The Role of Cutibacterium acnes in Sarcoidosis: From Antigen to Treatable Trait?
Abstract
:1. Introduction
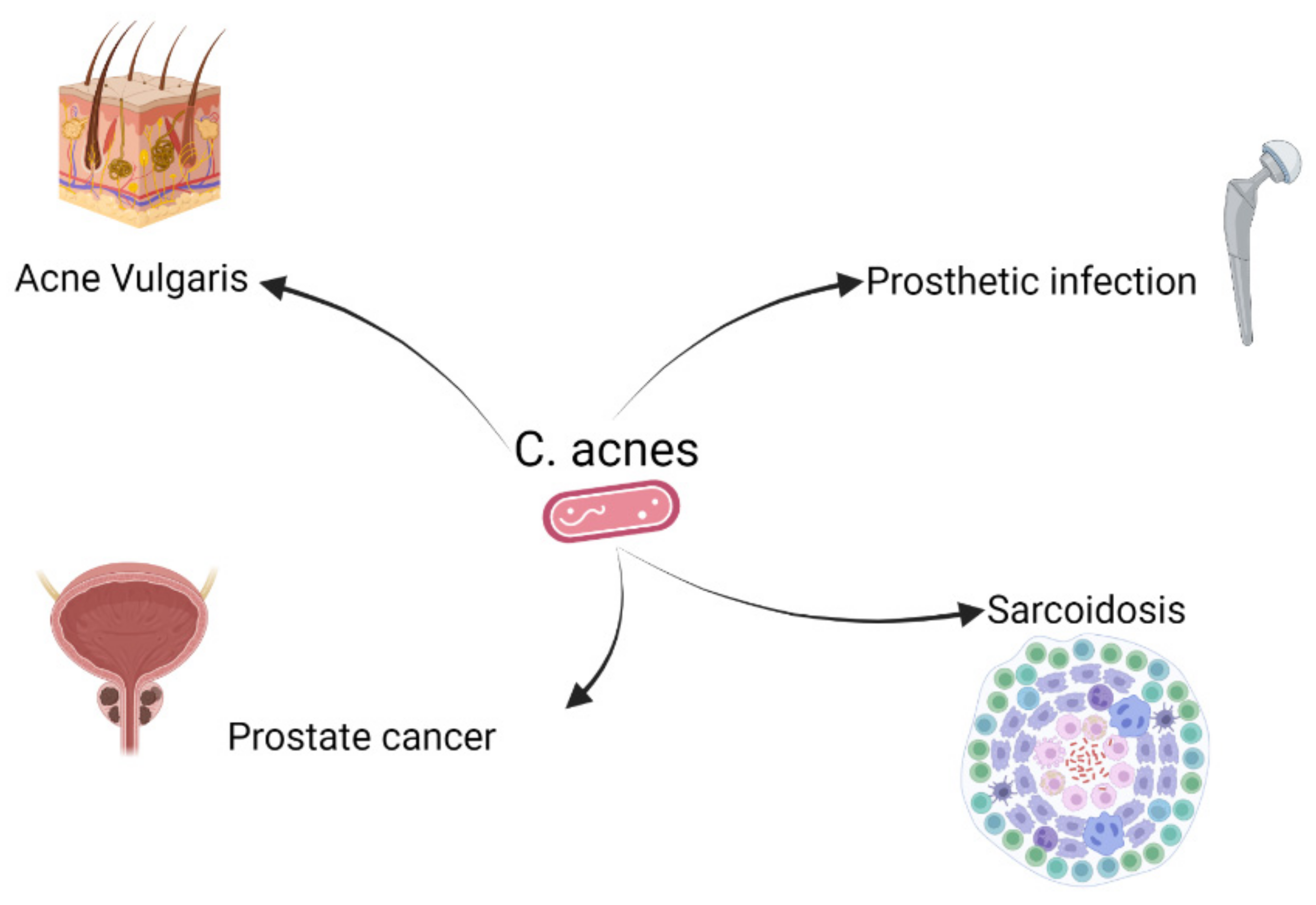
2. C. acnes and Different Diseases
3. Immunomodulatory Effect of C. acnes
3.1. Anti-Tumor Effect
3.2. C. acnes as a Vaccine Adjuvant
3.3. Innate Immunity
3.4. Adaptive Immunity
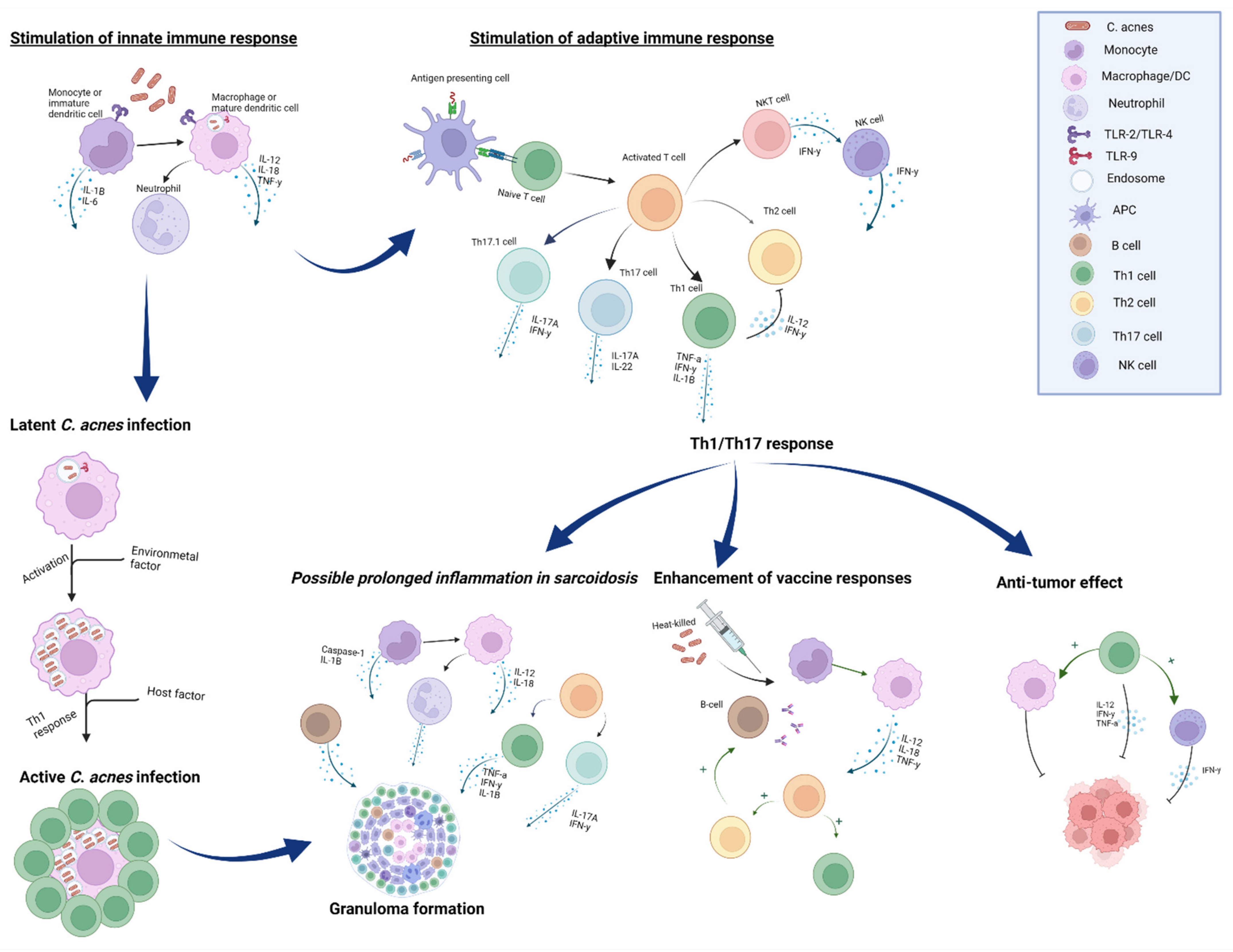
4. The Role of C. acnes in Sarcoidosis
4.1. Sarcoidosis and Possible Antigens
4.2. C. acnes as Antigen in Sarcoidosis
4.3. A Possible Role of C. acnes as a Mitogen in Sarcoidosis
5. Can the Presence of C. acnes in Patients with Sarcoidosis Be Seen as Treatable Trait?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Lambert, P. Propionibacterium acnes: Infection beyond the skin. Expert Rev. Anti-Infect. Ther. 2011, 9, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Portillo, M.E.; Corvec, S.; Borens, O.; Trampuz, A. Propionibacterium acnes: An underestimated pathogen in implant-associated infections. BioMed Res. Int. 2013, 2013, 804391. [Google Scholar] [CrossRef] [PubMed]
- McDowell, A.; Valanne, S.; Ramage, G.; Tunney, M.M.; Glenn, J.V.; McLorinan, G.C.; Bhatia, A.; Maisonneuve, J.-F.; Lodes, M.; Persing, D.H.; et al. Propionibacterium acnes types I and II represent phylogenetically distinct groups. J. Clin. Microbiol. 2005, 43, 326–334. [Google Scholar] [CrossRef]
- McDowell, A.; Nagy, I.; Magyari, M.; Barnard, E.; Patrick, S. The opportunistic pathogen Propionibacterium acnes: Insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS ONE 2013, 8, 70897. [Google Scholar] [CrossRef]
- McDowell, A.; Perry, A.L.; Lambert, P.A.; Patrick, S. A new phylogenetic group of Propionibacterium acnes. J. Med. Microbiol. 2008, 57, 218–224. [Google Scholar] [CrossRef]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 5–14. [Google Scholar] [CrossRef]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an opportunistic pathogen: An update of its virulence-associated factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Layton, A.M.; Dreno, B.; Gollnick, H.P.M.; Zouboulis, C.C. A review of the European Directive for prescribing systemic isotretinoin for acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 773–776. [Google Scholar] [CrossRef]
- Mondal, D.; Shenoy, S.R.; Mishra, S. Retinoic acid embryopathy. Int. J. Appl. Basic Med. Res. 2017, 7, 264–265. [Google Scholar] [CrossRef]
- Dessinioti, C.; Katsambas, A.D. The role of Propionibacterium acnes in acne pathogenesis: Facts and controversies. Clin. Dermatol. 2010, 28, 2–7. [Google Scholar] [CrossRef]
- Wang, Y.; Hata, T.R.; Tong, Y.L.; Kao, M.-S.; Zouboulis, C.C.; Gallo, R.L.; Huang, C.-M. The anti-inflammatory activities of propionibacterium acnes CAMP factor-targeted acne vaccines. J. Investig. Dermatol. 2018, 138, 2355–2364. [Google Scholar] [CrossRef]
- Achermann, Y.; Goldstein, E.J.C.; Coenye, T.; Shirtliff, M.E. Propionibacterium acnes: From commensal to opportunistic biofilm-associated implant pathogen. Clin. Microbiol. Rev. 2014, 27, 419–440. [Google Scholar] [CrossRef]
- Boisrenoult, P. Cutibacterium acnes prosthetic joint infection: Diagnosis and treatment. Orthop. Traumatol. Surg. Res. 2018, 104, S19–S24. [Google Scholar] [CrossRef]
- Brüggemann, H.; Salar-Vidal, L.; Gollnick, H.P.M.; Lood, R. A Janus-faced bacterium: Host-beneficial and -detrimental roles of cutibacterium acnes. Front. Microbiol. 2021, 12, 673845. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Tran, N.V.; Petty, P.M.; Johnson, C.H.; Walsh, M.F.; Bite, U.; Clay, R.P.; Mandrekar, J.N.; Piper, K.E.; Steckelberg, J.M.; et al. Pilot study of association of bacteria on breast implants with capsular contracture. J. Clin. Microbiol. 2009, 47, 1333–1337. [Google Scholar] [CrossRef]
- Ajdic, D.; Zoghbi, Y.; Gerth, D.; Panthaki, Z.J.; Thaller, S. The relationship of bacterial biofilms and capsular contracture in breast implants. Aesthetic Surg. J. 2016, 36, 297–309. [Google Scholar] [CrossRef]
- Conen, A.; Walti, L.N.; Merlo, A.; Fluckiger, U.; Battegay, M.; Trampuz, A. Characteristics and treatment outcome of cerebrospinal fluid shunt–associated infections in adults: A retrospective analysis over an 11-year period. Clin. Infect. Dis. 2008, 47, 73–82. [Google Scholar] [CrossRef]
- Piper, K.E.; Jacobson, M.J.; Cofield, R.H.; Sperling, J.W.; Sanchez-Sotelo, J.; Osmon, D.R.; McDowell, A.; Patrick, S.; Steckelberg, J.M.; Mandrekar, J.N.; et al. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J. Clin. Microbiol. 2009, 47, 1878–1884. [Google Scholar] [CrossRef]
- Levy, P.Y.; Fenollar, F.; Stein, A.; Borrione, F.; Cohen, E.; Lebail, B.; Raoult, D. Propionibacterium acnes postoperative shoulder arthritis: An emerging clinical entity. Clin. Infect. Dis. 2008, 46, 1884–1886. [Google Scholar] [CrossRef]
- Davidsson, S.; Mölling, P.; Rider, J.; Unemo, M.; Karlsson, M.G.; Carlsson, J.; Andersson, S.-O.; Elgh, F.; Söderquist, B.; Andrén, O. Frequency and typing of Propionibacterium acnes in prostate tissue obtained from men with and without prostate cancer. Infect. Agents Cancer 2016, 11, 26. [Google Scholar] [CrossRef]
- Bidaud, A.-L.; Karam, G.; Kandel-Aznar, C.; D’Epenoux, L.R.; Guillouzouic, A.; Bémer, P.; Leroy, A.-G.; Corvec, S. Low prevalence of Cutibacterium acnes in prostatic tissue biopsies in a French hospital. Anaerobe 2020, 66, 102286. [Google Scholar] [CrossRef]
- Erlandsson, A.; Carlsson, J.; Lundholm, M.; Fält, A.; Andersson, S.-O.; Andrén, O.; Davidsson, S. M2 macrophages and regulatory T cells in lethal prostate cancer. Prostate 2019, 79, 363–369. [Google Scholar] [CrossRef]
- Davidsson, S.; Andren, O.; Ohlson, A.-L.; Carlsson, J.; Andersson, S.-O.; Giunchi, F.; Rider, J.R.; Fiorentino, M. FOXP3+ regulatory T cells in normal prostate tissue, postatrophic hyperplasia, prostatic intraepithelial neoplasia, and tumor histological lesions in men with and without prostate cancer. Prostate 2018, 78, 40–47. [Google Scholar] [CrossRef]
- Idorn, M.; Køllgaard, T.; Kongsted, P.; Sengeløv, L.; Straten, P.T. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol. Immunother. 2014, 63, 1177–1187. [Google Scholar] [CrossRef]
- Roszkowski, W.; Ko, H.; Beuth, J.; Jeljaszewicz, J. Immunomodulation by propionibacteria. Zent. Bakteriol. 1990, 274, 289–298. [Google Scholar] [CrossRef]
- Michalak-Stoma, A.; Tabarkiewicz, J.; Olender, A.; Juszkiewicz-Borowiec, M.; Stoma, F.; Pietrzak, A.; Pozarowski, P.; Bartkowiak-Emeryk, M. The effect of Propionibacterium acnes on maturation of dendritic cells derived from acne patients’ peripherial blood mononuclear cells. Folia Histochem. Cytobiol. 2008, 46, 535–539. [Google Scholar] [CrossRef]
- Talib, W.H.; Saleh, S. Propionibacterium acnes augments antitumor, anti-angiogenesis and immunomodulatory effects of melatonin on breast cancer implanted in mice. PLoS ONE 2015, 10, e0124384. [Google Scholar] [CrossRef]
- Tsuda, K.; Yamanaka, K.; Linan, W.; Miyahara, Y.; Akeda, T.; Nakanishi, T.; Kitagawa, H.; Kakeda, M.; Kurokawa, I.; Shiku, H.; et al. Intratumoral injection of Propionibacterium acnes suppresses malignant melanoma by enhancing Th1 immune responses. PLoS ONE 2011, 6, e29020. [Google Scholar] [CrossRef]
- Dang, L.H.; Bettegowda, C.; Huso, D.L.; Kinzler, K.W.; Vogelstein, B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 15155–15160. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahasneh, A.M. Combination of Ononis hirta and Bifidobacterium longum decreases syngeneic mouse mammary tumor burden and enhances immune response. J. Cancer Res. Ther. 2012, 8, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Wu, C.-L.; Shiau, A.-L. Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis. Clin. Cancer Res. 2008, 14, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Buck, G.E.; Kelly, M.T. Investigation of the component of Propionibacterium acnes (Corynebacterium parvum) responsible for macrophage activation. Infect. Immun. 1980, 27, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.G.; Christie, G.H.; Scott, M.T. Biological effects of Corynebacterium parvum: IV. Adjuvant and inhibitory activities on B lymphocytes. Cell. Immunol. 1972, 7, 290–301. [Google Scholar] [CrossRef]
- Lichtenstein, A.; Bick, A.; Cantrell, J.; Zighelboim, J. Augmentation of NK activity by Corynebacterium parvum fractions in vivo and in vitro. Int. J. Immunopharmacol. 1983, 5, 137–144. [Google Scholar] [CrossRef]
- Disis, M.L. Immune regulation of cancer. J. Clin. Oncol. 2010, 28, 4531–4538. [Google Scholar] [CrossRef]
- Nakano, A.; Yoneyama, H.; Ueha, S.; Kitabatake, M.; Ishikawa, S.; Kawase, I.; Sugiyama, H.; Matsushima, K. Intravenous administration of MIP-1α with intra-tumor injection of P. acnes shows potent anti-tumor effect. Int. Immunopharmacol. 2007, 7, 845–857. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Knapp, R.C.; Mitchell, A.K.; Thurston, J.G.; Tucker, R.W.; Schlossman, S.F. Immunotherapy of a murine ovarian carcinoma with Corynebacterium parvum and specific heteroantiserum. I. Activation of peritoneal cells to mediate antibody-dependent cytotoxicity. J. Immunol. 1979, 123, 1945–1951. [Google Scholar]
- Knapp, R.C.; Berkowitz, R.S. Corynebacterium parvum as an immunotherapeutic agent in an ovarian cancer model. Am. J. Obstet. Gynecol. 1977, 128, 782–786. [Google Scholar] [CrossRef]
- Berek, J.S.; Cantrell, J.L.; Lichtenstein, A.K.; Hacker, N.F.; Knox, R.M.; Nieberg, R.K.; Poth, T.; Elashoff, R.M.; Lagasse, L.D.; Zighelboim, J. Immunotherapy with biochemically dissociated fractions of Propionibacterium acnes in a murine ovarian cancer model. Cancer Res. 1984, 44, 1871–1875. [Google Scholar]
- Roszkowski, W.; Ko, H.L.; Szmigielski, S.; Jeljaszewicz, J.; Pulverer, G. The correlation of susceptibility of different Propionibacterium strains to macrophage killing and antitumor activity. Med. Microbiol. Immunol. 1980, 169, 1–8. [Google Scholar] [CrossRef]
- Fujiwara, H.; Tsuchida, T.; Tsuji, Y.; Hamaoka, T. Preventive effect of Propionibacterium acnes on metastasis in mice rendered tolerant to tumor-associated transplantation antigens. GANN Jpn. J. Cancer Res. 1980, 71, 692–698. [Google Scholar]
- Teixeira, D.; Ishimura, M.E.; Apostolico, J.; Viel, J.M.; Passarelli, V.C.; Cunha-Neto, E.; Rosa, D.; Longo-Maugéri, I.M. Propionibacterium acnes enhances the immunogenicity of HIVBr18 human immunodeficiency virus-1 vaccine. Front. Immunol. 2018, 9, 177. [Google Scholar] [CrossRef]
- Squaiella-Baptistão, C.C.; Teixeira, D.; Mussalem, J.S.; Ishimura, M.E.; Longo-Maugéri, I.M. Modulation of Th1/Th2 immune responses by killed Propionibacterium acnes and its soluble polysaccharide fraction in a type I hypersensitivity murine model: Induction of different activation status of antigen-presenting cells. J. Immunol. Res. 2015, 2015, 132083. [Google Scholar] [CrossRef]
- Mussalem, J.S.; Squaiella-Baptistão, C.C.; Teixeira, D.; Yendo, T.M.; Thies, F.G.; Popi, A.F.; Mariano, M.; Longo-Maugéri, I.M. Adjuvant effect of killed Propionibacterium acnes on mouse peritoneal B-1 lymphocytes and their early phagocyte differentiation. PLoS ONE 2012, 7, e33955. [Google Scholar] [CrossRef]
- Paillot, R. A systematic review of the immune-modulators Parapoxvirus ovis and Propionibacterium acnes for the prevention of respiratory disease and other infections in the horse. Vet. Immunol. Immunopathol. 2013, 153, 1–9. [Google Scholar] [CrossRef]
- Vail, C.D.; Nestved, A.J.; Rollins, J.B.; Huff, G.K.; Hartgrove, T.B.; Evans, D.R.; Clapper, J.J.; Van Kampen, K.R.; Peters, B.A.; Hay, C.A. Adjunct treatment of equine respiratory disease complex (ERDC) with the Propionibacterium acnes, immunostimulant, EqStim®. J. Equine Vet. Sci. 1990, 10, 399–400. [Google Scholar] [CrossRef]
- Evans, D.R.; Brent Rollins, J.; Huff, G.K.; Hartgrove, T.B.; Van Kampen, K.R. Inactivated Propionibacterium acnes (immunoregulin) as adjunct to conventional therapy in the treatment of equine respiratory diseases. Equine Pract. 1998, 10, 17–21. [Google Scholar]
- Tchaptchet, S.; Gumenscheimer, M.; Kalis, C.; Freudenberg, N.; Hölscher, C.; Kirschning, C.J.; Lamers, M.; Galanos, C.; Freudenberg, M.A. TLR9-dependent and independent pathways drive activation of the immune system by Propionibacterium acnes. PLoS ONE 2012, 7, e39155. [Google Scholar] [CrossRef]
- Kalis, C.; Gumenscheimer, M.; Freudenberg, N.; Tchaptchet, S.; Fejer, G.; Heit, A.; Akira, S.; Galanos, C.; Freudenberg, M.A. Requirement for TLR9 in the immunomodulatory activity of Propionibacterium acnes. J. Immunol. 2005, 174, 4295–4300. [Google Scholar] [CrossRef]
- Holland, C.; Mak, T.N.; Zimny-Arndt, U.; Schmid, M.; Meyer, T.F.; Jungblut, P.R.; Brüggemann, H. Proteomic identification of secreted proteins of Propionibacterium acnes. BMC Microbiol. 2010, 10, 230. [Google Scholar] [CrossRef]
- Chen, Q.; Koga, T.; Uchi, H.; Hara, H.; Terao, H.; Moroi, Y.; Urabe, K.; Furue, M. Propionibacterium acnes-induced IL-8 production may be mediated by NF-κB activation in human monocytes. J. Dermatol. Sci. 2002, 29, 97–103. [Google Scholar] [CrossRef]
- Nagy, I.; Pivarcsi, A.; Koreck, A.; Széll, M.; Urbán, E.; Kemény, L. Distinct strains of Propionibacterium acnes induce selective human β-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J. Investig. Dermatol. 2005, 124, 931–938. [Google Scholar] [CrossRef]
- Yu, Y.; Champer, J.; Agak, G.W.; Kao, S.; Modlin, R.L.; Kim, J. Different Propionibacterium acnes phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J. Investig. Dermatol. 2016, 136, 2221–2228. [Google Scholar] [CrossRef]
- Kim, J.; Ochoa, M.-T.; Krutzik, S.R.; Takeuchi, O.; Uematsu, S.; Legaspi, A.J.; Brightbill, H.D.; Holland, D.; Cunliffe, W.J.; Akira, S.; et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 2002, 169, 1535–1541. [Google Scholar] [CrossRef]
- Tanabe, T.; Ishige, I.; Suzuki, Y.; Aita, Y.; Furukawa, A.; Ishige, Y.; Uchida, K.; Suzuki, T.; Takemura, T.; Ikushima, S.; et al. Sarcoidosis and NOD1 variation with impaired recognition of intracellular Propionibacterium acnes. Biochim. Biophys. Acta 2006, 1762, 794–801. [Google Scholar] [CrossRef]
- Fischer, N.; Mak, T.N.; Shinohara, D.B.; Sfanos, K.S.; Meyer, T.F.; Brüggemann, H. Deciphering the intracellular fate of Propionibacterium acnes in macrophages. BioMed Res. Int. 2013, 2013, 603046. [Google Scholar] [CrossRef]
- Yamamoto, K.; Uchida, K.; Furukawa, A.; Tamura, T.; Ishige, Y.; Negi, M.; Kobayashi, D.; Ito, T.; Kakegawa, T.; Hebisawa, A.; et al. Catalase expression of Propionibacterium acnes may contribute to intracellular persistence of the bacterium in sinus macrophages of lymph nodes affected by sarcoidosis. Immunol. Res. 2019, 67, 182–193. [Google Scholar] [CrossRef]
- Mussalem, J.S.; Vasconcelos, J.R.C.; Squaiella, C.C.; Ananias, R.Z.; Braga, E.G.; Rodrigues, M.M.; Longo-Maugéri, I.M. Adjuvant effect of the Propionibacterium acnes and its purified soluble polysaccharide on the immunization with plasmidial DNA containing a Trypanosoma cruzi gene. Microbiol. Immunol. 2006, 50, 253–263. [Google Scholar] [CrossRef]
- Mouser, P.E.; Seaton, E.D.; Chu, A.C.; Baker, B.S. Propionibacterium acnes-reactive T helper-1 cells in the skin of patients with acne vulgaris. J. Investig. Dermatol. 2003, 121, 1226–1228. [Google Scholar] [CrossRef]
- Kistowska, M.; Meier, B.; Proust, T.; Feldmeyer, L.; Cozzio, A.; Kuendig, T.; Contassot, E.; French, L.E. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J. Investig. Dermatol. 2015, 135, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [PubMed]
- Agak, G.W.; Qin, M.; Nobe, J.; Kim, M.-H.; Krutzik, S.R.; Tristan, G.R.; Elashoff, D.; Garbán, H.J.; Kim, J. Propionibacterium acnes induces an IL-17 response in Acne vulgaris that is regulated by vitamin A and vitamin D. J. Investig. Dermatol. 2014, 134, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Agak, G.W.; Kao, S.; Ouyang, K.; Qin, M.; Moon, D.; Butt, A.; Kim, J. Phenotype and antimicrobial activity of Th17 cells induced by Propionibacterium acnes strains associated with healthy and acne skin. J. Investig. Dermatol. 2018, 138, 316–324. [Google Scholar] [CrossRef]
- Kelhälä, H.-L.; Palatsi, R.; Fyhrquist, N.; Lehtimäki, S.; Väyrynen, J.; Kallioinen, M.; Kubin, M.E.; Greco, D.; Tasanen, K.; Alenius, H.; et al. IL-17/Th17 pathway is activated in acne lesions. PLoS ONE 2014, 9, e105238. [Google Scholar] [CrossRef]
- Eishi, Y. Etiologic link between sarcoidosis and Propionibacterium acnes. Respir. Investig. 2013, 51, 56–68. [Google Scholar] [CrossRef]
- Eishi, Y.; Suga, M.; Ishige, I.; Kobayashi, D.; Yamada, T.; Takemura, T.; Takizawa, T.; Koike, M.; Kudoh, S.; Costabel, U.; et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J. Clin. Microbiol. 2002, 40, 198–204. [Google Scholar] [CrossRef]
- Costabel, U.; Hunninghake, G.W. ATS/ERS/WASOG statement on sarcoidosis. Eur. Respir. J. 1999, 14, 735–737. [Google Scholar] [CrossRef]
- Pagán, A.J.; Ramakrishnan, L. The formation and function of granulomas. Annu. Rev. Immunol. 2018, 36, 639–665. [Google Scholar] [CrossRef]
- Nishiwaki, T.; Yoneyama, H.; Eishi, Y.; Matsuo, N.; Tatsumi, K.; Kimura, H.; Kuriyama, T.; Matsushima, K. Indigenous pulmonary Propionibacterium acnes primes the host in the development of sarcoid-like pulmonary granulomatosis in mice. Am. J. Pathol. 2004, 165, 631–639. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Costabel, U.; McDowell, A.; Guzman, J.; Uchida, K.; Ohashi, K.; Eishi, Y. Immunohistochemical detection of potential microbial antigens in granulomas in the diagnosis of sarcoidosis. J. Clin. Med. 2021, 10, 983. [Google Scholar] [CrossRef]
- Hawkins, C.; Shaginurova, G.; Shelton, D.A.; Herazo-Maya, J.D.; Oswald-Richter, K.A.; Rotsinger, J.E.; Young, A.; Celada, L.J.; Kaminski, N.; Sevin, C.; et al. Local and systemic CD4+ T cell exhaustion reverses with clinical resolution of pulmonary sarcoidosis. J. Immunol. Res. 2017, 2017, 3642832. [Google Scholar] [CrossRef]
- Rotsinger, J.E.; Celada, L.J.; Polosukhin, V.V.; Atkinson, J.B.; Drake, W.P. Molecular analysis of sarcoidosis granulomas reveals antimicrobial targets. Am. J. Respir. Cell Mol. Biol. 2016, 55, 128–134. [Google Scholar] [CrossRef]
- Chen, E.S.; Wahlström, J.; Song, Z.; Willett, M.H.; Wikén, M.; Yung, R.C.; West, E.E.; McDyer, J.F.; Zhang, Y.; Eklund, A.; et al. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J. Immunol. 2014, 181, 8784–8796. [Google Scholar] [CrossRef]
- Oswald-Richter, K.; Sato, H.; Hajizadeh, R.; Shepherd, B.E.; Sidney, J.; Sette, A.; Newman, L.S.; Drake, W.P. Mycobacterial ESAT-6 and katG are recognized by sarcoidosis CD4+ T cells when presented by the American sarcoidosis susceptibility allele, DRB1*1101. J. Clin. Immunol. 2010, 30, 157–166. [Google Scholar] [CrossRef]
- Suzuki, Y.; Uchida, K.; Takemura, T.; Sekine, M.; Tamura, T.; Furukawa, A.; Hebisawa, A.; Sakakibara, Y.; Awano, N.; Amano, T.; et al. Propionibacterium acnes-derived insoluble immune complexes in sinus macrophages of lymph nodes affected by sarcoidosis. PLoS ONE 2018, 13, e0192408. [Google Scholar] [CrossRef]
- Negi, M.; Takemura, T.; Guzman, J.; Uchida, K.; Furukawa, A.; Suzuki, Y.; Iida, T.; Ishige, I.; Minami, J.; Yamada, T.; et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod. Pathol. 2012, 25, 1284–1297. [Google Scholar] [CrossRef]
- Ishihara, M.; Ohno, S.; Ono, H.; Isogai, E.; Kimura, K.; Isogai, H.; Aoki, K.; Ishida, T.; Suzuki, K.; Kotake, S.; et al. Seroprevalence of anti-Borrelia antibodies among patients with confirmed sarcoidosis in a region of Japan where Lyme borreliosis is endemic. Graefe’s Arch. Clin. Exp. Ophthalmol. 1998, 236, 280–284. [Google Scholar] [CrossRef]
- Clarke, E.L.; Lauder, A.P.; Hofstaedter, C.E.; Hwang, Y.; Fitzgerald, A.S.; Imai, I.; Biernat, W.; Rękawiecki, B.; Majewska, H.; Dubaniewicz, A.; et al. Microbial lineages in sarcoidosis. A metagenomic analysis tailored for low–microbial content samples. Am. J. Respir. Crit. Care Med. 2018, 197, 225–234. [Google Scholar] [CrossRef]
- Terčelj, M.; Stopinšek, S.; Ihan, A.; Salobir, B.; Simčič, S.; Wraber, B.; Rylander, R. In vitro and in vivo reactivity to fungal cell wall agents in sarcoidosis. Clin. Exp. Immunol. 2011, 166, 87–93. [Google Scholar] [CrossRef]
- Suchankova, M.; Paulovicova, E.; Majer, I.; Tedlova, E.; Novosadova, H.; Tibenska, E.; Tedla, M.; Bucova, M. Increased antifungal antibodies in bronchoalveolar lavage fluid and serum in pulmonary sarcoidosis. Scand. J. Immunol. 2015, 81, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Greaves, S.A.; Ravindran, A.; Santos, R.G.; Chen, L.; Falta, M.T.; Wang, Y.; Mitchell, A.M.; Atif, S.M.; Mack, D.G.; Tinega, A.N.; et al. CD4+ T cells in the lungs of acute sarcoidosis patients recognize an Aspergillus nidulans epitope. J. Exp. Med. 2021, 218, e20210785. [Google Scholar] [CrossRef]
- Lim, C.X.; Weichhart, T. A fungal antigenic driver for Löfgren’s syndrome sarcoidosis. J. Exp. Med. 2021, 218, e20211572. [Google Scholar] [CrossRef]
- Beijer, E.; Meek, B.; Bossuyt, X.; Peters, S.; Vermeulen, R.C.H.; Kromhout, H.; Veltkamp, M. Immunoreactivity to metal and silica associates with sarcoidosis in Dutch patients. Respir. Res. 2020, 21, 141. [Google Scholar] [CrossRef]
- Fireman, E.; Bar Shai, A.; Alcalay, Y.; Ophir, N.; Kivity, S.; Stejskal, V. Identification of metal sensitization in sarcoid-like metal-exposed patients by the MELISA® lymphocyte proliferation test—A pilot study. J. Occup. Med. Toxicol. 2016, 11, 18. [Google Scholar] [CrossRef]
- Li, L.; Silveira, L.J.; Hamzeh, N.; Gillespie, M.; Mroz, P.M.; Mayer, A.S.; Fingerlin, T.E.; Maier, L.A. Beryllium-induced lung disease exhibits expression profiles similar to sarcoidosis. Eur. Respir. J. 2016, 47, 1797–1808. [Google Scholar] [CrossRef]
- Rossman, M.D.; Kreider, M.E. Is chronic beryllium disease sarcoidosis of known etiology? Sarcoidosis Vasc. Diffus. Lung Dis. 2003, 20, 104–109. [Google Scholar]
- Beijer, E.; Kraaijvanger, R.; Roodenburg, C.; Grutters, J.C.; Meek, B.; Veltkamp, M. Simultaneous testing of immunological sensitization to multiple antigens in sarcoidosis reveals an association with inorganic antigens specifically related to a fibrotic phenotype. Clin. Exp. Immunol. 2020, 203, 115–124. [Google Scholar] [CrossRef]
- Drent, M.; Bomans, P.; Van Suylen, R.; Lamers, R.; Bast, A.; Wouters, E. Association of man-made mineral fibre exposure and sarcoidlike granulomas. Respir. Med. 2000, 94, 815–820. [Google Scholar] [CrossRef]
- Üzmezoǧlu, B.; Şimşek, C.; Gülgösteren, S.; Gebeşoǧlu, B.; Sari, G.; Çelik, D. Sarcoidosis in iron-steel industry: Mini case series. Sarcoidosis Vasc. Diffus. Lung Dis 2017, 34, 365–372. [Google Scholar]
- Newman, L.S. Metals that cause sarcoidosis. Semin. Respir. Infect. 1998, 13, 212–220. [Google Scholar] [PubMed]
- Tomioka, H.; Kaneda, T.; Katsuyama, E.; Kitaichi, M.; Moriyama, H.; Suzuki, E. Elemental analysis of occupational granulomatous lung disease by electron probe microanalyzer with wavelength dispersive spectrometer: Two case reports. Respir. Med. Case Rep. 2016, 18, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Prezant, D.J.; Dhala, A.; Goldstein, A.; Janus, D.; Ortiz, F.; Aldrich, T.K.; Kelly, K.J. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest 1999, 116, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Kern, D.G.; Neill, M.A.; Wrenn, D.S.; Varone, J.C. Investigation of a unique time-space cluster of sarcoidosis in firefighters. Am. Rev. Respir. Dis. 1993, 148, 974–980. [Google Scholar] [CrossRef]
- Kajdasz, D.K.; Lackland, D.T.; Mohr, L.C.; Judson, M.A. A current assessment of rurally linked exposures as potential risk factors for sarcoidosis. Ann. Epidemiol. 2001, 11, 111–117. [Google Scholar] [CrossRef]
- Grunewald, J.; Kaiser, Y.; Ostadkarampour, M.; Rivera, N.V.; Vezzi, F.; Lötstedt, B.; Olsen, R.-A.; Sylwan, L.; Lundin, S.; Käller, M.; et al. T-cell receptor–HLA-DRB1 associations suggest specific antigens in pulmonary sarcoidosis. Eur. Respir. J. 2016, 47, 898–909. [Google Scholar] [CrossRef]
- Wahlström, J.; Dengjel, J.; Winqvist, O.; Targoff, I.; Persson, B.; Duyar, H.; Rammensee, H.-G.; Eklund, A.; Weissert, R.; Grunewald, J. Autoimmune T cell responses to antigenic peptides presented by bronchoalveolar lavage cell HLA-DR molecules in sarcoidosis. Clin. Immunol. 2009, 133, 353–363. [Google Scholar] [CrossRef]
- Wahlström, J.; Dengjel, J.; Persson, B.; Duyar, H.; Rammensee, H.-G.; Stevanoviδc, S.; Eklund, A.; Weissert, R.; Grunewald, J. Identification of HLA-DR–bound peptides presented by human bronchoalveolar lavage cells in sarcoidosis. J. Clin. Investig. 2007, 117, 3576–3582. [Google Scholar] [CrossRef]
- De Brouwer, B.; Veltkamp, M.; Wauters, C.A.; Grutters, J.C.; Janssen, R. Propionibacterium acnes isolated from lymph nodes of patients with sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis 2015, 32, 271–274. [Google Scholar]
- Ishige, I.; Eishi, Y.; Takemura, T.; Kobayashi, I.; Nakata, K.; Tanaka, I.; Nagaoka, S.; Iwai, K.; Watanabe, K.; Takizawa, T.; et al. Propionibacterium acnes is the most common bacterium commensal in peripheral lung tissue and mediastinal lymph nodes from subjects without sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2005, 22, 33–42. [Google Scholar]
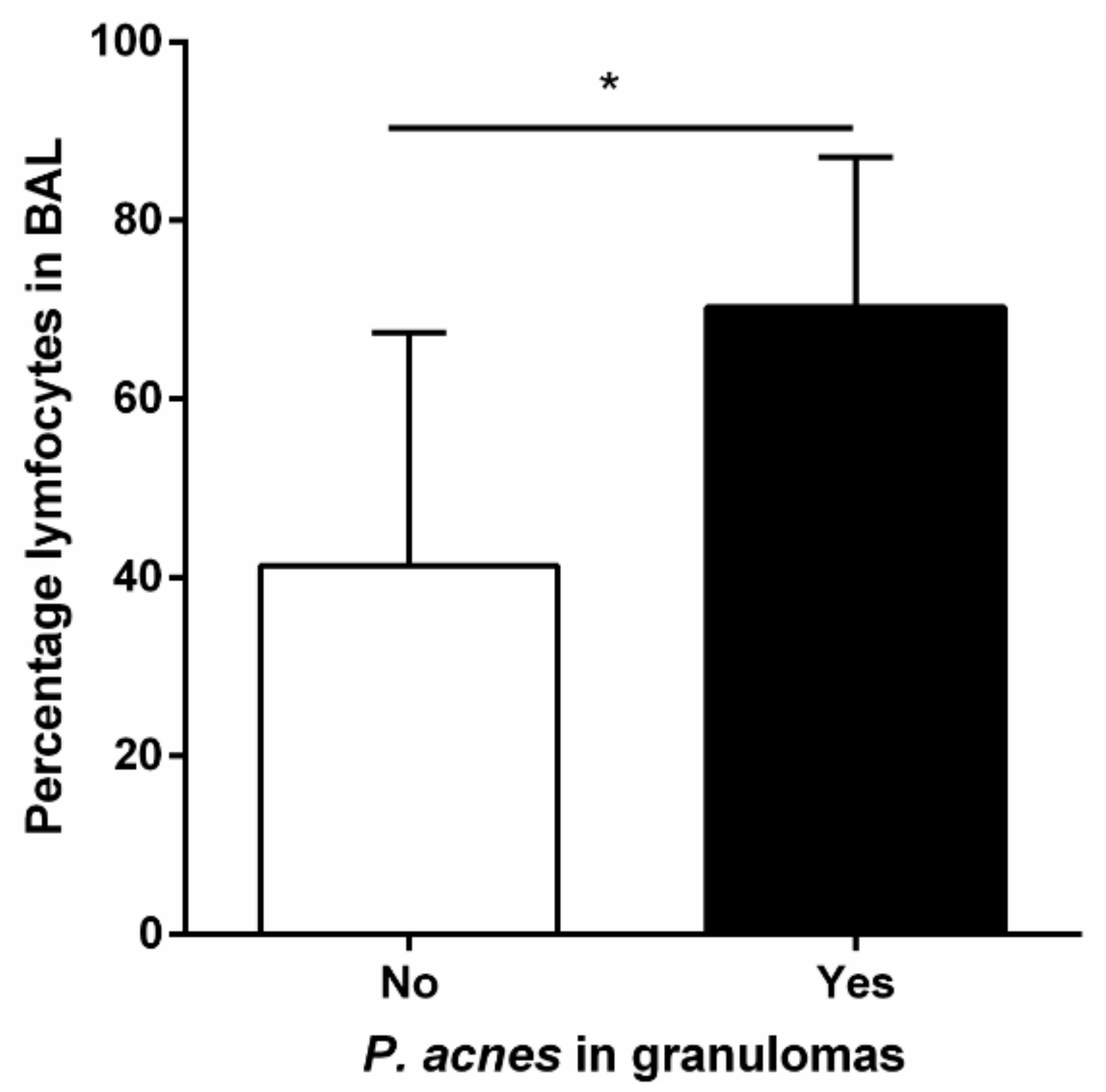
- Beijer, E.; Seldenrijk, K.; Eishi, Y.; Uchida, K.; Damen, J.; Grutters, J.C.; Veltkamp, M. Presence of Propionibacterium acnes in granulomas associates with a chronic disease course in Dutch sarcoidosis patients. ERJ Open Res. 2021, 7, 00486–02020. [Google Scholar] [CrossRef]
- Isshiki, T.; Matsuyama, H.; Sakamoto, S.; Honma, N.; Mikami, T.; Shibuya, K.; Eishi, Y.; Homma, S. Development of Propionibacterium acnes-associated sarcoidosis during etanercept therapy. Intern. Med. 2019, 58, 1473–1477. [Google Scholar] [CrossRef]
- Suzuki, T.; Fujita, A.; Takamori, M.; Murata, K.; Wada, A.; Miyamoto, M.; Yamamoto, Y.; Sakashita, K.; Tada, Y.; Suzuki, Y.; et al. Implication of immunohistochemistry for propionibacterium acnes in differential diagnosis of necrotizing granuloma. J. Pulm. Respir. Med. 2016, 6, 2–4. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Ishii, H.; Eishi, Y.; Uchida, K.; Yoshimura, M.; Iwasaki, A.; Fujita, M.; Nabeshima, K.; Watanabe, K. Histological differences between sarcoidosis and lung cancer-related sarcoid reaction. Respir. Investig. 2020, 58, 421–424. [Google Scholar] [CrossRef]
- Asakawa, N.; Uchida, K.; Sakakibara, M.; Omote, K.; Noguchi, K.; Tokuda, Y.; Kamiya, K.; Hatanaka, K.C.; Matsuno, Y.; Yamada, S.; et al. Immunohistochemical identification of Propionibacterium acnes in granuloma and inflammatory cells of myocardial tissues obtained from cardiac sarcoidosis patients. PLoS ONE 2017, 12, e0179980. [Google Scholar] [CrossRef]
- Ishige, I.; Usui, Y.; Takemura, T.; Eishi, Y.; Suga, M.; Ishige, I.; Kobayashi, D.; Yamada, T.; Takemura, T.; Takizawa, T.; et al. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet 1999, 354, 198–204. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Y.; Li, H. Role of Propionibacterium acnes in sarcoidosis: A meta-analysis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2013, 30, 262–267. [Google Scholar]
- Schupp, J.C.; Tchaptchet, S.; Lützen, N.; Engelhard, P.; Müller-Quernheim, J.; Freudenberg, M.A.; Prasse, A. Immune response to Propionibacterium acnes in patients with sarcoidosis—In vivo and in vitro. BMC Pulm. Med. 2015, 15, 75. [Google Scholar] [CrossRef]
- Vandenplas, O.; Depelchin, S.; Delaunois, L.; Delwiche, J.P.; Sibille, Y. Bronchoalveolar lavage immunoglobulin A and G and antiproteases correlate with changes in diffusion indices during the natural course of pulmonary sarcoidosis. Eur. Respir. J. 1994, 7, 1856–1864. [Google Scholar] [CrossRef]
- Furusawa, H.; Suzuki, Y.; Miyazaki, Y.; Inase, N.; Eishi, Y. Th1 and Th17 immune responses to viable Propionibacterium acnes in patients with sarcoidosis. Respir. Investig. 2012, 50, 104–109. [Google Scholar] [CrossRef]
- Yorozu, P.; Furukawa, A.; Uchida, K.; Akashi, T.; Kakegawa, T.; Ogawa, T.; Minami, J.; Suzuki, Y.; Awano, N.; Furusawa, H.; et al. Propionibacterium acnes catalase induces increased Th1 immune response in sarcoidosis patients. Respir. Investig. 2015, 53, 161–169. [Google Scholar] [CrossRef]
- Ebe, Y.; Ikushima, S.; Yamaguchi, T.; Kohno, K.; Azuma, A.; Sato, K.; Ishige, I.; Usui, Y.; Takemura, T.; Eishi, Y. Proliferative response of peripheral blood mononuclear cells and levels of antibody to recombinant protein from Propionibacterium acnes DNA expression library in Japanese patients with sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2000, 17, 256–265. [Google Scholar]
- Yamane, H.; Tachibana, I.; Takeda, Y.; Saito, Y.; Tamura, Y.; He, P.; Suzuki, M.; Shima, Y.; Yoneda, T.; Hoshino, S.; et al. Propionibacterium acnes-induced hepatic granuloma formation is impaired in mice lacking tetraspanin CD9. J. Pathol. 2005, 206, 486–492. [Google Scholar] [CrossRef]
- Beijer, E.; Seldenrijk, K.; Meek, B.; Damen, J.; Quanjel, M.J.R.; Grutters, J.C.; Veltkamp, M. Detection of Cutibacterium acnes in granulomas of patients with either hypersensitivity pneumonitis or vasculitis reveals that its presence is not unique for sarcoidosis. ERJ Open Res. 2021, 7, 00930-2020. [Google Scholar] [CrossRef]
- Khan, N.A.; Donatelli, C.V.; Tonelli, A.R.; Wiesen, J.; Neto, M.L.R.; Sahoo, D.; Culver, D.A. Toxicity risk from glucocorticoids in sarcoidosis patients. Respir. Med. 2017, 132, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Judson, M.A.; Chaudhry, H.; Louis, A.; Lee, K.; Yucel, R. The effect of corticosteroids on quality of life in a sarcoidosis clinic: The results of a propensity analysis. Respir. Med. 2015, 109, 526–531. [Google Scholar] [CrossRef]
- Gottlieb, J.E.; Israel, H.L.; Steiner, R.M.; Triolo, J.; Patrick, H. Outcome in sarcoidosis. Chest 1997, 111, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Minami, J.; Eishi, Y.; Ishige, Y.; Kobayashi, I.; Ishige, I.; Kobayashi, D.; Ando, N.; Uchida, K.; Ikeda, S.; Sorimachi, N.; et al. Pulmonary granulomas caused experimentally in mice by a recombinant trigger-factor protein of Propionibacterium acnes. J. Med. Dent. Sci. 2003, 50, 265–274. [Google Scholar]
- Miyazaki, E.; Ando, M.; Fukami, T.; Nureki, S.I.; Eishi, Y.; Kumamoto, T. Minocycline for the treatment of sarcoidosis: Is the mechanism of action immunomodulating or antimicrobial effect? Clin. Rheumatol. 2008, 27, 1195–1197. [Google Scholar] [CrossRef]
- Takemori, N.; Nakamura, M.; Kojima, M.; Eishi, Y. Successful treatment in a case of Propionibacterium acnes-associated sarcoidosis with clarithromycin administration: A case report. J. Med. Case Rep. 2014, 8, 15. [Google Scholar] [CrossRef]
- Ishibashi, K.; Eishi, Y.; Tahara, N.; Asakura, M.; Sakamoto, N.; Nakamura, K.; Takaya, Y.; Nakamura, T.; Yazaki, Y.; Yamaguchi, T.; et al. Japanese antibacterial drug management for cardiac sarcoidosis (J-ACNES): A multicenter, open-label, randomized, controlled study. J. Arrhythmia 2018, 34, 520–526. [Google Scholar] [CrossRef] [PubMed]

| Possible Antigens | Literature (References Are in Parentheses) |
|---|---|
| Infectious agents | |
| Mycobacterium tuberculosis | [72,73,74,75] |
| Cutibacterium acnes | [76,77] |
| Borrelia | [78] |
| Fungi | [79,80,81] |
| Aspergillus nidulans | [82,83] |
| Non-infectious environmental antigens | |
| Beryllium | [84,85,86,87,88] |
| Aluminum | [84,88,89] |
| Silica | [85,88,89,90] |
| copper | [91] |
| titanium | [85,89,91,92] |
| Combustible wood | [93,94,95] |
| Autoantigens | |
| vimentin | [96,97,98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraaijvanger, R.; Veltkamp, M. The Role of Cutibacterium acnes in Sarcoidosis: From Antigen to Treatable Trait? Microorganisms 2022, 10, 1649. https://doi.org/10.3390/microorganisms10081649
Kraaijvanger R, Veltkamp M. The Role of Cutibacterium acnes in Sarcoidosis: From Antigen to Treatable Trait? Microorganisms. 2022; 10(8):1649. https://doi.org/10.3390/microorganisms10081649
Chicago/Turabian StyleKraaijvanger, Raisa, and Marcel Veltkamp. 2022. "The Role of Cutibacterium acnes in Sarcoidosis: From Antigen to Treatable Trait?" Microorganisms 10, no. 8: 1649. https://doi.org/10.3390/microorganisms10081649
APA StyleKraaijvanger, R., & Veltkamp, M. (2022). The Role of Cutibacterium acnes in Sarcoidosis: From Antigen to Treatable Trait? Microorganisms, 10(8), 1649. https://doi.org/10.3390/microorganisms10081649






