The Impact of Antimicrobial Stewardship Training on Calf Producers’ Knowledge, Treatment Behaviors and Quantified Antimicrobial Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. The Intervention
2.4. Knowledge Assessment
2.5. Assessment of Treatment Accuracy
2.6. Quantification of Antimicrobial Use
2.7. Statistical Analysis
3. Results
3.1. Changes in Knowledge
3.2. Differences in Treatment Accuracy among Intervention and Control Farms
3.3. Differences in Quantified Antimicrobial Use
3.4. Production Information among Calf Cohorts
4. Discussion
4.1. Improvements in Knowledge of Antimicrobial Stewardship
4.2. Changes in Diagnostic Sensitivity and Specificity among Calf Personnel
4.3. Reduction in Quantified Antimicrobial Use
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FDA. The Judicious Use of Medically Important Antimicrobial Drugs in Food-Producing Animals; US Department of Health and Human Services: Rockville, MD, USA, 2010; Volume 6, pp. 1–19.
- Habing, G.; Djordjevic, C.; Schuenemann, G.M.; Lakritz, J. Understanding antimicrobial stewardship: Disease severity treatment thresholds and antimicrobial alternatives among organic and conventional calf producers. Prev. Vet. Med. 2016, 130, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosman, A.B.; Wagenaar, J.; Stegeman, A.; Vernooij, J.; Mevius, D. Quantifying Antimicrobial Resistance at Veal Calf Farms. PLoS ONE 2012, 7, e44831. [Google Scholar] [CrossRef] [Green Version]
- Creutzinger, K.; Pempek, J.; Habing, G.; Proudfoot, K.; Locke, S.; Wilson, D.; Renaud, D. Perspectives on the Management of Surplus Dairy Calves in the United States and Canada. Front. Vet. Sci. 2021, 8, 661453. [Google Scholar] [CrossRef]
- Shivley, C.; Lombard, J.; Urie, N.; Weary, D.; Von Keyserlingk, M. Management of preweaned bull calves on dairy operations in the United States. J. Dairy Sci. 2019, 102, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, H.; Finney, S.; Muñoz-Vargas, L.; Feicht, S.; Masterson, M.; Habing, G. Prevalence and Transmission of Antimicrobial Resistance in a Vertically Integrated Veal Calf Production System. Foodborne Pathog. Dis. 2017, 14, 711–718. [Google Scholar] [CrossRef]
- Tate, H.; Li, C.; Nyirabahizi, E.; Tyson, G.H.; Zhao, S.; Rice-Trujillo, C.; Jones, S.B.; Ayers, S.; M’Ikanatha, N.M.; Hanna, S.; et al. A National Antimicrobial Resistance Monitoring System Survey of Antimicrobial-Resistant Foodborne Bacteria Isolated from Retail Veal in the United States. J. Food Prot. 2021, 84, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Zou, G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Critically Important Antimicrobials for Human Medicine; 3rd Revision; World Health Organization: Geneva, Switzerland, 2012.
- Wu, C.-T.; Chen, C.-L.; Lee, H.-Y.; Chang, C.-J.; Liu, P.-Y.; Li, C.-Y.; Liu, M.-Y.; Liu, C.-H. Decreased antimicrobial resistance and defined daily doses after implementation of a clinical culture-guided antimicrobial stewardship program in a local hospital. J. Microbiol. Immunol. Infect. 2017, 50, 846–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Bergh, D.; Messina, A.P.; Goff, D.A.; van Jaarsveld, A.; Coetzee, R.; de Wet, Y.; Bronkhorst, E.; Brink, A.; Mendelson, M.; Richards, G.A.; et al. A pharmacist-led prospective antibiotic stewardship intervention improves compliance to community-acquired pneumonia guidelines in 39 public and private hospitals across South Africa. Int. J. Antimicrob. Agents 2020, 56, 106189. [Google Scholar] [CrossRef]
- Smith, R. Veterinary Clinical Epidemiology, 3rd ed.; Taylor and Francis: Abingdon, UK, 2006. [Google Scholar]
- Maier, G.U.; Rowe, J.D.; Lehenbauer, T.W.; Karle, B.M.; Williams, D.R.; Champagne, J.D.; Aly, S.S. Development of a clinical scoring system for bovine respiratory disease in weaned dairy calves. J. Dairy Sci. 2019, 102, 7329–7344. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; McClure, J.; Léger, D.; Dufour, S.; Sheldon, A.; Scholl, D.; Barkema, H. Antimicrobial use on Canadian dairy farms. J. Dairy Sci. 2012, 95, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Pol, M.; Ruegg, P. Relationship Between Antimicrobial Drug Usage and Antimicrobial Susceptibility of Gram-Positive Mastitis Pathogens. J. Dairy Sci. 2007, 90, 262–273. [Google Scholar] [CrossRef]
- Cheng, T.; Almeida, B.G.; Pempek, J.A.; Masterson, M.A.; Habing, G.G. The use of common antimicrobial agents in US veal calves. Zoonoses Public Health 2022, 69, 359–369. [Google Scholar] [CrossRef]
- Lardé, H.; Francoz, D.; Roy, J.-P.; Massé, J.; Archambault, M.; Paradis, M.; Dufour, S. Comparison of Quantification Methods to Estimate Farm-Level Usage of Antimicrobials Other than in Medicated Feed in Dairy Farms from Québec, Canada. Microorganisms 2021, 9, 1106. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, D.B.; De Buck, J.; Naqvi, S.A.; Liu, G.; Naushad, S.; Saini, V.; Barkema, H.W. Comparison of treatment records and inventory of empty drug containers to quantify antimicrobial usage in dairy herds. J. Dairy Sci. 2017, 100, 9736–9745. [Google Scholar] [CrossRef] [PubMed]
- Jarrige, N.; Cazeau, G.; Morignat, E.; Chanteperdrix, M.; Gay, E. Quantitative and qualitative analysis of antimicrobial usage in white veal calves in France. Prev. Vet. Med. 2017, 144, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Bokma, J.; Boone, R.; Deprez, P.; Pardon, B. Risk factors for antimicrobial use in veal calves and the association with mortality. J. Dairy Sci. 2019, 102, 607–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargeant, J.M.; O’Connor, A.M.; Gardner, I.A.; Dickson, J.S.; Torrence, M.E.; Dohoo, I.R.; Lefebvre, S.L.; Morley, P.S.; Ramirez, A.; Snedeker, K.; et al. The REFLECT Statement: Reporting Guidelines for Randomized Controlled Trials in Livestock and Food Safety: Explanation and Elaboration. Zoonoses Public Health 2010, 57, 105–136. [Google Scholar] [CrossRef] [Green Version]


| Module | Learning Objectives |
|---|---|
| Antibiotics and Antibiotic Resistance |
|
| Calf Health Assessments |
|
| Decision Tree Protocols |
|
| Study Group † | ||
|---|---|---|
| Rearing Periods |
Intervention (Mean TI100 ‡ ± SD §) |
Control (Mean TI100 ‡ ± SD §) |
| Group treatments | ||
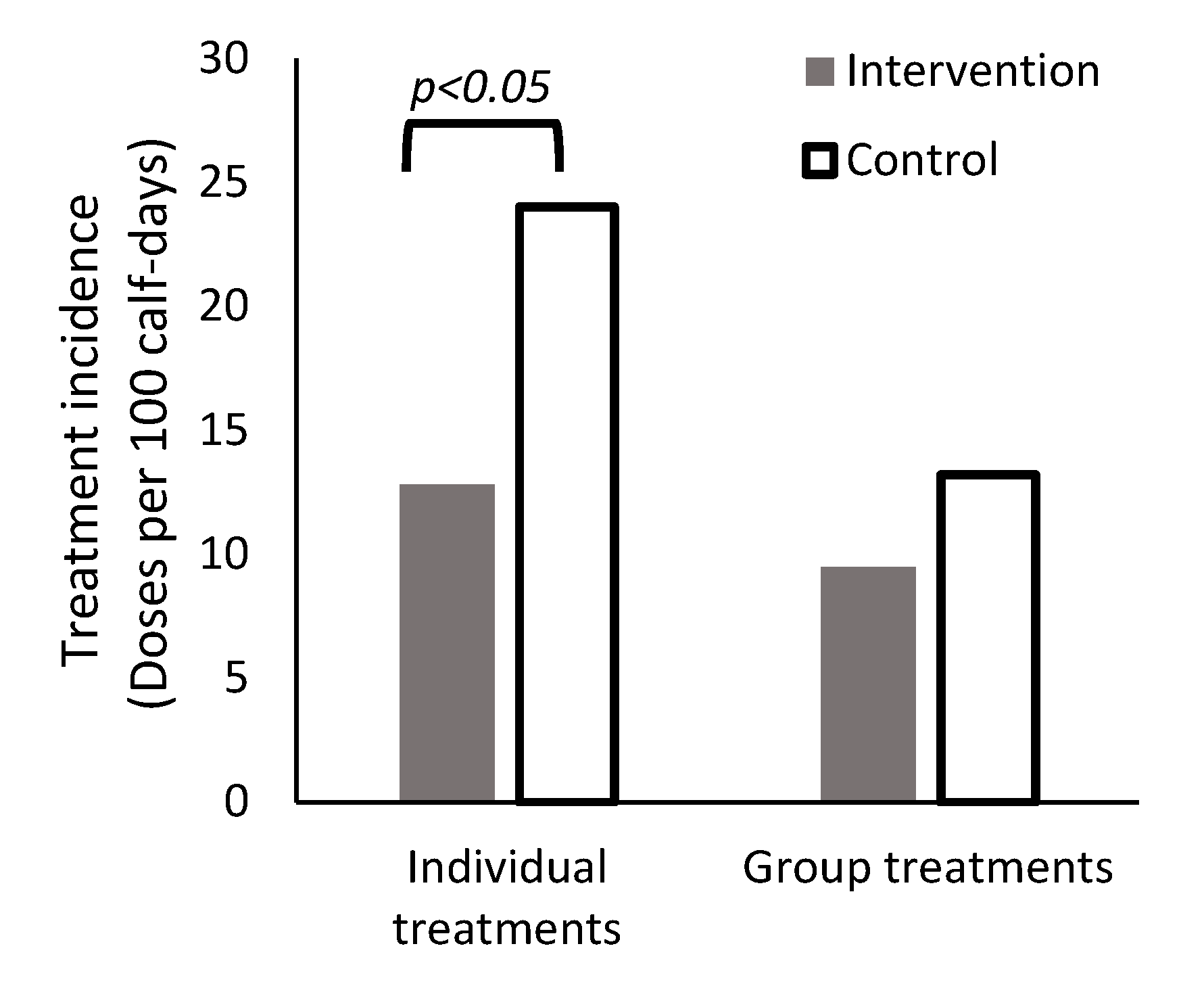
| Entire period | 9.48 ± 3.42 | 13.2 ± 5.06 |
| Day 1–21 | 80.6 ± 40.8 | 64.0 ± 24.3 |
| Day 22–63 | 21.5 ± 9.87 | 41.8 ± 20.5 |
| Day ≥ 64 | 2.65 ± 0.87 | 4.70 ± 4.35 |
| Individual treatments | ||
| Entire period | 12.8 ± 6.71 | 24.0 ± 6.17 |
| Day 1–21 | 101.2 ± 75.1 | 83.8 ± 39.5 |
| Day 22–63 | 29.6 ± 8.90 | 49.8 ± 16.1 |
| Day ≥ 64 | 3.68 ± 2.63 | 7.76 ± 5.21 |
| Study Group † | ||
|---|---|---|
| Antimicrobial Agents |
Intervention (Mean TI100 ‡ ± SD §) |
Control (Mean TI100 ‡ ± SD §) |
| Group treatments | ||
| Amoxicillin | 1.16 ± 0.40 | 1.21 ± 0.50 |
| Chlortetracycline HCl | 5.52 ± 2.05 | 5.01 ± 3.12 |
| Lincomycin–oral ¶ | 0.98 | 1.69 |
| Neomycin | 1.00 ± 0.93 | 3.50 ± 3.25 |
| Penicillin G potassium | 1.04 ± 0.86 | 2.71 ± 1.75 |
| Sulfamethoxazole + Trimethoprim | 0.73 ± 0.30 | 1.72 ± 0.97 |
| Tetracycline hydrochloride ¶ | 1.01 | 0.26 |
| Individual treatments | ||
| Amoxicillin | 2.35 ± 0.59 | 2.68 ± 0.82 |
| Ceftiofur sodium | 0.64 ± 0.37 | 0.23 ± 0.21 |
| Chlortetracycline HCl | 7.06 ± 7.20 | 10.12 ± 2.86 |
| Florfenicol | 0.16 ± 0.06 | 0.17 ± 0.04 |
| Lincomycin–parenteral | 1.03 ± 0.46 | 2.87 ± 4.25 |
| Lincomycin–oral ¶ | 0.03 | 1.65 |
| Neomycin | 1.09 ± 0.92 | 1.21 ± 0.64 |
| Penicillin G potassium | 0.90 ± 0.28 | 2.79 ± 1.12 |
| Penicillin G procaine | 1.93 ± 0.90 | 2.37 ± 0.97 |
| Spectinomycin | 0.14 ± 0.16 | 0.02 ± 0.00 |
| Sulfamethoxazole and trimethoprim | 0.80 ± 0.56 | 1.13 ± 1.05 |
| Tetracycline hydrochloride ¶ | 1.69 | - |
| Tildipirosin | 0.07 ± 0.05 | 0.12 ± 0.12 |
| Tulathromycin | 0.17 ± 0.14 | 0.22 |
| Tylosin | 0.11 ± 0.12 | 0.09 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pempek, J.; Masterson, M.; Portillo-Gonzalez, R.; Creutzinger, K.; Cheng, T.-Y.; Habing, G. The Impact of Antimicrobial Stewardship Training on Calf Producers’ Knowledge, Treatment Behaviors and Quantified Antimicrobial Use. Microorganisms 2022, 10, 1525. https://doi.org/10.3390/microorganisms10081525
Pempek J, Masterson M, Portillo-Gonzalez R, Creutzinger K, Cheng T-Y, Habing G. The Impact of Antimicrobial Stewardship Training on Calf Producers’ Knowledge, Treatment Behaviors and Quantified Antimicrobial Use. Microorganisms. 2022; 10(8):1525. https://doi.org/10.3390/microorganisms10081525
Chicago/Turabian StylePempek, Jessica, Martey Masterson, Rafael Portillo-Gonzalez, Kate Creutzinger, Ting-Yu Cheng, and Greg Habing. 2022. "The Impact of Antimicrobial Stewardship Training on Calf Producers’ Knowledge, Treatment Behaviors and Quantified Antimicrobial Use" Microorganisms 10, no. 8: 1525. https://doi.org/10.3390/microorganisms10081525
APA StylePempek, J., Masterson, M., Portillo-Gonzalez, R., Creutzinger, K., Cheng, T.-Y., & Habing, G. (2022). The Impact of Antimicrobial Stewardship Training on Calf Producers’ Knowledge, Treatment Behaviors and Quantified Antimicrobial Use. Microorganisms, 10(8), 1525. https://doi.org/10.3390/microorganisms10081525






