First Cases of Candida auris in a Referral Intensive Care Unit in Piedmont Region, Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Environmental Sampling
2.2. Microbiological Detection
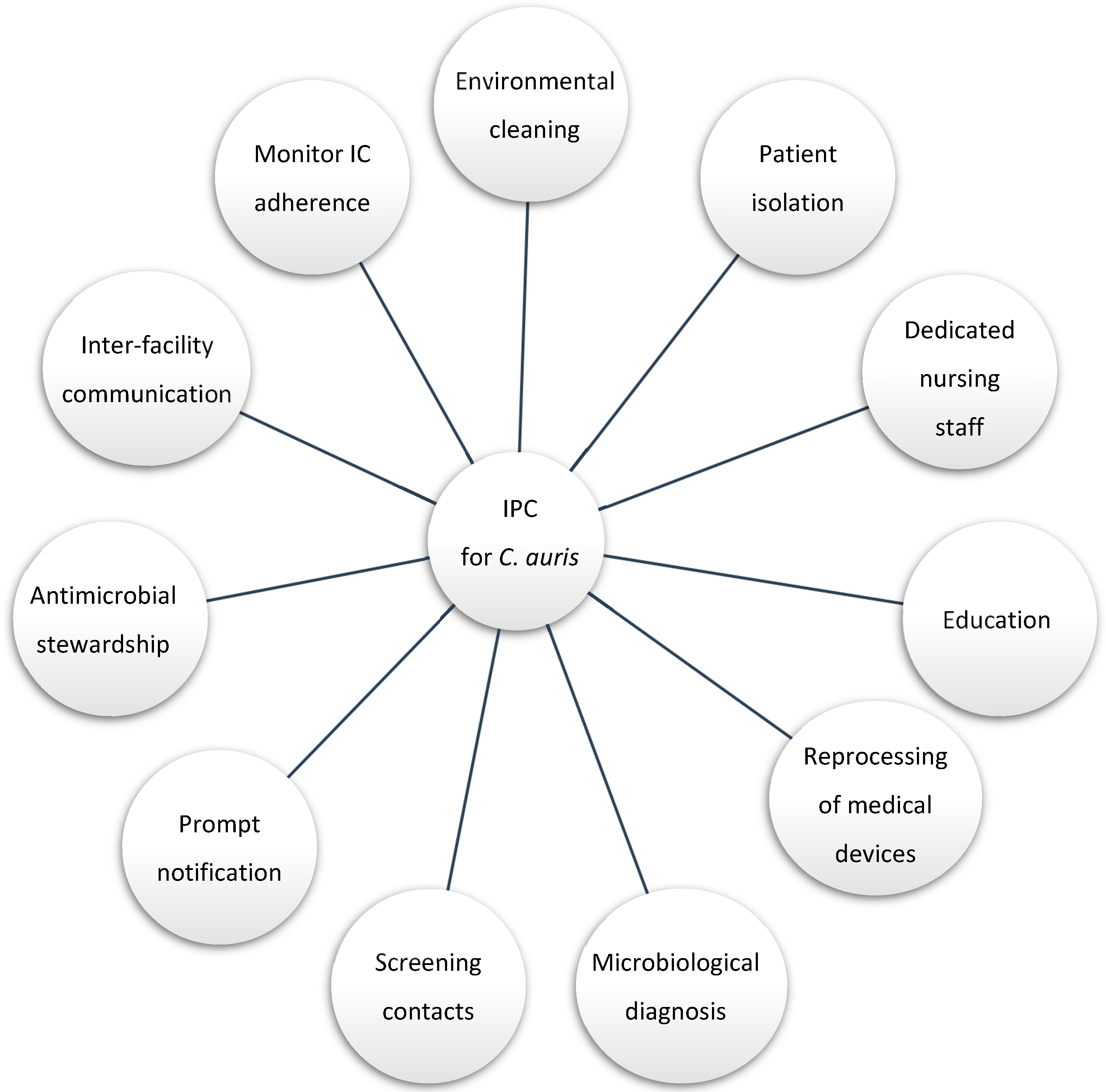
2.3. Infection Prevention and Control Interventions
3. Results
3.1. Patient Characteristics
3.2. Surveillance
3.3. Infection Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Sheppard, A.E.; Madder, H.; Moir, I.; Moroney, R.; Quan, T.P.; Griffiths, D.; George, S.; Butcher, L.; Morgan, M.; et al. A Candida auris Outbreak and Its Control in an Intensive Care Setting. N. Engl. J. Med. 2018, 379, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.S.N.; Walraven, C.J.; Lee, S.A. Candida auris: Disinfectants and Implications for Infection Control. Front. Microbiol. 2018, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Gutiérrez-Ocampo, E.; Villamizar-Peña, R.; Holguin-Rivera, Y.; Escalera-Antezana, J.P.; Alvarado-Arnez, L.E.; Bonilla-Aldana, D.K.; Franco-Paredes, C.; Henao-Martinez, A.F.; et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 34, 101623. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, V.; Cabanero-Navalon, M.D.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.C.; Salavert, M.; Tormo, M.; Pemán, J. What Do We Know about Candida auris? State of the Art, Knowledge Gaps, and Future Directions. Microorganisms 2021, 9, 2177. [Google Scholar] [CrossRef] [PubMed]
- Kadri, S.S. Key Takeaways From the U.S. CDC’s 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit. Care Med. 2020, 48, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.M.; Dinh, A.Q.; Tran, T.T.; Arenas, S.; Pronty, D.; Gershengorn, H.B.; Ferreira, T.; Arias, C.A.; Shukla, B.S. Candida auris Invasive Infections during a COVID-19 Case Surge. Antimicrob. Agents Chemother. 2021, 65, e01146-21. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.Y.; Le Pape, P.; Lopez, O.; Esquea, K.; Labiosa, A.L.; Alvarez-Moreno, C. Candida auris: A latent threat to criti-cally ill patients with COVID-19. Clin. Infect. Dis. 2020, 73, e2836–e2837. [Google Scholar] [CrossRef]
- Magnasco, L.; Mikulska, M.; Giacobbe, D.; Taramasso, L.; Vena, A.; Dentone, C.; Dettori, S.; Tutino, S.; Labate, L.; Di Pilato, V.; et al. Spread of Carbapenem-Resistant Gram-Negatives and Candida auris During the COVID-19 Pandemic in Critically Ill Patients: One Step Back in Antimicrobial Stewardship? Microorganisms 2021, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Berkow, E.L.; Chow, N.; Welsh, R.M. Candida auris for the Clinical Microbiology Laboratory: Not Your Grandfather’s Candida Species. Clin. Microbiol. Newsl. 2017, 39, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plachouras, D.; Lötsch, F.; Kohlenberg, A.; Monnet, D.L. The Candida auris survey collaborative group. Candida auris: Epidemiological situation, laboratory capacity and preparedness in the European Union and European Economic Area*, January 2018 to May 2019. Eurosurveillance 2020, 25, 2000240. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Zhu, F.; Jiang, W.; Wang, Y.; Quan, Y.; Zhang, G.; Gu, F.; Yang, Y. Retrospective Analysis of the Clinical Characteristics of Candida auris Infection Worldwide From 2009 to 2020. Front. Microbiol. 2021, 12, 658329. [Google Scholar] [CrossRef] [PubMed]
- Briano, F.; Magnasco, L.; Sepulcri, C.; Dettori, S.; Dentone, C.; Mikulska, M.; Ball, L.; Vena, A.; Robba, C.; Patroniti, N.; et al. Candida auris Candidemia in Critically Ill, Colonized Patients: Cumulative Incidence and Risk Factors. Infect. Dis. Ther. 2022, 11, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses|Clinical Infectious Diseases|Oxford Academic. Available online: https://academic.oup.com/cid/article/64/2/134/2706620 (accessed on 8 June 2022).
- USEPA. List P: Antimicrobial Products Registered with EPA for Claims against Candida auris. 28 October 2020. Available online: https://www.epa.gov/pesticide-registration/list-p-antimicrobial-products-registered-epa-claims-against-candida-auris (accessed on 9 June 2022).
- Ong, C.W.; Chen, S.C.; Clark, J.E.; Halliday, C.L.; Kidd, S.E.; Marriott, D.J.; Marshall, C.L.; Morris, A.J.; Morrissey, C.O.; Roy, R.; et al. Diagnosis, management and prevention of Candida auris in hospitals: Position statement of the Australasian Society for Infectious Diseases. Intern. Med. J. 2019, 49, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Infection Prevention and Control for Candida auris|Candida auris|Fungal Diseases|CDC. 12 July 2021. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html (accessed on 9 June 2022).
- Screening for Candida auris Colonization|Candida auris|Fungal Diseases|CDC. 29 May 2020. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-screening.html (accessed on 9 June 2022).
- Snyder, G.M.; Wright, S.B. The Epidemiology and Prevention of Candida auris. Curr. Infect. Dis. Rep. 2019, 21, 19. [Google Scholar] [CrossRef]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- De Cássia Orlandi Sardi, J.; Silva, D.R.; Mendes-Giannini, M.J.S.; Rosalen, P.L. Candida auris: Epidemiology, risk factors, virulence, resistance, and therapeutic options. Microb. Pathog. 2018, 125, 116–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcoceba, E.; Gómez, A.; Lara-Esbrí, P.; Oliver, A.; Beltrán, A.F.; Ayestarán, I.; Muñoz, P.; Escribano, P.; Guinea, J. Fluconazole-resistant Candida parapsilosis clonally related genotypes: First report proving the presence of endemic isolates harbouring the Y132F ERG11 gene substitution in Spain. Clin. Microbiol. Infect. 2022, 28, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
| ID | Sex, Age | Hospital Stay (days) | ICU stay (days) | Death | Comorbidities | COVID-19 | Site of Isolation (1) | Site of Isolation (2) | Subsequent Infection Type | Antifungal Treatment for C. auris | Mechanical Ventilation | Steroids | Immuno-Modulatory Agents | Previous Broad-Spectrum ATB | Previous Antifungal tp | Other Infections | Microorganism |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M 44 | 47 | 35 | Yes | Autoimune disease, respiratory disease, smoker | No | skin | skin | Colonization | No | Yes | Yes | Yes | Yes | Yes | CAP | S. marcescensPJP |
| 2 | F 58 | 35 | 31 | No | Smoker, HTA | No | urine | skin | Colonization | No | Yes | No | No | Yes | No | VAP | P. aeruginosa, S. marcescens |
| 3 | M 64 | 100+ | 35 | No | N/A | Yes | skin | - | Colonization-Suspected UTI | Yes—Anidulafungin | Yes | Yes | No | Yes | No | VAP/BSI | M. morgani/KPC, E.faecalis |
| 4 | M 64 | 16 | 14 | Yes | Respiratory disease, smoker, HTA, DMNID | Yes | skin | - | Colonization | No | Yes | Yes | No | Yes | Yes | VAP/BSI/CAPA | A.baumannii + KP/E.faecium VRE |
| 5 | F 49 | 25 | 22 | Yes | Respiratory disease, HTA, DMNID, autoimmune disease | Yes | skin | - | Colonization | No | Yes | Yes | No | Yes | No | VAP | A.baumannii + KP ESBL |
| 6 | M 57 | 28 | 27 | Yes | Autoimmune disease | Yes | urine | - | Colonization | No | Yes | Yes | Yes | Yes | Yes | VAP/BSI | P. aeruginosa/C. albicans |
| 7 | F 55 | 100+ | 100+ | No | HTA, hemathological disease, malignancy | Yes | respiratory tract | blood | Colonization-Infection | Yes—Anidulafungin, Ambisome | Yes | Yes | Yes | Yes | Yes | VAP/BSI | KPC/C. albicans |
| 8 | F 58 | 66 | 65 | No | respiratory disease, HTA, DMNID, autoimmune disease | Yes | Skin | - | Colonization | No | Yes | Yes | No | Yes | Yes | VAP/BSI | MRSA/KPC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corcione, S.; Montrucchio, G.; Shbaklo, N.; De Benedetto, I.; Sales, G.; Cedrone, M.; Vita, D.; Costa, C.; Zozzoli, S.; Zaccaria, T.; et al. First Cases of Candida auris in a Referral Intensive Care Unit in Piedmont Region, Italy. Microorganisms 2022, 10, 1521. https://doi.org/10.3390/microorganisms10081521
Corcione S, Montrucchio G, Shbaklo N, De Benedetto I, Sales G, Cedrone M, Vita D, Costa C, Zozzoli S, Zaccaria T, et al. First Cases of Candida auris in a Referral Intensive Care Unit in Piedmont Region, Italy. Microorganisms. 2022; 10(8):1521. https://doi.org/10.3390/microorganisms10081521
Chicago/Turabian StyleCorcione, Silvia, Giorgia Montrucchio, Nour Shbaklo, Ilaria De Benedetto, Gabriele Sales, Martina Cedrone, Davide Vita, Cristina Costa, Susanna Zozzoli, Teresa Zaccaria, and et al. 2022. "First Cases of Candida auris in a Referral Intensive Care Unit in Piedmont Region, Italy" Microorganisms 10, no. 8: 1521. https://doi.org/10.3390/microorganisms10081521
APA StyleCorcione, S., Montrucchio, G., Shbaklo, N., De Benedetto, I., Sales, G., Cedrone, M., Vita, D., Costa, C., Zozzoli, S., Zaccaria, T., Silvestre, C., Cavallo, R., Brazzi, L., & De Rosa, F. G. (2022). First Cases of Candida auris in a Referral Intensive Care Unit in Piedmont Region, Italy. Microorganisms, 10(8), 1521. https://doi.org/10.3390/microorganisms10081521









