The Effect of Different Substrates on the Morphological Features and Polyols Production of Endomyces magnusii Yeast during Long-Lasting Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Growth Conditions
2.2. Cell Viability and Vitality Assays
2.2.1. Spotting Test
2.2.2. Staining with Methyl Blue
2.2.3. CLS Assay
2.2.4. Flow Cytometry
2.3. Cell Respiration
2.4. Carbohydrate Analysis
2.5. Statistical Analyses
3. Results
3.1. Growth Features of the E.magnusii Yeasts upon the Cultivation Using Different Substrates
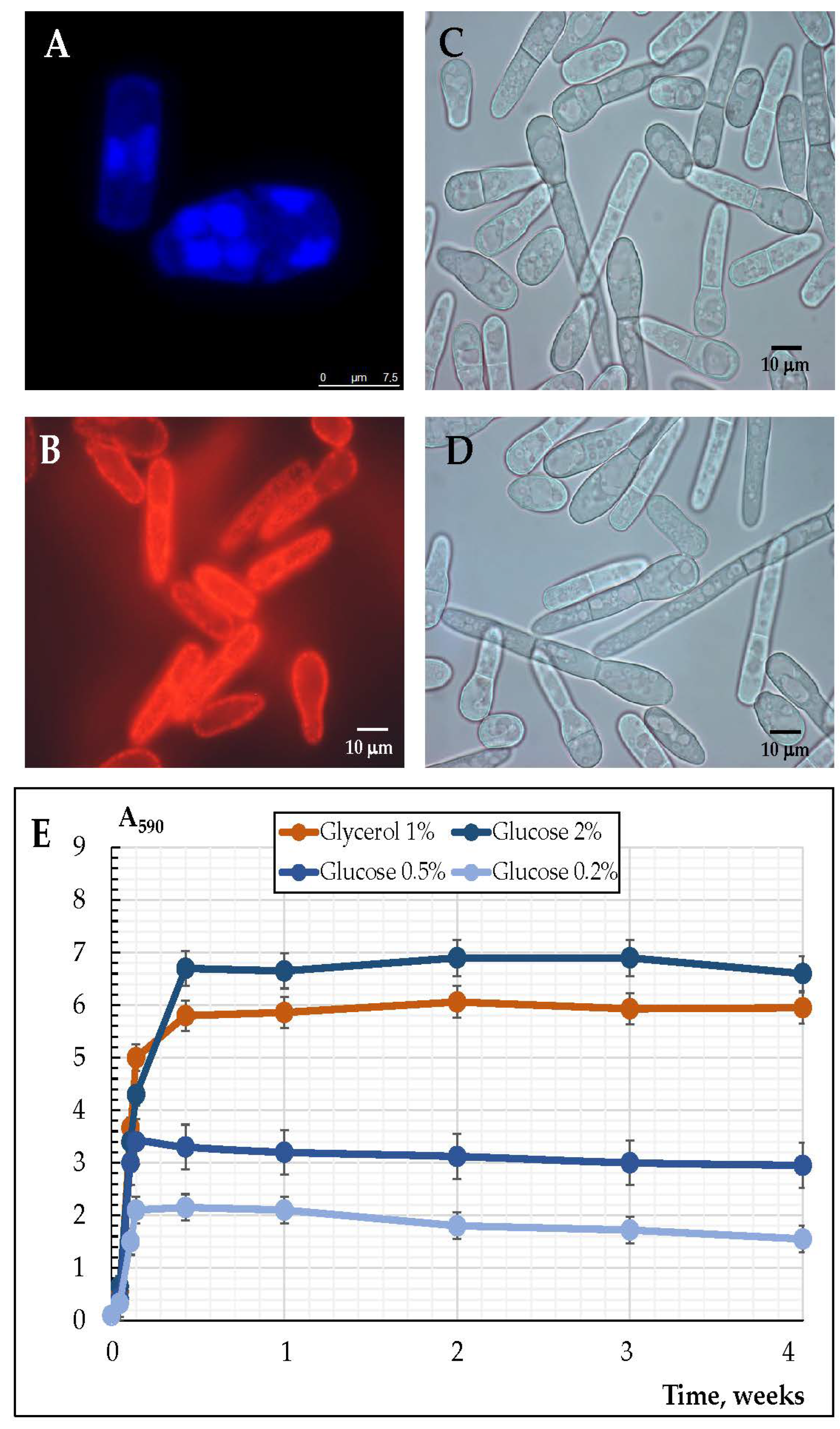
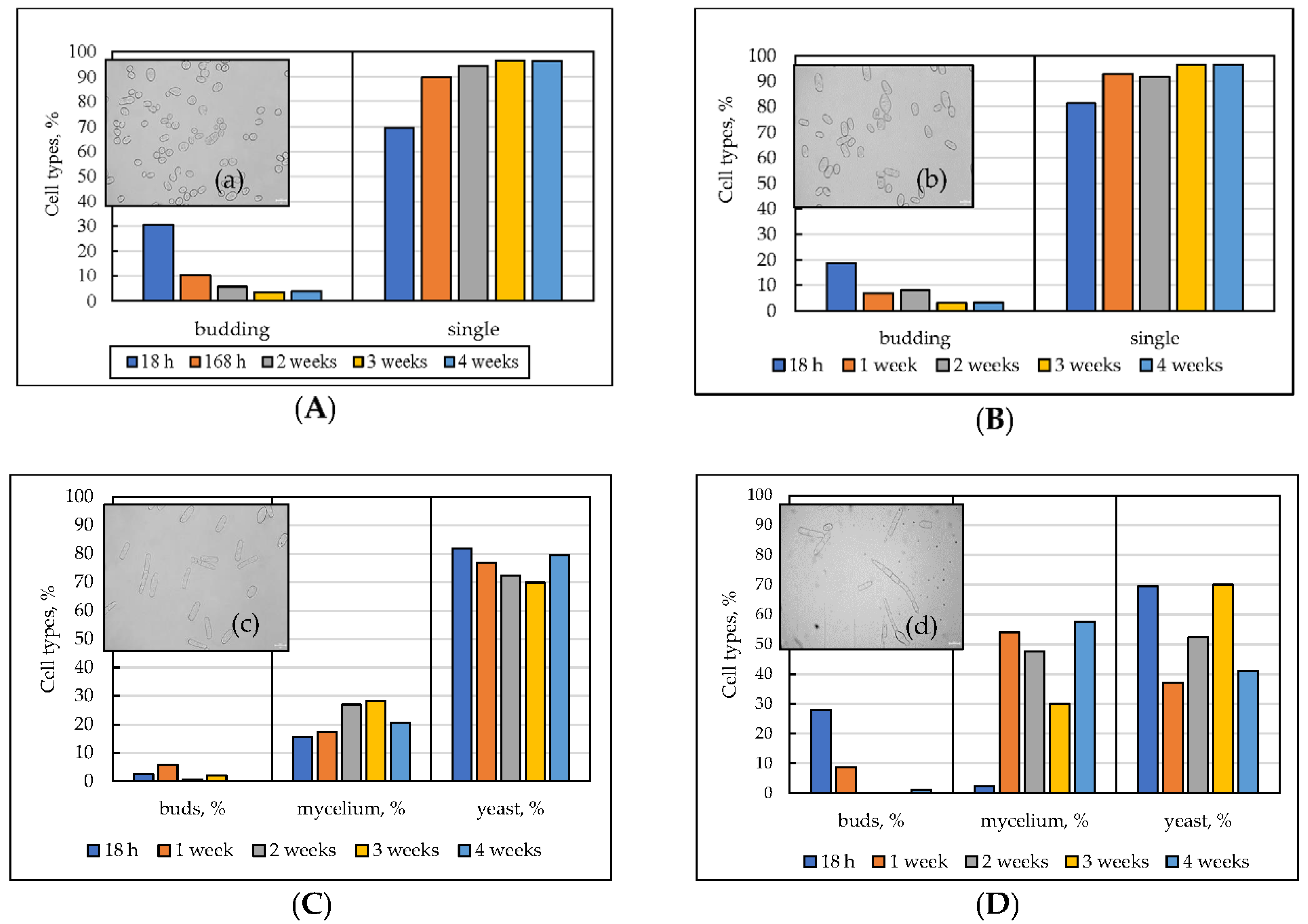
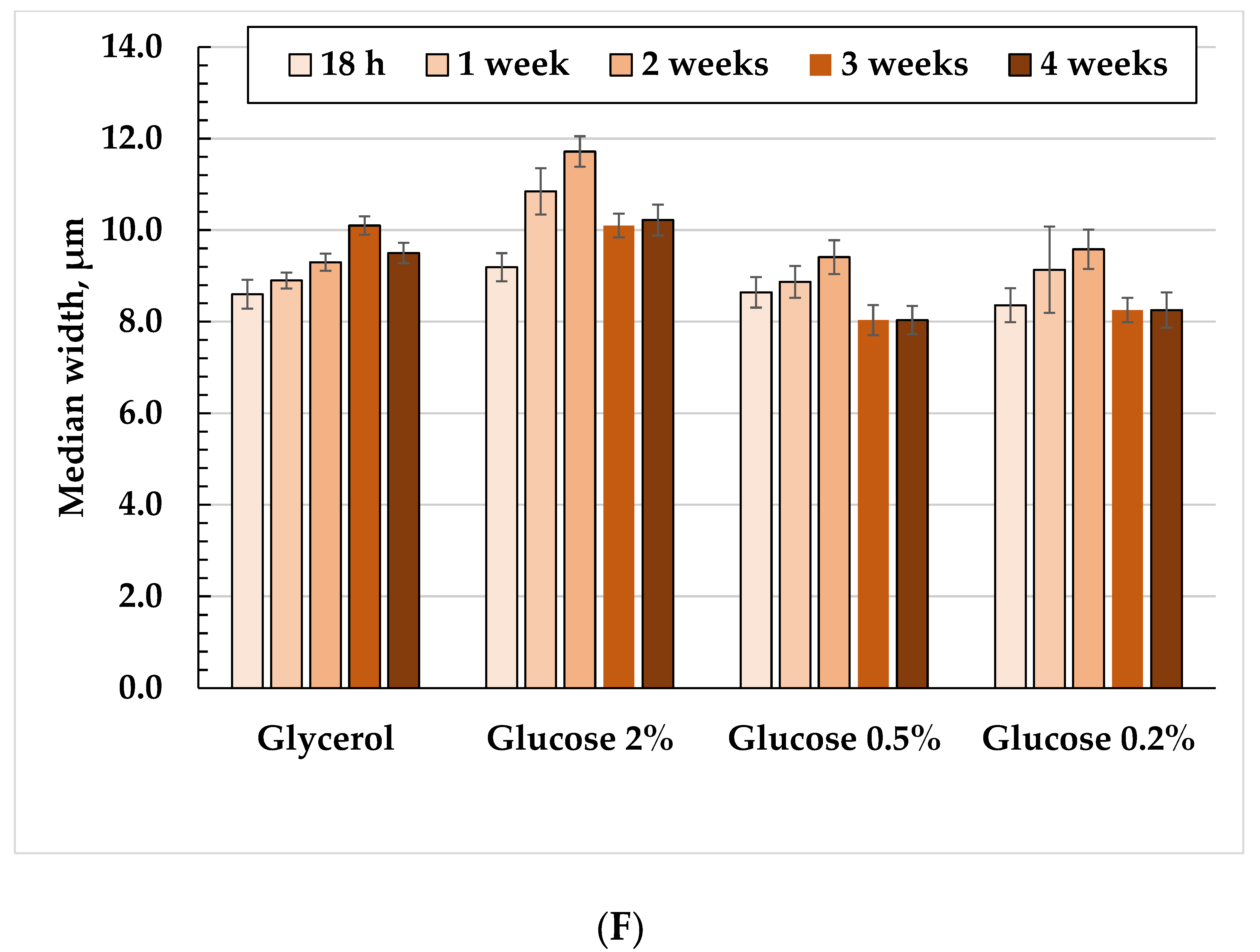
3.2. The Cell Morphology upon Long-Lasting Cultivation Using Different Substrates
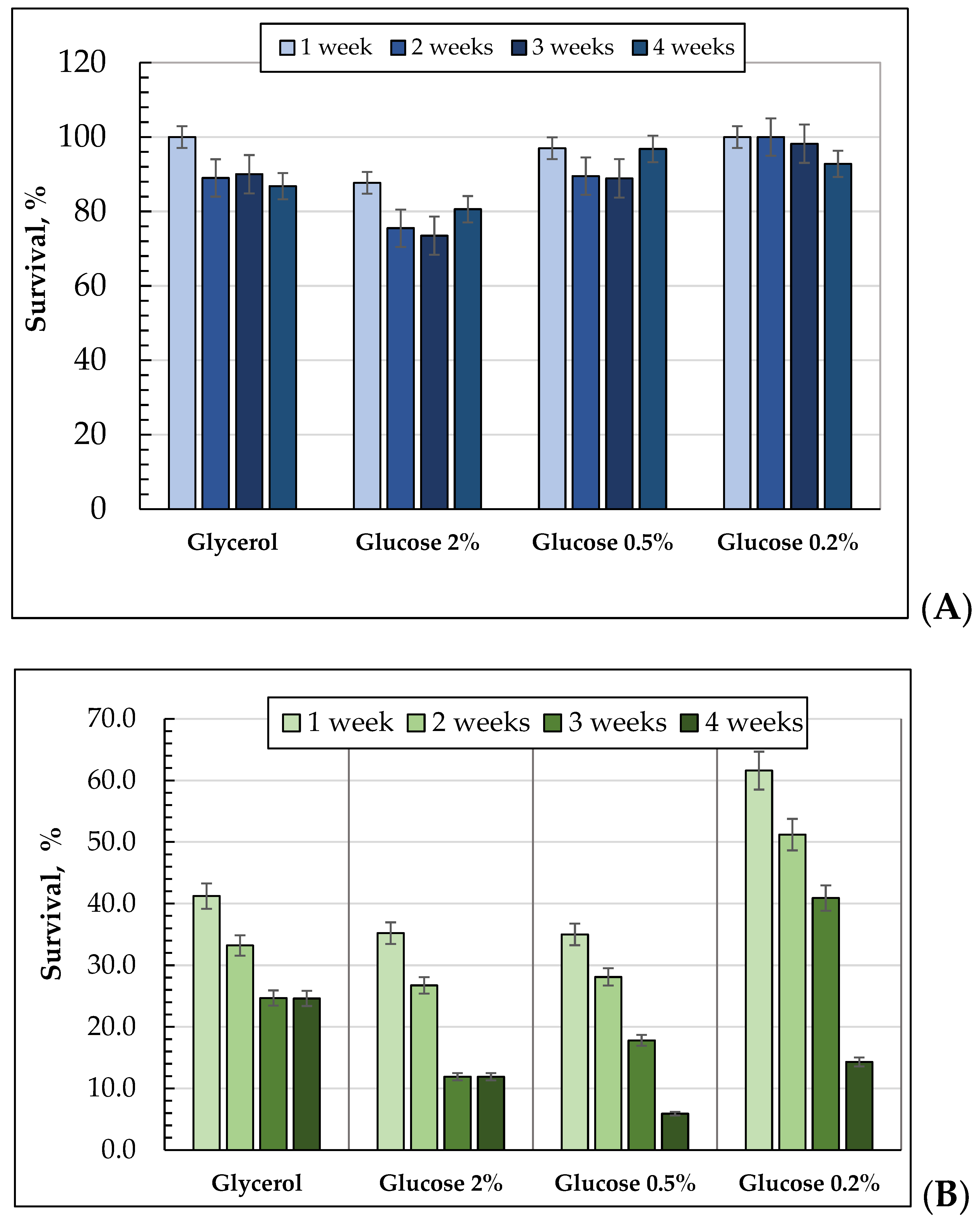
3.3. The Dynamics of the Survival of the E. magnusii Yeast during the Long-Term Cultivation Using Different Substrates
3.4. The Respiratory Features of the E. magnusii Yeast upon Long-Lasting Cultivations on Different Substrates
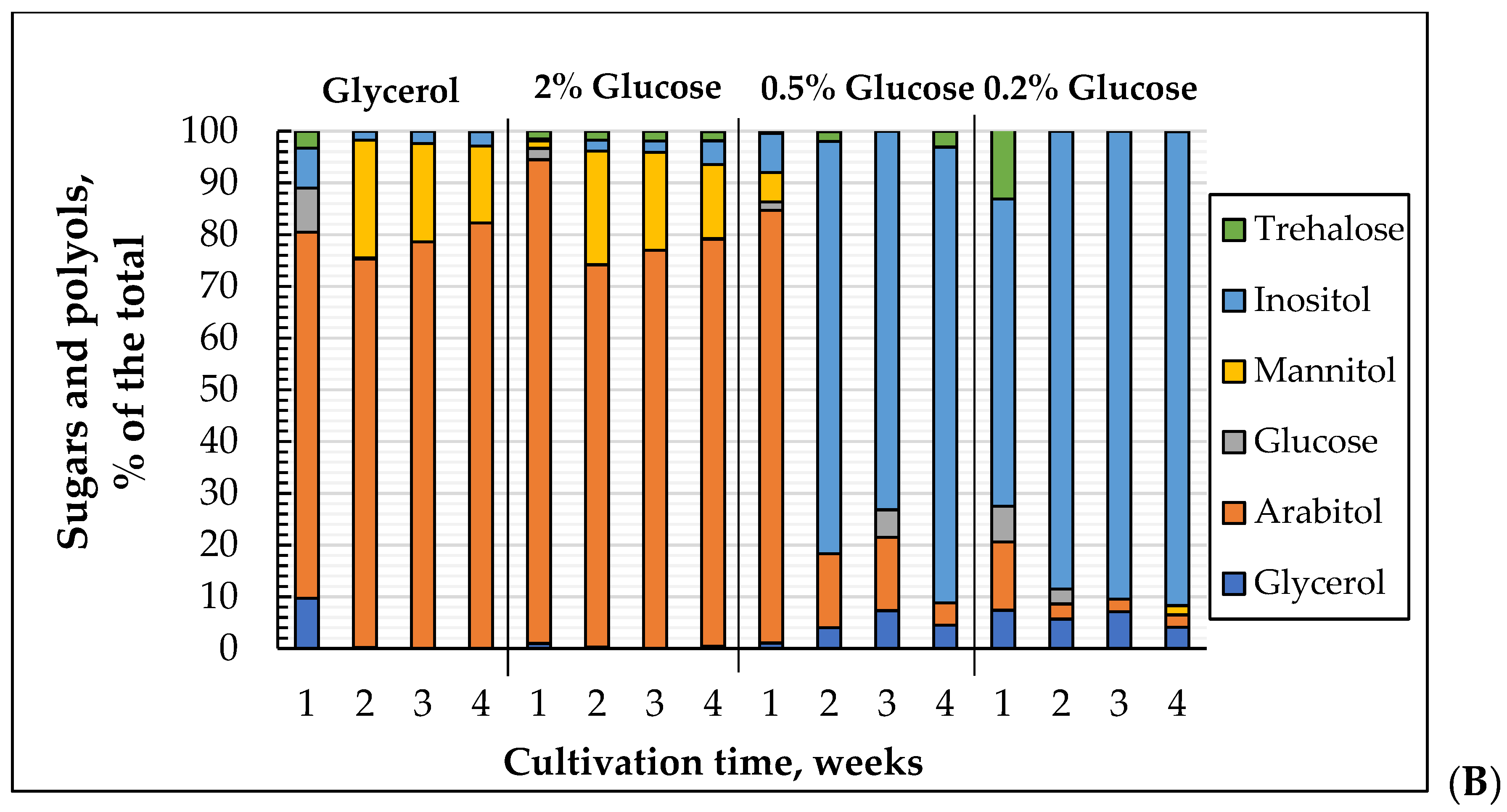
3.5. The Dynamics of Soluble Carbohydrate Profile Changes in the E. magnusii Yeast upon Long-Lasting Cultivation on Different Substrates
4. Discussion
5. Conclusions Remarks
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Denham, H. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Harman, D. Free radical theory of aging: An update: Increasing the functional life span. Ann. N. Y. Acad. Sci. 2006, 1067, 10–21. [Google Scholar] [CrossRef]
- Enriquez-Hesles, E.; Smith, D.L., Jr.; Maqani, N.; Wierman, M.B.; Sutcliffe, M.D.; Fine, R.D.; Kalita, A.; Santos, S.M.; Muehlbauer, M.J.; Bain, J.R.; et al. A cell-nonautonomous mechanism of yeast chronological aging regulated by caloric restriction and one-carbon metabolism. J. Biol. Chem. 2021, 296, 100125. [Google Scholar] [CrossRef]
- Flanagan, E.W.; Most, J.; Mey, J.T.; Redman, L.M. Calorie Restriction and Aging in Humans. Annu. Rev. Nutr. 2020, 40, 105–133. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, E.; Toniolo, L. The potential of calorie restriction and calorie restriction mimetics in delaying aging: Focus on experimental models. Nutrients 2021, 13, 2346. [Google Scholar] [CrossRef] [PubMed]
- Gabandé-Rodríguez, E.; Gómez de Las Heras, M.M.; Mittelbrunn, M. Control of inflammation by calorie restriction mimetics: On the crossroad of autophagy and mitochondria. Cells 2019, 9, 82. [Google Scholar] [CrossRef]
- Longo, V.D.; Shadel, G.S.; Kaeberlein, M.; Kennedy, B. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 2012, 16, 18–31. [Google Scholar] [CrossRef]
- He, C.; Zhou, C.; Kennedy, B.K. The yeast replicative aging model. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 2690–2696. [Google Scholar] [CrossRef]
- Mirisola, M.G.; Longo, V.D. Yeast chronological lifespan: Longevity regulatory genes and mechanisms. Cells 2022, 11, 1714. [Google Scholar] [CrossRef]
- Mohammad, K.; Baratang Junio, J.A.; Tafakori, T.; Orfanos, E.; Titorenko, V.I. Mechanisms that link chronological aging to cellular quiescence in budding yeast. Int. J. Mol. Sci. 2020, 21, 4717. [Google Scholar] [CrossRef]
- Lin, S.J.; Kaeberlein, M.; Andalis, A.A.; Sturtz, L.A.; Defossez, P.A.; Culotta, V.C.; Fink, G.R.; Guarente, L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 2002, 418, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Schleit, J.; Wasko, B.M.; Kaeberlein, M. Yeast as a model to understand the interaction between genotype and the response to calorie restriction. FEBS Lett. 2012, 586, 2868–2873. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Suarez, J.A.; Longo, V.D. Protein and amino acid restriction, aging and disease: From yeast to humans. Trends Endocrinol. Metab. 2014, 25, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Cummings, N.E.; Lamming, D.W. Regulation of metabolic health and aging by nutrient-sensitive signaling pathways. Mol. Cell. Endocrinol. 2017, 455, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Sohal, R.S.; Weindruch, R. Oxidative Stress, Caloric Restriction, and Aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Reverter-Branchat, G.; Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative damage to specific proteins in replicative and chronological aged Saccharomyces cerevisiae: Common targets and prevention by calorie restriction. J. Biol. Chem. 2004, 279, 31983–31989. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L., Jr.; McClure, J.M.; Matecic, M.; Smith, J.S. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the sirtuins. Aging Cell 2007, 6, 649–662. [Google Scholar] [CrossRef]
- Mei, S.C.; Brenner, C. Calorie restriction-mediated replicative lifespan extension in yeast is non-cell autonomous. PLoS Biol. 2015, 13, e1002048. [Google Scholar] [CrossRef]
- Lin, S.J. Requirement of NAD and SIR2 for Life-Span Extension by Calorie Restriction in Saccharomyces cerevisiae. Science 2000, 289, 2126–2128. [Google Scholar] [CrossRef]
- Kaeberlein, M.; Powers, R.W.; Steffen, K.K.; Westman, E.A.; Hu, D.; Dang, N.; Kennedy, B.K. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 2005, 310, 1193–1196. [Google Scholar] [CrossRef]
- Cameroni, E.; Hulo, N.; Roosen, J.; Winderickx, J.; Virgilio, C.D. The novel yeast PAS kinase Rim15 orchestrates G(0)-associated antioxidant defense. Cell Cycle 2004, 3, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Agarval, S.; Sharma, S.; Agrawal, V.; Roy, N. Caloric restriction augments ROS defense in S. cerevisiae by a Sir2p independent mechanism. Free Radic. Res. 2005, 39, 55–62. [Google Scholar] [CrossRef]
- Sharma, S.; Agrawal, V.; Roy, N. Mitochondria-mediated hormetic response in life span extension of calorie-restricted Saccharomyces cerevisiae. AGE 2011, 33, 143–154. [Google Scholar] [CrossRef]
- Herker, E.; Jungwirth, H.; Lehmann, K.A.; Maldener, C.; Fröhlich, K.U.; Wissing, S.; Büttner, S.; Fehr, M.; Sigrist, S.; Madeo, F. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 2004, 16, 501–507. [Google Scholar] [CrossRef]
- Gray, J.V.; Petsko, G.A.; Johnston, G.C.; Ringe, D.; Singer, R.A.; Werner-Washburne, M. “Sleeping beauty”: Quiescence in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2004, 68, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Buttner, S.; Aragon, A.D.; Thomas, J.A.; Meirelles, O.; Jaetao, J.E.; Benn, D.; Ruby, S.W.; Veenhuis, M.; Madeo, F.; et al. Isolation of quiescent and nonquiescent cells from yeast stationary-phase cultures. J. Cell Biol. 2006, 174, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Arlia-Ciommo, A.; Piano, A.; Svistkova, V.; Mohtashami, S.; Titorenko, V.I. Mechanisms underlying the anti-aging and anti-tumor effects of lithocholic bile acid. Int. J. Mol. Sci. 2014, 15, 16522–16543. [Google Scholar] [CrossRef] [PubMed]
- Arlia-Ciommo, A.; Leonov, A.; Piano, A.; Svistkova, V.; Titorenko, V.I. Cell-autonomous mechanisms of chronological aging in the yeast Saccharomyces cerevisiae. Microb. Cell 2014, 1, 163–178. [Google Scholar] [CrossRef]
- Arlia-Ciommo, A.; Leonov, A.; Beach, A.; Richard, V.R.; Bourque, S.D.; Burstein, M.T.; Kyryakov, P.; Gomez-Perez, A.; Koupaki, O.; Feldman, R.; et al. Caloric restriction delays yeast chronological aging by remodeling carbohydrate and lipid metabolism, altering peroxisomal and mitochondrial functionalities, and postponing the onsets of apoptotic and liponecrotic modes of regulated cell death. Oncotarget 2018, 9, 16163–16184. [Google Scholar] [CrossRef][Green Version]
- Isakova, E.P.; Matushkina, I.N.; Popova, T.N.; Dergacheva, D.I.; Gessler, N.N.; Klein, O.I.; Semenikhina, A.V.; Deryabina, Y.I.; La Porta, N.; Saris, N.L. Metabolic Remodeling during Long-Lasting Cultivation of the Endomyces magnusii Yeast on Oxidative and Fermentative Substrates. Microorganisms 2020, 8, 91. [Google Scholar] [CrossRef]
- Deryabina, Y.I.; Bazhenova, E.N.; Saris, N.E.; Zvyagilskaya, R.A. Ca(2+) efflux in mitochondria from the yeast Endomyces magnusii. J. Biol. Chem. 2001, 276, 47801–47806. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Shadel, G. Extension of chronological life span by reduced TOR signaling requires down-regulation of Sch9p and involves increased mitochondrial OXPHOS complex density. Aging 2009, 1, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P. How does hormesisimpact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Kwolek-Mirek, M.; Zadrag-Tecza, R. Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res. 2014, 14, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Deryabina, Y.; Isakova, E.; Antipov, A.; Saris, N.E. The inhibitors of antioxidant cell enzymes induce permeability transition in yeast mitochondria. J. Bioenerg. Biomembr. 2013, 45, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, M. Determination of blood sugar. J. Biol. Chem. 1945, 160, 69–73. [Google Scholar] [CrossRef]
- Brobst, K.M.; Tai, H. Determination of chlorohydrins in hydroxypropyl starch ethers. J. Assoc. Anal. Chem. 1971, 54, 1093–1094. [Google Scholar] [CrossRef]
- Norman, K.L.; Shively, C.A.; De La Rocha, A.J.; Mutlu, N.; Basu, S.; Cullen, P.J.; Kumar, A. Inositol polyphosphates regulate and predict yeast pseudohyphal growth phenotypes. PLoS Genet. 2018, 14, e1007493. [Google Scholar] [CrossRef]
- Mitchell, A.P. Dimorphism and virulence in Candida albicans. Curr. Opin. Microbiol. 1998, 1, 687–692. [Google Scholar] [CrossRef]
- Madhani, H.D.; Fink, G.R. The control of filamentous differentiation and virulence in fungi. Trends Cell Biol. 1998, 8, 348–353. [Google Scholar] [CrossRef]
- Berman, J.; Sudbery, P.E. Candida albicans: A molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 2002, 3, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Okagaki, L.H.; Strain, A.K.; Nielsen, J.N.; Charlier, C.; Baltes, N.J.; Chretien, F.; Heitman, J.; Dromer, F.; Nielsen, K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010, 6, e1000953. [Google Scholar] [CrossRef]
- Gimeno, C.J.; Ljungdahl, P.O.; Styles, C.A.; Fink, G.R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: Regulation by starvation and RAS. Cell 1992, 68, 1077–1090. [Google Scholar] [CrossRef]
- Lo, W.S.; Dranginis, A.M. FLO11, a yeast gene related to the STA genes, encodes a novel cell surface flocculin. J. Bacteriol. 1996, 178, 7144–7151. [Google Scholar] [CrossRef] [PubMed]
- Cullen, P.J.; Sprague, G.F. Glucose depletion causes haploid invasive growth in yeast. Proc. Natl. Acad. Sci. USA 2000, 97, 13461–13463. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Dobry, C.J.; McCown, P.J.; Kumar, A. Large-scale analysis of yeast filamentous growth by systematic gene disruption and overexpression. Mol. Biol. Cell 2008, 19, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Ryan, O.; Shapiro, R.S.; Kurat, C.F.; Mayhew, D.; Baryshnikova, A.; Chin, B.; Lin, Z.; Cox, M.J.; Vizeacoumar, F.; Cheung, D.; et al. Global gene deletion analysis exploring yeast filamentous growth. Science 2012, 337, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Shively, C.A.; Eckwahl, M.J.; Dobry, C.J.; Mellacheruvu, D.; Nesvizhskii, A.; Kumar, A. Genetic networks inducing invasive growth in Saccharomyces cerevisiae identified through systematic genome-wide overexpression. Genetics 2013, 193, 1297–1310. [Google Scholar] [CrossRef]
- Carlson, M. Glucose repression in yeas. Curr. Opin. Microbiol. 1999, 2, 202–207. [Google Scholar] [CrossRef]
- Pronk, J.; Yde Steensma, H.; van Dijken, J.P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 1996, 12, 1607–1633. [Google Scholar] [CrossRef]
- DeRisi, J.L.; Iyer, V.R.; Brown, P.O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 1997, 278, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, A.; Liu, J.; Schroeder, E.A.; Shadel, G.S.; Barrientos, A. Mitochondrial respiratory thresholds regulate yeast chronological life span and its extension by caloric restriction. Cell Metab. 2012, 16, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Rogov, A.G.; Zvyagilskaya, R.A. Physiological role of alternative oxidase (from yeasts to plants). Biochemistry 2015, 80, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Fabrizio, P.; Hu, J.; Ge, H.; Cheng, C.; Li, L.; Longo, V.D. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008, 4, 0139–0149. [Google Scholar] [CrossRef]
- Sánchez-Fresneda, R.; Guirao-Abad, J.P.; Argüelles, A.; González-Párraga, P.; Valentín, E.; Argüelles, J.C. Specific stress-induced storage of trehalose, glycerol and D-arabitol in response to oxidative and osmotic stress in Candida albicans. Biochem. Biophys. Res. Commun. 2012, 430, 1334–1339. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Iwata, H.; Yoshida, J.; Ogihara, J.; Kato, J.; Kasumi, T. Metabolic correlation between polyol and energy-storing carbohydrate under osmotic and oxidative stress condition in Moniliella megachiliensis. J. Biosci. Bioeng. 2015, 120, 405–410. [Google Scholar] [CrossRef]
- Eleutherio, E.; Panek, A.; De Mesquita, J.F.; Trevisol, E.; Magalhães, R. Revisiting yeast trehalose metabolism. Curr. Genet. 2015, 61, 263–274. [Google Scholar] [CrossRef]
- Benaroudj, N.; Lee, D.H.; Goldberg, A.L. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J. Biol. Chem. 2001, 276, 24261–24267. [Google Scholar] [CrossRef]
- Ianutsevich, E.A.; Danilova, O.A.; Tereshina, V.M.; Groza, N.V. Membrane lipids and cytosol carbohydrates in Aspergillus niger under osmotic, oxidative, and cold impact. Microbiology 2016, 85, 302–310. [Google Scholar] [CrossRef]
- Feofilova, E.P.; Mysyakina, I.S.; Usov, A.I.; Kochkina, G.A. Trehalose: Chemical structure, biological functions, and practical application. Microbiology 2014, 83, 184–194. [Google Scholar] [CrossRef]
- Koganti, S.; Kuo, T.M.; Kurtzman, C.P.; Smith, N.; Ju, L.K. Production of arabitol from glycerol: Strain screening and study of factors aecting production yield. Appl. Microbiol. Biotechnol. 2011, 90, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, T.; Ohtaguchi, K. Effect of temperature on D-arabitol production from lactose by Kluyveromyceslactis. J. Ind. Microbiol. Biotechnol. 2011, 38, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Voegele, R.T.; Hahn, M.; Lohaus, G.; Link, T.; Heiser, I.; Mendgen, K. Possible roles for mannitol and mannitol dehydrogenase in the biotrophic plant pathogen Uromycesfabae. Plant Physiol. 2005, 137, 190–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solomon, P.S.; Waters, O.D.; Oliver, R.P. Decoding the mannitol enigma in filamentous fungi. Trends Microbiol. 2007, 15, 257–262. [Google Scholar] [CrossRef]
- Patel, T.K.; Williamson, J.D. Mannitol in plants, fungi, and plant-fungal interactions. Trends Plant Sci. 2016, 21, 486–497. [Google Scholar] [CrossRef]
- Saha, B.C.; Racine, F.M. Biotechnological production of mannitol and its applications. Appl. Microbiol. Biotechnol. 2011, 89, 879–891. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, T.; Wei, W.; Mu, W.; Miao, M. Production of Mannitol from a High Concentration of Glucose by Candida parapsilosis SK26.001. Appl. Biochem. Biotechnol. 2017, 181, 391–406. [Google Scholar] [CrossRef]
- Rakicka, M.; Rywińska, A.; Cybulski, K.; Rymowicz, W. Enhanced production of erythritol and mannitol by Yarrowia lipolytica in media containing surfactants. Braz. J. Microbiol. 2016, 47, 417–423. [Google Scholar] [CrossRef]
- Kordowska-Wiater, M. Production of arabitol by yeasts: Current status and future prospects. J. Appl. Microbiol. 2015, 119, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Tamura, T.; Takaoka, C.; Harada, K.; Kobayashi, A.; Mukai, Y.; Fukusaki, E. Metabolomics-based systematic prediction of yeast lifespan and its application for semirational screening of ageing-related mutants. Aging Cell 2010, 9, 616–625. [Google Scholar] [CrossRef]
- Sarup, P.; Pedersen, S.M.; Nielsen, N.C.; Malmendal, A.; Loeschcke, V. The metabolic profile of long-lived Drosophila melanogaster. PLoS ONE 2012, 7, e47461. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Campisi, J. From Ancient Pathways to Aging Cells-Connecting Metabolism and Cellular Senescence. Cell Metab. 2016, 23, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Galluzzi, L.; Freije, J.M.; Madeo, F.; Kroemer, G. Metabolic control of longevity. Cell 2016, 166, 802–821. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, K.; Titorenko, V.I. Caloric restriction creates a metabolic pattern of chronological aging delay that in budding yeast differs from the metabolic design established by two other geroprotectors. Oncotarget 2021, 12, 608–625. [Google Scholar] [CrossRef]



| Source of Changes in the Cells Length | SS | df | MS | F | p-Value | F Critical |
|---|---|---|---|---|---|---|
| Substrate used | 30,285.72 | 3 | 10,095.24 | 396.44 | 1.20 × 10−201 | 2.61 |
| Age of the culture | 4604.02 | 4 | 1151.01 | 45.20 | 2.26 × 10−36 | 2.38 |
| Interaction of the factors | 2139.72 | 12 | 178.31 | 7.00 | 1.37 × 10−12 | 1.76 |
| Overlooked effects | 50,419.62 | 1980 | 25.47 | - | - | - |
| Total | 87,449.089 | 1999 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokoreva, A.S.; Isakova, E.P.; Tereshina, V.M.; Klein, O.I.; Gessler, N.N.; Deryabina, Y.I. The Effect of Different Substrates on the Morphological Features and Polyols Production of Endomyces magnusii Yeast during Long-Lasting Cultivation. Microorganisms 2022, 10, 1709. https://doi.org/10.3390/microorganisms10091709
Kokoreva AS, Isakova EP, Tereshina VM, Klein OI, Gessler NN, Deryabina YI. The Effect of Different Substrates on the Morphological Features and Polyols Production of Endomyces magnusii Yeast during Long-Lasting Cultivation. Microorganisms. 2022; 10(9):1709. https://doi.org/10.3390/microorganisms10091709
Chicago/Turabian StyleKokoreva, Anastasia S., Elena P. Isakova, Vera M. Tereshina, Olga I. Klein, Natalya N. Gessler, and Yulia I. Deryabina. 2022. "The Effect of Different Substrates on the Morphological Features and Polyols Production of Endomyces magnusii Yeast during Long-Lasting Cultivation" Microorganisms 10, no. 9: 1709. https://doi.org/10.3390/microorganisms10091709
APA StyleKokoreva, A. S., Isakova, E. P., Tereshina, V. M., Klein, O. I., Gessler, N. N., & Deryabina, Y. I. (2022). The Effect of Different Substrates on the Morphological Features and Polyols Production of Endomyces magnusii Yeast during Long-Lasting Cultivation. Microorganisms, 10(9), 1709. https://doi.org/10.3390/microorganisms10091709





