Abstract
Candida auris is an emerging healthcare-associated infection that can easily cause dissemination in hospitals through colonizing the skin and contaminating environmental surfaces, especially in Intensive Care Units (ICU). Difficulties with identification of this organism, uncertainty about routes of transmission and antifungals resistance have impacted significantly outbreak detection and management. Here, we describe our experience with colonization/infection of C. auris among critically ill patients, admitted to a referral ICU of a University Hospital, in a transitional period (July 2021–March 2022) between management of non-COVID-19 and COVID-19 patients due to the reconversion of the ICU between two waves. A total of 8 patients presented colonization from C. auris, and two of them developed invasive infection from C. auris. The fungal pathogen was cultured from different sites: the skin (7 isolates), urine (2), respiratory tract (1), blood (1). The median time from admission to first detection is 24 days with 100% of patients requiring mechanical ventilation. All 8 patients received broad-spectrum antibiotic therapy for bacterial infections before identification of C. auris; 62.5% of the patients had prior antifungal exposure; 87.5% received steroids; 37.5% patients used immunomodulatory; and 75% had severe COVID-19 illness prior to C. auris identification. Only two cases (25%) were treated with antifungals as C. auris infections (1 patient for suspected UTI; 1 patient with candidemia). Infection control measures, including rapid microbiological identification, contact isolation, screening of contacts, antisepsis of colonized patients, dedicated equipment, cleaning and disinfection of the environment and subsequent follow-up sampling, remain essential in critically ill patients. Our experience highlights the importance of establishing a multidisciplinary model and bundling of practices for preventing C. auris’ spread.
1. Introduction
Since its first description in 2009, Candida auris has been a serious public health threat. Invasive Fungal Infection (IFI) caused by this species has been described in more than 40 countries [1]. Due to its high multidrug resistance [1], transmissibility and long persistence in the hospital environments [2,3,4], it is considered a serious global threat causing outbreaks and deep-seated infections [5].
C. auris combines all the essential characteristics for a pathogen to pose a threat to public health: potential to spread through horizontal transmission; ability to cause serious and life-threatening infections; multi-resistance profile and limitations for optimal treatment [1].
Nowadays, little evidence on its pathogenicity and the complex host–pathogen interactions is available [1]. Progress in its identification with definite diagnostic molecular or spectrometry tools is crucial but is not equally available in hospitals and countries.
Several risk factors were related to the development of infection, especially in previous colonized patients, and treatment options remain really scant [1]. In 2019, the Center for Disease Control and Prevention of the United States (CDC) considered C. auris infection an urgent threat for international public health in the field of multidrug resistant microorganisms [6]. New epidemiological alerts have been released in view of the increase in healthcare-associated C. auris cases in the context of the COVID-19 pandemic, worldwide and in Italy [7,8,9,10].
The latest ECDC survey reports cases of C. auris in 9 countries in the EU/EEA, including Italy, where an outbreak in Liguria (North-West Italy) is still ongoing [11].
In a worldwide retrospective study of clinical characteristics of C. auris, there was a higher proportion of men, premature babies and elderly people [12]. The proportions of patients with underlying diseases such as diabetes, kidney disease, trauma and ear disease were also high. More than half of patients had a history of central venous catheter use and a history of broad-spectrum antibiotic use. As previously said, recently, a sharp rise in new cases of C. auris colonization and infection has been reported, especially during the ongoing COVID-19 pandemic probably due to the increased vulnerability of SARS-CoV-2-infected patients with severe respiratory and immune damage [13].
Several diagnostic and therapeutic challenges have been reported with C. auris: first, C. auris may be misidentified by conventional phenotypic methods; second, most of th isolates are resistant to fluconazole, a subset of the C. auris strain that has high minimum inhibitory concentrations (MICs) to amphotericin B and echinocandins, and some C. auris isolates are resistant to all antifungal classes [14]. In terms of treatment, it constitutes the only fungal species able to be resistant to azoles, amphotericin B and echinocandins, although clinical data seem to suggest a more differentiated pattern of antifungal susceptibility [5,14].
From an infection-control perspective, daily cleaning and disinfection are recommended for patients’ rooms because C. auris persists on surfaces. The Center for Disease Control and Prevention (CDC) recommends using an Environmental Protection Agency (EPA)-registered hospital-grade disinfectant that is active against C. auris listed on List P [15]. Moreover, screening contacts of identified cases for C. auris is essential to contain the organism’s spreading.
We report here the first cases of C. auris in our ICU, among critically ill patients, in a transitional period between management of non-COVID-19 and COVID-19 patients, focusing on clinical characteristics of eight ICU cases, microbiological detection methods, infection control procedures and mortality rates.
2. Materials and Methods
We describe eight cases of colonization or infection caused by C. auris observed between July 2021 and March 2022 in an 8-bed intensive care unit of a 1200-bed academic hospital with primary and secondary referral (AOU Città della Salute e della Scienza, Turin, Italy).
Surveillance cultures (urine culture, tracheal aspirate, rectal swab) are performed weekly. C. auris was not routinely sought, except for patients with previous contiguity with infected/colonized cases. In those cases, surveillance cultures (urine culture, tracheal aspirate, rectal swab) were performed weekly. The study was approved by the Local Ethical Committee (Prot.n. 0008191).
2.1. Environmental Sampling
SRK Copan swabs (Brescia, Italy) were used for environmental sampling. Samples from healthcare workers’ hands were collected after contacting an infected or colonized patient. Twenty swabs from the hands, one from a cell phone and three from hands with gloves were collected.
Surface swabs were collected to evaluate the efficacy of cleaning interventions. Forty-two samples were obtained from high-touch surfaces such as doorknobs, light switches, keyboards, screens, difficult-to-disinfect sites such as and machine-equipped zones. To avoid false results related to health-care workers, samples were collected during 3 different working days.
The swab was rotated between the thumb and forefinger during the sweeping action to maximize the uptake of the surface material. The samples were transported to the laboratory for analysis within 2 h in a cool box at 1–4 °C. The samples had the possibility to be refrigerated at 2–8 °C for up to 24 h before laboratory analysis. In the laboratory, the swab was mixed using the vortex to release sample material and make an even suspension before the culture.
2.2. Microbiological Detection
Culture-based approaches remain the mainstay of the laboratory diagnosis of C. auris. Candida isolates from clinical swabs were plated on BD Sabouraud Agar with gentamicin and chloramphenicol agar plates (Becton Dickinson GmbH, Heidelberg, Germany) and identified using chromogenic agar with five days at 37 °C implemented for the incubation protocol (Brilliance Candida Agar, Thermo Scientific, Basingstoke, UK). Non-C.albicans isolates including C. auris were identified to the species level by MALDI-TOF (Bruker, Bremen, Germany) using the Biotyper v4.1.100 software. MIC determination was conducted by microbroth dilution according to the standard EUCAST with the commercial method MICRONAUT-AM antifungal agents MIC (Bruker, Bremen, Germany).
2.3. Infection Prevention and Control Interventions
Following the finding of a positive urine culture for C. auris of a patient during surveillance standard screening, the Infection Control Office proceeded to identify an operative protocol capable of defining other potential cases and recommended a rigorous application of control measures. These measures included: rapid microbiological identification, isolation or cohort of cases, screening of contacts, antisepsis of colonized patients, cleaning and disinfection of the environment and subsequent follow-up sampling, according to available guidelines [16,17,18].
To remind about contact isolation, an educative poster was put with an indication to reduce the number of entries to the minimum, even to healthcare personnel. Educational activities were conducted about monitoring, contact precautions for non-ICU staff, while it was also recommended to use dedicated, disposable equipment where applicable and immediately disinfect/sterilize reusable equipment. Skin antisepsis was conducted for colonized patients with disposable wipes of non-alcoholic 2% chlorhexidine gluconate solution on alternate days.
Screening of patients was performed from axillary, inguinal and tracheal and nostril swabs. Screening was conducted for all close contacts including: all patients who shared the same hospital room in the same hospitalization period with the index case; those who were cared for by the same healthcare staff who handled the index case; and those who occupied the same bed of the index case despite cleaning and disinfection. Screening was repeated weekly in contacts who had a negative result. In addition, all patients hospitalized in the same period were weekly screened for fungi in respiratory and urine samples. In case of the positivity of fungal growth, a specific culture was carried out to rule out C. auris.
3. Results
3.1. Patient Characteristics
Overall, eight patients with C. auris colonization or infection were observed. Four (50%) patients were male; the median age of the population was 57.5 years old (IQR 52–61), with several comorbidities. Population characteristics are described in Table 1. Of note, 75% of patients were admitted to the ICU due to COVID-19-related acute respiratory distress syndrome (ARDS). Considering patients’ severity, the median Charlson Comorbidity Index was 3.5, while the median SOFA score was 7 (IQR 6–9), and the median SAPS II at ICU admission was 36.5 (IQR 30–40). The median hospital of stay was 41 days (IQR 26.5–83), and the median ICU stay was 33 days (IQR 24.5–50). The median sites of C. auris colonization were 1 (IQR 1–3), and the median time from admission to first C. auris detection was 24 days, with 100% of patients requiring mechanical ventilation. In 5 patients (62.5%), skin was the site of C. auris isolation; in 2 (25%), it was urine; in 1 (12.5%), it was the respiratory tract. The isolates were resistant to fluconazole with a MIC >128 μg/mL. The MIC was interpreted according to the criteria of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) of non-species-related breakpoints for Candida.

Table 1.
Characteristics of C. auris colonized/infected patients.
All eight patients received broad-spectrum antibiotic therapy for bacterial infections before identification of C. auris; 62.5% (5) had prior antifungal exposure (with 4/5 of them having previous colonization of other Candida species); 87.5% received steroids; 37.5% of patients used immunomodulatory drugs.
Out of eight patients with colonization, two cases (25%) were simultaneously infected and treated with antifungals as a part of empirical broad spectrum antibiotic treatment. In fact, one patient had candidemia due to C. auris, and another one presented with persistent fever and a possible urinary tract infection. Overall, the crude 28-day mortality rate was 50%.
3.2. Surveillance
In total, 66 environmental samples were collected. A total of 44 were positive for Gram-positive polymicrobial flora. However, they were normal cutaneous microbiota with a non-significant microbial load. Four positive samples of Enterobacteriaceae were revealed from closets in the corridor, which is considered as a clean area, the trolley and drawers. Of them, three had a bacterial load >50 CFU and were identified as Klebsiella pneumonia carbapenemase (KPC). Swabs from health-care workers’ hands were negative. Positivity for C. auris was never found on the surfaces investigated.
3.3. Infection Control
Daily environmental cleaning and disinfection were intensified for up to four times a day and in case of spills or visible dirt. High-touchable surfaces were disinfected using a chlorine-based solution with concentrations not lower than 1000 ppm. Terminal cleaning at the patient’s discharge was conducted with a dilution of 5000 ppm, followed by a nebulization with hydrogen peroxide [15,16,17]. Materials and furnishing that are metallic and/or intolerant to chlorine were treated with a disinfectant based on didecyldimethylammonium chloride and chlorhexidine digluconate. Finally, they were disinfected by passing a cloth soaked in chlorine-based solution. Follow-up sampling was carried post-disinfection. Figure 1 summarizes the multi-disciplinary infection control measures conducted to contain the spread of C. auris.
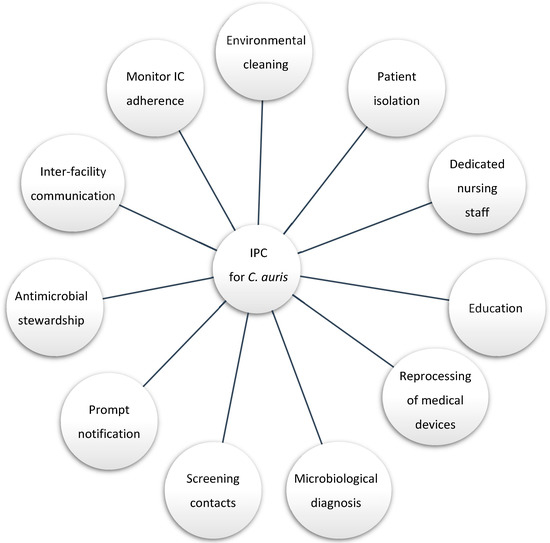
Figure 1.
Infection control practice (IPC) and stewardship cornerstone for C. auris management.
4. Discussion
The case series of C. auris here reported in the ICU represented to date the first outlined phenomenon in Piedmont, Italy.
Considering our cohort, it is crucial to mention the severity of the patients, such as the prolonged hospital stay of colonized/infected patients. Even though no risk factor was identified with this analysis, two-thirds of patients had COVID-19-related ARDS, requiring mechanical ventilation in line with what was recently reported by Briano et al. [13]. This finding suggests that a therapy with steroids or immunomodulatory agents could be a risk factor for C. auris isolation. Moreover, the use of broad-spectrum prior to C. auris isolation in all cases might suggest it as risk factor for C. auris acquisition similar to documented risk factors for Candida spp. invasive infections [19,20,21].
We also underline that, in our case series, many patients were immunosuppressed for prolonged hospitalizations and multiple complications, and presented simultaneous or previous presence of other infections, especially from Gram-negative, difficult-to-treat pathogens. In this sense, in addition to the already mentioned use of antimicrobials, and relevance of infection control measures, other factors may have influenced the selection of the microbiome, such as parenteral/enteral nutrition or previous bacterial colonizations or infections. Actually, the fact of colonization with C. auris is linked to the frailty of the patients due to comorbidities, invasive procedures and long hospital stays.
Nonetheless, it is not elucidated if a prior use of antifungals agents including echinocandins or a previous Candida spp. infection might induce a C. auris colonization or if it is rather due to clonal dispersion phenomenon as recently proven by a study on C. parapsilosis isolates harboring the Y132F ERG11 gene substitution that demonstrated that resistance to fluconazole was not attributable to prior azole use but rather to a group of fluconazole-resistant C. parapsilosis that have become endemic [22]. In our case series, two-thirds of C. auris patients received previous antifungals, of which more than a half were echinocandins. Interestingly, even though two isolated strains of C. auris showed in vitro resistance to all antifungals including echinocandins, only one case had a prior exposure to echinocandins. Although the development of infection and candidemia has been associated with multisite colonization as an independent risk factor in critically ill patients [13], in our cases, we did not observe a higher frequency of colonized sites in the two patients who developed infections.
One possible explanation for C. auris’ spread at our institution could be the high rate of transferred patients from peripheral hospitals during the COVID-19 pandemic as well as the high number of incoming patients from other peripheral regions for transplant evaluation and procedures, in which C. auris was previously described [13]. An essential aspect in infection control might be in fact the management of patient transfers between different hospitals: In case of the need for inter-hospital transfers, it is important that all hospitals can guarantee an adequate diagnostic standard.
Limitations of our report include the lack of comparison with controls not colonized by C. auris to confirm if colonization is a robust risk factor. In addition, the limitation due to environmental sampling meant that it was not possible to evaluate all possible surfaces that may have contributed to the spread. It is probable that the dissemination of the case series is an outbreak due to different factors such as: organism resistance, hospital transfers, invasive procedures and critically-ill patients, invasive procedures and breaches in infection control. Yet another limitation is that we could not identify the source of the transmission among different patients and molecularly or genomically type the similarity of the clone to confirm the modality of the spread.
5. Conclusions
In conclusion, we report here the first case series of C. auris in a regional referral ICU. In our case series, patients developed C. auris colonization after a long hospital stay in patients transferred from different hospitals with several comorbidities and previous bacterial infections. Few invasive infections were reported, supporting the finding that C. auris is a relevant infection control issue especially in the setting of fragile, critically ill patients, but its clinical role in determining invasive infections needs further data. Mortality in colonized patients was high (50%); however, the low number of invasive infections does not support a correlation between the pathogen and mortality.
Author Contributions
Conceptualization, S.C. and F.G.D.R.; methodology, S.C., G.M. and N.S.; investigation, I.D.B. and N.S.; resources, I.D.B., D.V., N.S., G.S., M.C., C.C., S.Z., T.Z., C.S., R.C. and L.B.; data curation, N.S.; writing—original draft preparation, I.D.B. and N.S.; writing—review and editing, S.C. and G.M.; supervision, F.G.D.R. and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Local Ethical Committee (Prot.n. 0008191).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
Due to patient confidentiality, raw data will be available upon request with a compelling reason.
Acknowledgments
All authors acknowledge the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Sheppard, A.E.; Madder, H.; Moir, I.; Moroney, R.; Quan, T.P.; Griffiths, D.; George, S.; Butcher, L.; Morgan, M.; et al. A Candida auris Outbreak and Its Control in an Intensive Care Setting. N. Engl. J. Med. 2018, 379, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.S.N.; Walraven, C.J.; Lee, S.A. Candida auris: Disinfectants and Implications for Infection Control. Front. Microbiol. 2018, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Gutiérrez-Ocampo, E.; Villamizar-Peña, R.; Holguin-Rivera, Y.; Escalera-Antezana, J.P.; Alvarado-Arnez, L.E.; Bonilla-Aldana, D.K.; Franco-Paredes, C.; Henao-Martinez, A.F.; et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 34, 101623. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, V.; Cabanero-Navalon, M.D.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.C.; Salavert, M.; Tormo, M.; Pemán, J. What Do We Know about Candida auris? State of the Art, Knowledge Gaps, and Future Directions. Microorganisms 2021, 9, 2177. [Google Scholar] [CrossRef] [PubMed]
- Kadri, S.S. Key Takeaways From the U.S. CDC’s 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit. Care Med. 2020, 48, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.M.; Dinh, A.Q.; Tran, T.T.; Arenas, S.; Pronty, D.; Gershengorn, H.B.; Ferreira, T.; Arias, C.A.; Shukla, B.S. Candida auris Invasive Infections during a COVID-19 Case Surge. Antimicrob. Agents Chemother. 2021, 65, e01146-21. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.Y.; Le Pape, P.; Lopez, O.; Esquea, K.; Labiosa, A.L.; Alvarez-Moreno, C. Candida auris: A latent threat to criti-cally ill patients with COVID-19. Clin. Infect. Dis. 2020, 73, e2836–e2837. [Google Scholar] [CrossRef]
- Magnasco, L.; Mikulska, M.; Giacobbe, D.; Taramasso, L.; Vena, A.; Dentone, C.; Dettori, S.; Tutino, S.; Labate, L.; Di Pilato, V.; et al. Spread of Carbapenem-Resistant Gram-Negatives and Candida auris During the COVID-19 Pandemic in Critically Ill Patients: One Step Back in Antimicrobial Stewardship? Microorganisms 2021, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Berkow, E.L.; Chow, N.; Welsh, R.M. Candida auris for the Clinical Microbiology Laboratory: Not Your Grandfather’s Candida Species. Clin. Microbiol. Newsl. 2017, 39, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plachouras, D.; Lötsch, F.; Kohlenberg, A.; Monnet, D.L. The Candida auris survey collaborative group. Candida auris: Epidemiological situation, laboratory capacity and preparedness in the European Union and European Economic Area*, January 2018 to May 2019. Eurosurveillance 2020, 25, 2000240. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Zhu, F.; Jiang, W.; Wang, Y.; Quan, Y.; Zhang, G.; Gu, F.; Yang, Y. Retrospective Analysis of the Clinical Characteristics of Candida auris Infection Worldwide From 2009 to 2020. Front. Microbiol. 2021, 12, 658329. [Google Scholar] [CrossRef] [PubMed]
- Briano, F.; Magnasco, L.; Sepulcri, C.; Dettori, S.; Dentone, C.; Mikulska, M.; Ball, L.; Vena, A.; Robba, C.; Patroniti, N.; et al. Candida auris Candidemia in Critically Ill, Colonized Patients: Cumulative Incidence and Risk Factors. Infect. Dis. Ther. 2022, 11, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses|Clinical Infectious Diseases|Oxford Academic. Available online: https://academic.oup.com/cid/article/64/2/134/2706620 (accessed on 8 June 2022).
- USEPA. List P: Antimicrobial Products Registered with EPA for Claims against Candida auris. 28 October 2020. Available online: https://www.epa.gov/pesticide-registration/list-p-antimicrobial-products-registered-epa-claims-against-candida-auris (accessed on 9 June 2022).
- Ong, C.W.; Chen, S.C.; Clark, J.E.; Halliday, C.L.; Kidd, S.E.; Marriott, D.J.; Marshall, C.L.; Morris, A.J.; Morrissey, C.O.; Roy, R.; et al. Diagnosis, management and prevention of Candida auris in hospitals: Position statement of the Australasian Society for Infectious Diseases. Intern. Med. J. 2019, 49, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Infection Prevention and Control for Candida auris|Candida auris|Fungal Diseases|CDC. 12 July 2021. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html (accessed on 9 June 2022).
- Screening for Candida auris Colonization|Candida auris|Fungal Diseases|CDC. 29 May 2020. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-screening.html (accessed on 9 June 2022).
- Snyder, G.M.; Wright, S.B. The Epidemiology and Prevention of Candida auris. Curr. Infect. Dis. Rep. 2019, 21, 19. [Google Scholar] [CrossRef]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- De Cássia Orlandi Sardi, J.; Silva, D.R.; Mendes-Giannini, M.J.S.; Rosalen, P.L. Candida auris: Epidemiology, risk factors, virulence, resistance, and therapeutic options. Microb. Pathog. 2018, 125, 116–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcoceba, E.; Gómez, A.; Lara-Esbrí, P.; Oliver, A.; Beltrán, A.F.; Ayestarán, I.; Muñoz, P.; Escribano, P.; Guinea, J. Fluconazole-resistant Candida parapsilosis clonally related genotypes: First report proving the presence of endemic isolates harbouring the Y132F ERG11 gene substitution in Spain. Clin. Microbiol. Infect. 2022, 28, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).