Zrt2 [83]. Zinc is transported in vacuoles by two zinc transporters, Zrt3 and Zrc1. The transport of zinc is also regulated by the transcription factor Zap1 [84]. The mitochondrial carrier gene MTM1 can maintain zinc homeostasis via regulating Zap1, Zrt1, and Zrc1 expression [85].
4. Detoxification Mechanism of Trace Elements in Yeast
Trace elements become toxic when the concentration of trace elements exceeds the tolerance threshold of yeast cells, and the toxicity is specific. The toxic microelements destroy plasma membrane, bind in a non-specific manner with biomolecules, and interfere with the homeostasis of base metals by competing with their normal transport and buffering systems, resulting in toxic effects which impede yeast growth and metabolism [86]. The toxicity of microelements in cells can cause oxidative stress [87], base damage [88], and altered DNA repair [89], as well as inhibiting enzyme function and interfering with proliferation [90]. Additionally, the cell cycle process is also affected [91], and apoptosis or differentiation of protein function is also impaired [92]. In response to microelements toxicity, yeast cells block cell cycle progression, altering gene expression and metabolism, and tweaking transport processes to protect cell and gene integrity [93]. We summarize essentiality and toxicity values of trace elements in the human body (Table 1).

Table 1.
Summary of essentiality and toxicity data for metal trace elements.
In the case of metallic chromium, tetravalent chromium is considered the most toxic form. Compared to the tetravalent form, the toxicity of trivalent chromium is very low, and the rapid reduction of tetravalent chromium by intracellular reducing agents (such as ascorbic acid, cysteine, glutathione) to trivalent chromium produces reactive oxygen species (ROS) and chromium in the pentavalent and hexavalent states [94]. ROS combines with proteins, hydrocarbons, and nucleic acids to attack and destroy macromolecules in cells, resulting in protein oxidation, lipid peroxidation, and DNA damage, especially base oxidation and single-chain breakage [93]. Long-term exposure to chromium in its hexavalent state is associated with an increased risk of lung cancer. Throughout the long evolutionary process, organisms must strictly maintain the homeostasis and balance of intracellular metal trace elements in order to adapt to the changing environment.
4.1. Mechanism of Glutathione Detoxification
Glutathione is the most abundant non-protein thiol tripeptide, which mainly exists in eukaryotic cells. In cells, glutathione exists mainly in either reduced (GSH) or oxidized (GSSG) form/state [95]. Moreover, glutathione is a crucial redox buffer, reducing the adverse effects of oxidative stress, protecting the mitochondrial macromolecules from the harmful effects of ROS [96], and participating in DNA repair [97].
In S. cerevisiae, the enzymes (glutamylcysteine synthase (GSH1) and glutathione synthase (GSH2)) catalyze the continuous glutathione synthesis from three precursor amino acids molecules, viz., glutamate, cysteine, and glycine [98,99]. The thiol group (-SH) in glutathione binds to heavy metals so that heavy metals cannot disrupt cellular metabolic activities [100]. Glutathione is a protein containing cysteine, and cysteine residues can bind metals to overcome the toxicity caused by metals [101]. For selenium, glutathione can not only bind to selenium through cysteine residues, but also is closely related to the generation of nano-selenium. In S. cerevisiae, tetravalent selenium ions are easily spontaneously reacted with reduced glutathione to form selenide glutathione (GS-Se-SG) and GSSG [102]. Among them, selenide glutathione was converted to glutathione [103]. Glutathione is catalyzed by superoxide dismutase to convert into elemental selenium (Se0), This pathway is widely believed to be the main way for S. cerevisiae to reduce selenite to form biological elemental nano-selenium [104].
Thorsen et al. [105] studied the mechanism of arsenic resistance in S. cerevisiae and observed that the glutathione biosynthesis pathway was induced by exposure of yeast to arsenic. They also identified the core transcriptional regulators as Yap1p and Met4p, which regulate glutathione synthesis. Lafaye et al. [106] proved that the high production of glutathione in yeast was essential for the detoxification of heavy metals such as cadmium.
4.2. Vacuolar Metal Transporter
Yeast and other organisms detoxify heavy metals by chelating the metal with a ligand. There are heavy metal transporters on the vacuole, which transport the metal–protein bound complexes to the vacuole in order to block heavy metals and prevent them from damaging the normal physiological function of the cell. Metal ions enter the organelles such as vacuoles, mitochondria, endoplasmic reticulum, or Golgi apparatus through highly active membrane transporters that isolate metal ion chelates and prevent them from exerting toxic effects [107]. The CCC1 transport system for iron and manganese ions affects the accumulation of iron and manganese ions in vacuoles. It involves the transfer of iron from the cytosol to the vacuole. The overexpression of CCC1 leads to decreased iron content in the cytosol and increased iron content in vacuoles. In contrast, the loss of CCC1 leads to a decrease in iron content and iron storage in vacuoles, thereby affecting the level of iron concentration in the cytosol and cell growth [108]. Yeast cadmium factor 1 (Ycf1) sequesters heavy metals and glutathione into the vacuole to combat cell stress. Ycf1 performs this protective function when conjugated with toxic heavy metals such as cadmium, mercury, or lead into vacuoles [109]. In addition, YCF1 oxidizes glutathione to maintain cellular redox homeostasis and reduce metal-induced cell damage.
4.3. Metallothionein and Phytochelin
In addition to glutathione, certain other proteins also chelate with heavy metals for detoxification purposes, including small metal-binding peptides known as phytochelatins (c-Glu–Cys)nGly) and metallothioneins (small cysteine-rich proteins). These metal-chelating proteins neutralize the toxic effect of free metal ions by binding with them inside the cells.
Phytochelatins (PCs) and the vacuolar membrane transporter (SpHMT1) play an important role in the tolerance of heavy metal stress in clonal yeast. PCs are synthesized from glutathione in yeast cells through plant chelate peptide synthase. PCs, as ligands of intracellular heavy metals, form a low molecular weight complex in the cytoplasm, which can enter vacuoles through SpHMT1 and further form stable (phytochelatins–membrane transporter) PC-metal high molecular weight complexes. This sequential process is considered to be the main mode of vacuolar compartmentalization of heavy metals and the main mechanism for heavy metal tolerance among higher plants and fungi [110]. In S. cerevisiae, only PC2 ((γ-glutamylcysteinyl)2-glycine) can be synthesized by vacuolar serine carboxypeptidase CPC and CPY, which transport the high molecular weight complex to the vacuoles mainly through vacuolar membrane protein YCF1 to achieve tolerance to heavy metals. In a study by Matthias et al. [94], glutathione was found to affect zinc homeostasis, with an increased phytochelatins concentration being related to lower free zinc levels in vacuoles, indicating that phytochelatins are important for zinc buffering in S. cerevisiae.
Metallothioneins are a kind of metal-binding protein characterized by more cystine residues and low molecular weight. Metallothionein is a universal conjugate against metal cytotoxicity, which prevents the free metal ions from destroying the cells through chelation [111]. It has the capacity to buffer intracellular metal. The amino and carboxyl groups of metallothionein can bind to metal cations [101]. In addition, the high binding capacity of metallothionein is that it is rich in thiol groups. The thiol group has higher binding ability to metal than the amino group and the carboxyl group [112]. CUP1 is a metallothionein found in S. cerevisiae, which has been shown to protect yeast cells against oxidants [113]. Ruta et al. [114] have successfully carried out the heterogenically expression of the metallothionein gene from Arabidopsis thaliana and the metallothionein gene of Thlaspi Caerulescens in S. cerevisiae cells, which increased the resistance of S. cerevisiae towards Cu2+, Zn2+, Cd2+, and other heavy metals. In vitro and in vivo studies have shown that low initial doses of cadmium are protective against subsequent high doses of cadmium. This is because cadmium induces the synthesis and storage of metallothioneins in the liver and kidneys [115].
In general, the detoxification mechanisms of yeast for heavy metals include expression of related genes (GSH1 and GSH2, etc.) and proteins (glutathione, etc.) to alleviate oxidative stress [116], transport of heavy metals out of the cell (PCA1, etc.) or into the vacuole by related transporters (YCF1 and BPT1, etc.) [117], and bind metallothionein [118] and phytochelatins [119] with heavy metal ions to reduce their toxicity and adverse effects (Figure 1). The following table summarizes the metal detoxification mechanism and related genes (Table 2).
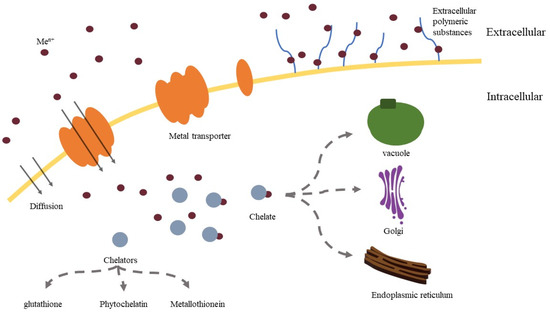
Figure 1.
Model for transport and detoxification mechanism of metal trace elements by yeast.

Table 2.
Resistance mechanisms of yeast towards heavy metal ions.
5. Five Yeasts Enriched with Trace Elements Obtained by Metabolic Engineering
Recently, there have been a number of studies focused on the enrichment of heavy metals in yeast, including optimization of the fermentation process and culture medium system, mutation, and other methods to obtain yeast tolerant to heavy metals, but still, the metal enrichment of yeast has not been greatly improved. As an example, consider chromium-enriched yeast. Yeast enriches chromium by about 1 g/kg, and the highest amount only reaches 4 g/kg [41,124,125]. Several chromium-enriched yeasts only adsorb chromium on the surface of their cells or, more simply, a mixture of yeast and chromium. Until inorganic chromium is converted into active chromium in order to supplement the chromium source, the utilization rate is not improved. The low concentration of metal trace elements in yeast causes a lot of wastewaters containing heavy metals to be produced during yeast production, which is not friendly to the environment. Therefore, many studies have been dedicated to improving the metal enrichment of yeast. Metabolic modification is a possibility, in addition to traditional methods, which is expected to greatly improve metal enrichment. Previous studies on genes related to metal enrichment in yeast have found that the transport and detoxification mechanisms of yeast are intimately related to metal enrichment. Zhao et al. [126] found that 108 yeast single-gene deletion mutants were found to be sensitive to ZnCl2 through genome-wide screening, of which 64 mutants had higher intracellular zinc content than the wild type when the concentration of zinc in the medium is high. Berrak et al. [127] obtained yeast mutants that were resistant to metals such as Fe, Zn, Cr, and Co through a reverse metabolic engineering strategy by chemical mutation and iron stress application and found that the expression of phosphate transporter gene PHO84 and iron transporter FTR1 in mutants was down-regulated, and the expression of related genes which are dealing with oxidative stress was found to be up-regulated.
The metal transport and detoxification mechanisms of yeast have been described in detail above. Considering their close relationship and the enrichment of metal trace elements, strategies for metabolic engineering of yeast may also include overexpression of membrane transporter activity, overexpression of metal-isolating organelle transporters (e.g., vacuolar transporters), deletion of export transporter genes, and enhancement of detoxification pathways to enhance metal tolerance by yeast [122].
Overexpression of the membrane transport can enhance the transport of microelement ions into yeast cells. More trace elements enter yeast cells and stimulate them to produce more glutathione and other proteins. Activated metal protein chelates are formed when these proteins combine with metal trace elements [128]. In order to prevent the excess of metal trace elements and metal toxicity in yeast cells, it is extremely crucial to enhance the detoxification pathway of yeast and the synthesis of metal-chelating high-affinity ligands, such as glutathione pathway, metallothionein, and PC as mentioned above [63,93]. Metal chelators not only alleviate the cytotoxicity caused by excessive metal but also chelate with metal and reduce the adverse effects caused by them. Finally, the organelle transporters on the surface of vacuoles, Golgi apparatus, and endoplasmic reticulum membrane were overexpressed in order to transport metals and protein chelates into organelles for isolation [108].To improve gene expression, not only can key metabolic pathway genes be overexpressed, but multiple copies of genes can also be constructed. In yeast, multicopy expression of genes may increase the transport of metal trace elements, although it is also possible that yeast with a high copy number of genes has low metal enrichment capability.
Many studies have carried out metabolic engineering on transporters (Table 3). Overexpression of the sulphate osmotic enzymes Sul1 and Sul2 shows a more than five-fold increase in metal absorption of chromate [122]. The overexpression of ZRT1 and ZRT2 transporters greatly improved the transport capacity of Zn by about 10-fold. On the other hand, overexpression of CTR1 and CTR3 greatly promoted copper transport by about 10-fold [122]. SMF1 has a wide range of metal specificity and is often optimized and engineered to enhance the absorption of metals including Fe and Ni [128]. Express hyperactive Aft1 alleles Aft1-1up lead to iron cells accumulating up to four-fold more endogenous iron than Aft1-expressing cells [79]. The co-expression of cell membrane transporter (SMF1) and CCC1 increased the uptake of Mn and Cd more than 10-fold [122].

Table 3.
Metabolic engineering to enrich trace elements by S. cerevisiae.
Enhancing the detoxification mechanism of glutathione is an important method of increasing the metal tolerance in yeast. Many studies have explored the glutathione pathway and discussed the methods to effectively enhance this pathway. Perrone et al. [132] reported that a number of genes (PEP12, UBP6, etc.) are involved in the production of glutathione. Previously, Hara et al. [103] cultivated an engineered strain of S. cerevisiae whose glutathione biosynthesis was boosted by overexpression of MET16, which showed a content of intracellular glutathione 1.5 times higher than that of the parent strain. Many studies have been reported where maximizing glutathione production was discussed. These mainly involved two promising approaches: increasing the biomass concentration of glutathione producing yeast and increasing the intracellular glutathione content [98]. Aside from chelating metal itself, glutathione can also produce metallothionein and phytochelatin to further chelate metal detoxification. Although the content of proteins related to detoxification such as glutathione can be increased through metabolic engineering, people rarely use their enhancement to achieve higher trace element enrichment in S. cerevisiae. The detoxification metabolism of many microelements is similar, and some transporters have the same recognition and transport functions for multiple microelement ions or microelement ions of the same valence state. S. cerevisiae is a model eukaryote. The technical tools (CRISPR/Cas9 [133], etc.) and expression cassette elements (promoters [134] and terminators, etc., [135]) necessary for metabolic engineering transformation have been fully reported and studied, and the metabolic engineering transformation of S. cerevisiae has been successfully carried out. In industry, engineered S. cerevisiae has been successfully applied in the production of terpenes [136] and fatty acid derivatives [137]. As a result, we can rearrange yeast-related genes or integrate foreign genes through metabolic engineering. As a result, a more efficient production strategy for trace element nutritional supplements might be applied with reduced production period and pollution costs.
6. Conclusions and Future Perspectives
A lack of trace elements can lead to a variety of health problems. Trace elements, such as chromium, are difficult to ingest from food, so nutritional supplements are needed to assist ingestion. More and more metal trace element nutritional supplements are needed to improve the deficiency of metal trace elements in the diet. The advantages of yeast-enriched trace elements include high efficiency, green, stability, and extensive nutrition, thus making them ideal for supplementing trace elements in humans.
Combined with the previous description of microelements transport and detoxification mechanism, we have reason to believe that S. cerevisiae can be engineered to hyper accumulate metals efficiently by overexpression of certain transporter genes and evolving native metal transporters and engineering mechanisms for metal detoxification. Engineered yeasts have more applications beyond nutritional supplements because of their enhanced ability to enrich metals. Yeast is also a common raw material for making bread, beer, and many other foods. To make food rich in trace elements, yeast rich in trace elements can be substituted for ordinary yeast. Meanwhile, the metallic taste of the micronutrient supplement can also be improved by the flavor of the food. Furthermore, adding microelement-enriched yeast to feed can improve the immunity of poultry and livestock, reducing the use of antibiotics. Similarly, metabolized yeast can be applied to soil or wastewater contaminated by heavy metals.
Nevertheless, an accumulation of trace metal elements among consumers from metabolized yeast still poses food safety issues. Since metal-enriched yeast food supplements are not authorized, more information is required for their bioavailability. Furthermore, what the metal active substances in the yeast enriched with metal trace elements are and whether these substances can be isolated from yeast to discover a new method to detoxify the yeast remain unclear. As an alternative to the overexpression of import transporters, the elimination of export transporters can also improve metal retention in yeast. However, at present, the export transport mechanism of yeast is not well understood, and research is scarce. This may be a new research direction and a means to further enhance metal enrichment in yeast. Metal and protein chelate are transported by a transporter on the organelle membrane. It is possible that this transporter is localized in the inner membrane of the cell for expression so that yeast can reverse transport active trace elements throughout the body, which may help solve the current safety risk for yeast as a nutritional supplement.
At the same time, whether new forms of trace element supplements, such as nano-chromium and nano-iron and the evaluation of their safety and the comparison of their absorption and utilization efficiency can be obtained, will become the future exploration direction.
As a result of the low enrichment of metal trace elements by yeast, even the yeast with the highest enrichment at present still has a low metal conversion rate in the culture medium, and it requires a certain high concentration environment to achieve such enrichment. The result will be a large amount of waste raw materials and waste liquids containing heavy metals. In addition to the above strategy, yeast can also be used to improve the utilization of raw metal. Metals with low-affinity transport pathways can absorb metals in environments with low metal concentrations. In the future, the low-affinity transport pathway of yeast can be metabolically modified to make yeast achieve high enrichment and high conversion rates at low metal concentrations. In addition to saving raw materials, this method can be more environmentally friendly.
Author Contributions
Conceptualization, J.S.; investigation, S.X.; resources, Y.J. and H.W.; writing—original draft preparation, S.X.; writing—review and editing, Y.D. and K.Y.; visualization, S.X.; supervision, W.Y.; project administration, W.Y.; funding acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Zhejiang Provincial Natural Science Foundation of China (Grant Nos. LY19C010005) and National Natural Science Foundation of China (Grant Nos. 31900497).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Squitti, R.; Negrouk, V.; Perera, M.; Llabre, M.M.; Ricordi, C.; Rongioletti, M.C.A.; Mendez, A.J. Serum copper profile in patients with type 1 diabetes in comparison to other metals. J. Trace Elem. Med. Biol. 2019, 56, 156–161. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Toward practical prevention of type 2 diabetes. Med. Hypotheses 2000, 54, 786–793. [Google Scholar] [CrossRef]
- Via, M.; Scurlock, C.; Raikhelkar, J.; Di Luozzo, G.; Mechanick, J.I. Chromium Infusion Reverses Extreme Insulin Resistance in a Cardiothoracic ICU Patient. Nutr. Clin. Pract. 2008, 23, 325–328. [Google Scholar] [CrossRef]
- Anderson, R.A. Chromium in the prevention and control of diabetes. Diabetes Metab. 2000, 26, 22–27. [Google Scholar] [PubMed]
- Moeini, M.M.; Bahrami, A.; Ghazi, S.; Targhibi, M.R. The effect of different levels of organic and inorganic chromium supplementation on production performance, carcass traits and some blood parameters of broiler chicken under heat stress condition. Biol. Trace Elem. Res. 2011, 144, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Bin-Jumah, M.; Abd El-Hack, M.E.; Abdelnour, S.A.; Hendy, Y.A.; Ghanem, H.A.; Alsafy, S.A.; Khafaga, A.F.; Noreldin, A.E.; Shaheen, H.; Samak, D.; et al. Potential use of chromium to combat thermal stress in animals: A review. Sci. Total Environ. 2020, 707, 135996. [Google Scholar] [CrossRef] [PubMed]
- Shubham, K.; Anukiruthika, T.; Dutta, S.; Kashyap, A.V.; Moses, J.A.; Anandharamakrishnan, C. Iron deficiency anemia: A comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends Food Sci. Technol. 2020, 99, 58–75. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.P.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martinez-Pastor, M. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Brown, K.H.; Peerson, J.M.; Rivera, J.; Allen, L.H. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2002, 75, 1062–1071. [Google Scholar] [CrossRef]
- Marger, L.; Schubert, C.R.; Bertrand, D. Zinc: An underappreciated modulatory factor of brain function. Biochem. Pharmacol. 2014, 91, 426–435. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simano, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Vadalasetty, K.P.; Lukasiewicz, M.; Jaworski, S.; Wierzbicki, M.; Chwalibog, A.; Sawosz, E. Effect of different levels of copper nanoparticles and copper sulphate on performance, metabolism and blood biochemical profiles in broiler chicken. J. Anim. Physiol. Anim. Nutr. 2018, 102, E364–E373. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Y.; Liu, H.; Zhu, Y.-F.; Pinson, S.R.M.; Lin, H.-X.; Guerinot, M.L.; Zhao, F.-J.; Salt, D.E. Natural variation in a molybdate transporter controls grain molybdenum concentration in rice. New Phytol. 2019, 221, 1983–1997. [Google Scholar] [CrossRef]
- Yadav, A.; Kon, K.; Kratosova, G.; Duran, N.; Ingle, A.P.; Rai, M. Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: Progress and key aspects of research. Biotechnol. Lett. 2015, 37, 2099–2120. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Salitre, L.; Roman-Gutierrez, A.; Contreras-Lopez, E.; Bautista-Avila, M.; Rodriguez-Serrano, G.; Gonzalez-Olivares, L. Promising Use of Selenized Yeast to Develop New Enriched Food: Human Health Implications. Food Rev. Int. 2021, 1–18. [Google Scholar] [CrossRef]
- Cannas, D.; Loi, E.; Serra, M.; Firinu, D.; Valera, P.; Zavattari, P. Relevance of Essential Trace Elements in Nutrition and Drinking Water for Human Health and Autoimmune Disease Risk. Nutrients 2020, 12, 2074. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Acute human toxicity and mortality after selenium ingestion: A review. J. Trace Elem. Med. Biol. 2020, 58, 126435. [Google Scholar] [CrossRef]
- Bocquet, A.; Barouki, R.; Briend, A.; Chouraqui, J.P.; Darmaun, D.; Feillet, F.; Frelut, M.L.; Guimber, D.; Lapillonne, A.; Peretti, N.; et al. Potential toxicity of metal trace elements from food in children. Arch. Pediatr. 2021, 28, 173–177. [Google Scholar] [CrossRef]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Cheng, N.Z.; Bryden, N.A.; Polansky, M.M.; Cheng, N.P.; Chi, J.M.; Feng, J.G. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes 1997, 46, 1786–1791. [Google Scholar] [CrossRef]
- Zhang, L.H.; Chu, C.C. Selenium Uptake, Transport, Metabolism, Reutilization, and Biofortification in Rice. Rice 2022, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mamtani, R.; Stern, P.; Dawood, I.; Cheema, S. Metals and disease: A global primary health care perspective. J. Toxicol. 2011, 2011, 319136. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Abente, G.; Locutura-Ruperez, J.; Fernandez-Navarro, P.; Martin-Mendez, I.; Bel-Lan, A.; Nunez, O. Compositional analysis of topsoil metals and its associations with cancer mortality using spatial misaligned data. Environ. Geochem. Health 2018, 40, 283–294. [Google Scholar] [CrossRef]
- Zemrani, B.; Bines, J.E. Recent insights into trace element deficiencies: Causes, recognition and correction. Curr. Opin. Gastroenterol. 2020, 36, 110–117. [Google Scholar] [CrossRef]
- Amoroso, L. The Second International Conference on Nutrition: Implications for Hidden Hunger. In Hidden Hunger: Malnutrition and the First 1000 Days of Life: Causes, Consequences and Solutions; World Review of Nutrition and Dietetics; Biesalski, H.K., Black, R.E., Eds.; Karger Medical and Scientific Publishers: Basel, Switzerland, 2016; Volume 115, pp. 142–152. [Google Scholar]
- Liu, L.; Cui, W.M.; Zhang, S.W.; Kong, F.H.; Pedersen, M.A.; Wen, Y.; Lv, J.P. Effect of glucose tolerance factor (GTF) from high chromium yeast on glucose metabolism in insulin-resistant 3T3-L1 adipocytes. RSC Adv. 2015, 5, 3482–3490. [Google Scholar] [CrossRef]
- Loubieres, Y.; de Lassence, A.; Bernier, M.; Vieillard-Baron, A.; Schmitt, J.M.; Page, B.; Jardin, F. Acute, fatal, oral chromic acid poisoning. J. Toxicol. -Clin. Toxicol. 1999, 37, 333–336. [Google Scholar] [CrossRef]
- Anderson, R.A.; Bryden, N.A.; Polansky, M.M.; Gautschi, K. Dietary chromium effects on tissue chromium concentrations and chromium absorption in rats. J. Trace Elem. Exp. Med. 1996, 9, 11–25. [Google Scholar] [CrossRef]
- Kieliszek, M.; Blazejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef]
- Cao, H.; Su, R.; Hu, G.; Li, C.; Guo, J.; Pan, J.; Tang, Z. In vivo effects of high dietary copper levels on hepatocellular mitochondrial respiration and electron transport chain enzymes in broilers. Br. Poult. Sci. 2016, 57, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Hao, J.; Wang, W.; Han, Z.; Li, G.; Zhang, L.; Zhao, X.; Yu, G. Insulin Sensitizing Effects of Oligomannuronate-Chromium (III) Complexes in C2C12 Skeletal Muscle Cells. PLoS ONE 2011, 6, e24598. [Google Scholar] [CrossRef] [PubMed]
- McAdory, D.; Rhodes, N.R.; Briggins, F.; Bailey, M.M.; Di Bona, K.R.; Goodwin, C.; Vincent, J.B.; Rasco, J.F. Potential of Chromium(III) Picolinate for Reproductive or Developmental Toxicity Following Exposure of Male CD-1 Mice Prior to Mating. Biol. Trace Elem. Res. 2011, 143, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. Recent developments in the biochemistry of chromium(III). Biol. Trace Elem. Res. 2004, 99, 1–16. [Google Scholar] [CrossRef]
- Costa, M.; Murphy, A. Overview of Chromium(III) Toxicology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 341–359. [Google Scholar]
- Kieliszek, M.; Bano, I. Selenium as an Important Factor in Various Disease States—A Review. Excli J. 2022, 21, 948–966. [Google Scholar] [CrossRef]
- Hu, Z.; Cheng, Y.; Suzuki, N.; Guo, X.; Xiong, H.; Ogra, Y. Speciation of Selenium in Brown Rice Fertilized with Selenite and Effects of Selenium Fertilization on Rice Proteins. Int. J. Mol. Sci. 2018, 19, 3494. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Lapena, D.; Kosa, G.; Hansen, L.D.; Mydland, L.T.; Passoth, V.; Horn, S.J.; Eijsink, V.G.H. Production and characterization of yeasts grown on media composed of spruce-derived sugars and protein hydrolysates from chicken by-products. Microb. Cell Factories 2020, 19, 19. [Google Scholar] [CrossRef]
- Demirci, A.; Pometto, A.L. Enhanced organically bound chromium yeast production. J. Agric. Food Chem. 2000, 48, 531–536. [Google Scholar] [CrossRef]
- Batic, M.; Raspor, P. Effect of cultivation mode on a bioprocess for chromium yeast biomass enrichment. Pflug. Arch. -Eur. J. Physiol. 2000, 439, R73–R75. [Google Scholar] [CrossRef]
- Goldhaber, S.B. Trace element risk assessment: Essentiality vs. toxicity. Regul. Toxicol. Pharmacol. 2003, 38, 232–242. [Google Scholar] [CrossRef]
- Tompkins, T.A.; Renard, N.E.; Kiuchi, A. Clinical evaluation of the bioavailability of zinc-enriched yeast and zinc gluconate in healthy volunteers. Biol. Trace Elem. Res. 2007, 120, 28–35. [Google Scholar] [CrossRef]
- Krol, B.; Slupczynska, M.; Kinal, S.; Bodarski, R.; Tronina, W.; Monka, M. Bioavailability of Organic and Inorganic Sources of Chromium in Broiler Chicken Feeds. J. Elem. 2017, 22, 283–294. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of chromium-enriched biomass of Yarrowia lipolytica as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2020, 18, e06005. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Keil, C.; Pallasdies, L.; Schmacht, M.; Senz, M.; Nissen, J.; Kieserling, H.; Drusch, S.; Haase, H. Zinc availability from zinc-enriched yeast studied with an in vitro digestion/Caco-2 cell culture model. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2022, 71, 126934. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-Y.; Liu, Z.-Y.; Jia, Z.-P.; Wei, Y.-R.; Xie, D.-D.; Zhang, J.; Wang, B.; Zhang, X.-G. A novel preparation for siderophore-assisted copper and zinc enrichment in yeast. J. Food Process. Preserv. 2021, e16131. [Google Scholar] [CrossRef]
- von Haehling, S.; Ebner, N.; Evertz, R.; Ponikowski, P.; Anker, S.D. Iron Deficiency in Heart Failure An Overview. JACC-Heart Fail. 2019, 7, 36–46. [Google Scholar] [CrossRef]
- Sabatier, M.; Egli, I.; Hurrell, R.; Hoppler, M.; Gysler, C.; Georgeon, S.; Mukherje, R.; Richon, P.-A.; Vigo, M.; Foman, J.T.; et al. Iron bioavailability from fresh cheese fortified with iron-enriched yeast. Eur. J. Nutr. 2017, 56, 1551–1560. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M. The Use of Iron-Enriched Yeast for the Production of Flatbread. Molecules 2021, 26, 5204. [Google Scholar] [CrossRef]
- Raguzzi, F.; Lesuisse, E.; Crichton, R.R. Iron storage in Saccharomyces cerevisiae. FEBS Lett. 1988, 231, 253–258. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Guo, X.N.; He, X.P.; Zhang, B.R.; Liu, S.G. Construction of a high-biomass, iron-enriched yeast strain and study on distribution of iron in the cells of Saccharomyces cerevisiae. Biotechnol. Lett. 2004, 26, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Karunasinghe, N.; Han, D.Y.; Zhu, S.T.; Duan, H.; Ko, Y.J.; Yu, J.F.; Triggs, C.M.; Ferguson, L.R. Effects of Supplementation with Selenium, as Selenized Yeast, in a Healthy Male Population from New Zealand. Nutr. Cancer -Int. J. 2013, 65, 355–366. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Xu, T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with Se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Mix, H.; Carlson, B.A.; Grabowski, P.J.; Gladyshev, V.N.; Berry, M.J.; Hatfield, D.L. Evidence for direct roles of two additional factors, SECp43 and soluble liver antigen, in the selenoprotein synthesis machinery. J. Biol. Chem. 2005, 280, 41568–41575. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Blazejak, S.; Placzek, M. Spectrophotometric evaluation of selenium binding by Saccharomyces cerevisiae ATCC MYA-2200 and Candida utilis ATCC 9950 yeast. J. Trace Elem. Med. Biol. 2016, 35, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Khoei, N.S.; Lampis, S.; Zonaro, E.; Yrjala, K.; Bernardi, P.; Vallini, G. Insights into selenite reduction and biogenesis of elemental selenium nanoparticles by two environmental isolates of Burkholderia fungorum. New Biotechnol. 2017, 34, 1–11. [Google Scholar] [CrossRef]
- Guo, X.-N.; He, X.-X.; Zhang, L.-B.; Cheng, Y.-F.; Bai, X.-M.; Wang, Z.-Y.; He, X.-P. Enhancement of Copper Uptake of Yeast Through Systematic Optimization of Medium and the Cultivation Process of Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2022, 94, 1857–1870. [Google Scholar] [CrossRef]
- Vinson, J.A.; Tompkins, T.A.; Agbor, G.A. Comparative bioavailability of mineral-enriched gluconates and yeast in rat liver after depletion-repletion feeding. Biol. Trace Elem. Res. 2007, 118, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the Art for the Biosorption Process-a Review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef]
- Mameeva, O.G.; Podgorsky, V.S. Cr (VI) ion uptake by the yeast S. cerevisiae UCM Y-1968 and its protoplasts. In Proceedings of the 18th International Biohydrometallurgy Symposium, Bariloche, Argentina, 13–17 September 2009; pp. 593–596. [Google Scholar]
- Diep, P.; Mahadevan, R.; Yakunin, A.F. Heavy Metal Removal by Bioaccumulation Using Genetically Engineered Microorganisms. Front. Bioeng. Biotechnol. 2018, 6, 157. [Google Scholar] [CrossRef]
- Li, Z.S.; Szczypka, M.; Lu, Y.P.; Thiele, D.J.; Rea, P.A. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J. Biol. Chem. 1996, 271, 6509–6517. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshanee, M.; Das, S. Biosorption and removal of toxic heavy metals by metal tolerating bacteria for bioremediation of metal contamination: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 104686. [Google Scholar] [CrossRef]
- Stout, M.D.; Nyska, A.; Collins, B.J.; Witt, K.L.; Kissling, G.E.; Malarkey, D.E.; Hooth, M.J. Chronic toxicity and carcinogenicity studies of chromium picolinate monohydrate administered in feed to F344/N rats and B6C3F1 mice for 2 years. Food Chem. Toxicol. 2009, 47, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Pas, M.; Milacic, R.; Draslar, K.; Pollak, N.; Raspor, P. Uptake of chromium(III) and chromium(VI) compounds in the yeast cell structure. Biometals 2004, 17, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Pinson, B.; Merle, M.; Franconi, J.M.; Daignan-Fornier, B. Low affinity orthophosphate carriers regulate PHO gene expression independently of internal orthophosphate concentration in Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 35273–35280. [Google Scholar] [CrossRef]
- Pereira, Y.; Lagniel, G.; Godat, E.; Baudouin-Cornu, P.; Junot, C.; Labarre, J. Chromate Causes Sulfur Starvation in Yeast. Toxicol. Sci. 2008, 106, 400–412. [Google Scholar] [CrossRef]
- Protchenko, O.; Ferea, T.; Rashford, J.; Tiedeman, J.; Brown, P.O.; Botstein, D.; Philpott, C.C. Three cell wall mannoproteins facilitate the uptake of iron in Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 49244–49250. [Google Scholar] [CrossRef]
- Dix, D.R.; Bridgham, J.T.; Broderius, M.A.; Byersdorfer, C.A.; Eide, D.J. The Fet4 Gene Encodes the Low-Affinity Fe(II) Transport Protein of Saccharomyces-Cerevisiae. J. Biol. Chem. 1994, 269, 26092–26099. [Google Scholar] [PubMed]
- Lesuisse, E.; Simon-Casteras, M.; Labbe, P. Siderophore-mediated iron uptake in Saccharomyces cerevisiae: The SIT1 gene encodes a ferrioxamine B permease that belongs to the major facilitator superfamily. Microbiology 1998, 144, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Georgatsou, E.; Alexandraki, D. 2 Distinctly Regulated Genes are Required for Ferric Reduction, the first Step of Iron Uptake in Saccharomyces-Cerevisiae. Mol. Cell. Biol. 1994, 14, 3065–3073. [Google Scholar] [CrossRef][Green Version]
- Stearman, R.; Yuan, D.S.; YamaguchiIwai, Y.; Klausner, R.D.; Dancis, A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science 1996, 271, 1552–1557. [Google Scholar] [CrossRef]
- Martinez-Pastor, M.T.; Perea-Garcia, A.; Puig, S. Mechanisms of iron sensing and regulation in the yeast Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2017, 33, 75. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Alonso, L.; Wittmaack, N.; Mulet, I.; Martinez-Garay, C.A.; Fita-Torro, J.; Jesus Lozano, M.; Romero, A.M.; Garcia-Ferris, C.; Teresa Martinez-Pastor, M.; Puig, S. Molecular strategies to increase yeast iron accumulation and resistance. Metallomics 2018, 10, 1245–1256. [Google Scholar] [CrossRef]
- Zhao, H.; Eide, D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc. Natl. Acad. Sci. USA 1996, 93, 2454–2458. [Google Scholar] [CrossRef]
- Zhao, H.; Eide, D. The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J. Biol. Chem. 1996, 271, 23203–23210. [Google Scholar] [CrossRef]
- Van Ho, A.; Ward, D.M.; Kaplan, J. Transition metal transport in yeast. Annu. Rev. Microbiol. 2002, 56, 237–261. [Google Scholar] [CrossRef]
- Eide, D.J. Homeostatic and Adaptive Responses to Zinc Deficiency in Saccharomyces cerevisiae. J. Biol. Chem. 2009, 284, 18565–18569. [Google Scholar] [CrossRef]
- MacDiarmid, C.W.; Gaither, L.A.; Eide, D. Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 2000, 19, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Wang, L.; Wu, J.; Simth, N.; Zhang, L.; Wang, Y.; Wu, X. MTM1 plays an important role in the regulation of zinc tolerance in Saccharomyces cerevisiae. J. Trace Elem. Med. Biol. 2021, 66, 126759. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-J.; Kim, Y.-S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Yadav, K.K.; Rajasekharan, R. Effect of zinc deprivation on the lipid metabolism of budding yeast. Curr. Genet. 2017, 63, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, J.; Shi, W.; Shi, W.; Liu, H.; Wu, X. Lead Induces Genotoxicity via Oxidative Stress and Promoter Methylation of DNA Repair Genes in Human Lymphoblastoid TK6 Cells. Med. Sci. Monit. 2018, 24, 4295–4304. [Google Scholar] [CrossRef]
- Chen, F.; Shi, X.L. Intracellular signal transduction of cells in response to carcinogenic metals. Crit. Rev. Oncol. Hematol. 2002, 42, 105–121. [Google Scholar] [CrossRef]
- Harris, G.K.; Shi, X. Signaling by carcinogenic metals and metal-induced reactive oxygen species. Mutat. Res. -Fundam. Mol. Mech. Mutagen. 2003, 533, 183–200. [Google Scholar] [CrossRef]
- Sumner, E.R.; Shanmuganathan, A.; Sideri, T.C.; Willetts, S.A.; Houghton, J.E.; Avery, S.V. Oxidative protein damage causes chromium toxicity in yeast. Microbiology 2005, 151, 1939–1948. [Google Scholar] [CrossRef]
- Wysocki, R.; Tamas, M.J. How Saccharomyces cerevisiae copes with toxic metals and metalloids. FEMS Microbiol. Rev. 2010, 34, 925–951. [Google Scholar] [CrossRef]
- Shi, X.L.; Mao, Y.; Knapton, A.D.; Ding, M.; Rojanasakul, Y.; Gannett, P.M.; Dalal, N.; Liu, K.J. Reaction of Cr(VI) with Ascorbate and Hydrogen-Peroxide Generates Hydroxyl Radicals and Causes Dna-Damage-Role of A Cr(IV)-Mediated Fenton-Like Reaction. Carcinogenesis 1994, 15, 2475–2478. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yokoyama, A.; Tsuji, T.; Ikeshima, E.; Nakashima, K.; Ikushima, S.; Kobayashi, C.; Yoshida, S. Identification and characterization of genes involved in glutathione production in yeast. J. Biosci. Bioeng. 2011, 112, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Straffon, M.J.; Jang, T.-Y.; Higgins, V.J.; Grant, C.M.; Dawes, I.W. The essential and ancillary role of glutathione in Saccharomyces cerevisiae analysed using a grande gsh1 disruptant strain. FEMS Yeast Res. 2001, 1, 57–65. [Google Scholar] [PubMed]
- Dannenmann, B.; Lehle, S.; Hildebrand, D.G.; Kuebler, A.; Grondona, P.; Schmid, V.; Holzer, K.; Froeschl, M.; Essmann, F.; Rothfuss, O.; et al. High Glutathione and Glutathione Peroxidase-2 Levels Mediate Cell-Type-Specific DNA Damage Protection in Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2015, 4, 886–898. [Google Scholar] [CrossRef]
- Li, Y.; Wei, G.Y.; Chen, J. Glutathione: A review on biotechnological production. Appl. Microbiol. Biotechnol. 2004, 66, 233–242. [Google Scholar] [CrossRef]
- Wu, A.L.; Moyerowley, W.S. GSH1, which encodes gamma-glutamylcysteine synthetase, is a target gene for yap-1 transcriptional regulation. Mol. Cell. Biol. 1994, 14, 5832–5839. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuca, K.; Musilek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Herrero, E.; Wellinger, R.E. Yeast as a model system to study metabolic impact of selenium compounds. Microb. Cell 2015, 2, 139–149. [Google Scholar] [CrossRef]
- Hara, K.Y.; Kiriyama, K.; Inagaki, A.; Nakayama, H.; Kondo, A. Improvement of glutathione production by metabolic engineering the sulfate assimilation pathway of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2012, 94, 1313–1319. [Google Scholar] [CrossRef]
- Kessi, J.; Hanselmann, K.W. Similarities between the abiotic reduction of selenite with glutathione and the dissimilatory reaction mediated by Rhodospirillum rubrum and Escherichia coli. J. Biol. Chem. 2004, 279, 50662–50669. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, M.; Lagniel, G.; Kristiansson, E.; Junot, C.; Nerman, O.; Labarre, J.; Tamas, M.J. Quantitative transcriptome, proteome, and sulfur metabolite profiling of the Saccharomyces cerevisiae response to arsenite. Physiol. Genom. 2007, 30, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lafaye, A.; Junot, C.; Pereira, Y.; Lagniel, G.; Tabet, J.C.; Ezan, E.; Labarre, J. Combined proteome and metabolite-profiling analyses reveal surprising insights into yeast sulfur metabolism. J. Biol. Chem. 2005, 280, 24723–24730. [Google Scholar] [CrossRef] [PubMed]
- Culotta, V.C.; Luk, E. The many highways for intracellular trafficking of metals. J. Inorg. Biochem. 2003, 96, 9. [Google Scholar] [CrossRef]
- Li, L.T.; Chen, O.S.; Ward, D.M.; Kaplan, J. CCC1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem. 2001, 276, 29515–29519. [Google Scholar] [CrossRef]
- Khandelwal, N.K.; Millan, C.R.; Zangari, S.I.; Avila, S.; Williams, D.; Thaker, T.M.; Tomasiak, T.M. The structural basis for regulation of the glutathione transporter Ycf1 by regulatory domain phosphorylation. Nat. Commun. 2022, 13, 1278. [Google Scholar] [CrossRef]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef]
- Ruta, L.L.; Farcasanu, I.C. Interaction between Polyphenolic Antioxidants and Saccharomyces cerevisiae Cells Defective in Heavy Metal Transport across the Plasma Membrane. Biomolecules 2020, 10, 1512. [Google Scholar] [CrossRef]
- Sheng, Y.; Cao, H.; Li, Y.; Zhang, Y. Effects of sulfide on sulfate reducing bacteria in response to Cu(II), Hg(II) and Cr(VI) toxicity. Chin. Sci. Bull. 2011, 56, 862–868. [Google Scholar] [CrossRef]
- Kommuguri, U.N.; Bodiga, S.; Sankuru, S.; Bodiga, V.L. Copper deprivation modulates CTR1 and CUP1 expression and enhances cisplatin cytotoxicity in Saccharomyces cerevisiae. J. Trace Elem. Med. Biol. 2012, 26, 13–19. [Google Scholar] [CrossRef]
- Ruta, L.L.; Lin, Y.F.; Kissen, R.; Nicolau, I.; Neagoe, A.D.; Ghenea, S.; Bones, A.M.; Farcasanu, I.C. Anchoring plant metallothioneins to the inner face of the plasma membrane of Saccharomyces cerevisiae cells leads to heavy metal accumulation. PLoS ONE 2017, 12, e0178393. [Google Scholar] [CrossRef]
- Gaddipati, J.P.; Rajeshkumar, N.V.; Grove, J.C.; Maharaj, S.V.M.; Centeno, J.A.; Maheshwari, R.K.; Jonas, W.B. Low-Dose Cadmium Exposure Reduces Human Prostate Cell Transformation in Culture and Up-Regulates Metallothionein and MT-1G mRNA. Nonlinearity Biol. Toxicol. Med. 2003, 1, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Clark, A.B.; Slebos, R.J.C.; Al-Refai, H.; Taylor, J.A.; Kunkel, T.A.; Resnick, M.A.; Gordenin, D.A. Cadmium is a mutagen that acts by inhibiting mismatch repair. Nat. Genet. 2003, 34, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Paumi, C.M.; Chuk, M.; Snider, J.; Stagljar, I.; Michaelis, S. ABC Transporters in Saccharomyces cerevisiae and Their Interactors: New Technology Advances the Biology of the ABCC (MRP) Subfamily. Microbiol. Mol. Biol. Rev. 2009, 73, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Winge, D.R.; Nielson, K.B.; Gray, W.R.; Hamer, D.H. Yeast metallothionein. Sequence and metal-binding properties. J. Biol. Chem. 1985, 260, 14464–14470. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Adle, D.J.; Sinani, D.; Kim, H.; Lee, J. A cadmium-transporting P-1B-type ATPase in yeast Saccharomyces cerevisiae. J. Biol. Chem. 2007, 282, 947–955. [Google Scholar] [CrossRef]
- Salt, D.E.; Wagner, G.J. Cadmium Transport across Tonoplast of Vesicles from Oat Roots-Evidence For A CD2+/H+ Antiport Activity. J. Biol. Chem. 1993, 268, 12297–12302. [Google Scholar] [CrossRef]
- Sun, G.L.; Reynolds, E.E.; Belcher, A.M. Designing yeast as plant-like hyperaccumulators for heavy metals. Nat. Commun. 2019, 10, 5080. [Google Scholar] [CrossRef]
- Wuenschmann, J.; Krajewski, M.; Letzel, T.; Huber, E.M.; Ehrmann, A.; Grill, E.; Lendzian, K.J. Dissection of glutathione conjugate turnover in yeast. Phytochemistry 2010, 71, 54–61. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; He, X.; Zhang, P.; Chai, Z. Selection of a high-biomass, chromium-rich yeast strain and optimization of cultivation conditions. J. Ind. Microbiol. Biotechnol. 2001, 27, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Ksheminska, H.; Fedorovych, D.; Babyak, L.; Yanovych, D.; Kaszycki, P.; Koloczek, H. Chromium(III) and (VI) tolerance and bioaccumulation in yeast: A survey of cellular chromium content in selected strains of representative genera. Process. Biochem. 2005, 40, 1565–1572. [Google Scholar] [CrossRef]
- Zhao, Y.-y.; Cao, C.-l.; Liu, Y.-l.; Wang, J.; Li, J.; Li, S.-y.; Deng, Y. Identification of the Genetic Requirements for Zinc Tolerance and Toxicity in Saccharomyces cerevisiae. G3-Genes Genomes Genet. 2020, 10, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Balaban, B.G.; Yilmaz, U.; Alkim, C.; Topaloglu, A.; Kisakesen, H.I.; Holyavkin, C.; Cakar, Z.P. Evolutionary Engineering of an Iron-Resistant Saccharomyces cerevisiae Mutant and Its Physiological and Molecular Characterization. Microorganisms 2020, 8, 43. [Google Scholar] [CrossRef]
- Manatschal, C.; Ehrnstorfer, I.A.; Dutzler, R. Structural and Mechanistic Basis of Proton-Coupled Metal Ion Transport in the SLC11/NRAMP Family. Biophys. J. 2017, 112, 22A. [Google Scholar] [CrossRef][Green Version]
- Ofiteru, A.M.; Ruta, L.L.; Rotaru, C.; Dumitru, I.; Ene, C.D.; Neagoe, A.; Farcasanu, I.C. Overexpression of the PHO84 gene causes heavy metal accumulation and induces Ire1p-dependent unfolded protein response in Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 2012, 94, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, V.; Hillestrom, P.R.; Kapolna, E.; Larsen, E.H.; Olsson, L. Metabolic and bioprocess engineering for production of selenized yeast with increased content of seleno-methylselenocysteine. Metab. Eng. 2011, 13, 282–293. [Google Scholar] [CrossRef]
- Sorribes-Dauden, R.; Martinez-Pastor, M.T.; Puig, S. Expression of a Truncated Yeast Ccc1 Vacuolar Transporter Increases the Accumulation of Endogenous Iron. Genes 2021, 12, 1120. [Google Scholar] [CrossRef]
- Perrone, G.G.; Grant, C.M.; Dawes, I.W. Genetic and environmental factors influencing glutathione homeostasis in Saccharomyces cerevisiae. Mol. Biol. Cell 2005, 16, 218–230. [Google Scholar] [CrossRef]
- Rainha, J.; Rodrigues, J.L.; Rodrigues, L.R. CRISPR-Cas9: A Powerful Tool to Efficiently Engineer Saccharomyces cerevisiae. Life 2021, 11, 13. [Google Scholar] [CrossRef]
- Lee, M.E.; DeLoache, W.C.; Cervantes, B.; Dueber, J.E. A Highly Characterized Yeast Toolkit for Modular, Multipart Assembly. ACS Synth. Biol. 2015, 4, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Curran, K.A.; Karim, A.S.; Gupta, A.; Alper, H.S. Use of expression-enhancing terminators in Saccharomyces cerevisiae to increase mRNA half-life and improve gene expression control for metabolic engineering applications. Metab. Eng. 2013, 19, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Buijs, N.A.; Zhu, Z.; Qin, J.; Siewers, V.; Nielsen, J. Production of fatty acid-derived oleochemicals and biofuels by synthetic yeast cell factories. Nat. Commun. 2016, 7, 11709. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).