Biosurfactant from Bacillus subtilis DS03: Properties and Application in Cleaning Out Place System in a Pilot Sausages Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of the Biosurfactant Crude Extract
2.3. Evaluation of Physical-Chemical Properties of BCE
2.4. Anti-Adhesion Assay on Polystyrene Surface
2.5. Antimicrobial Activity of BCE: Time-Kill Curve
2.6. Design of the Cleaning Out-of-Place System (COP) Using BCE
2.6.1. Description of the COP Cleaning Method
2.6.2. Microbiological Verification
2.7. Statistics
3. Results and Discussion
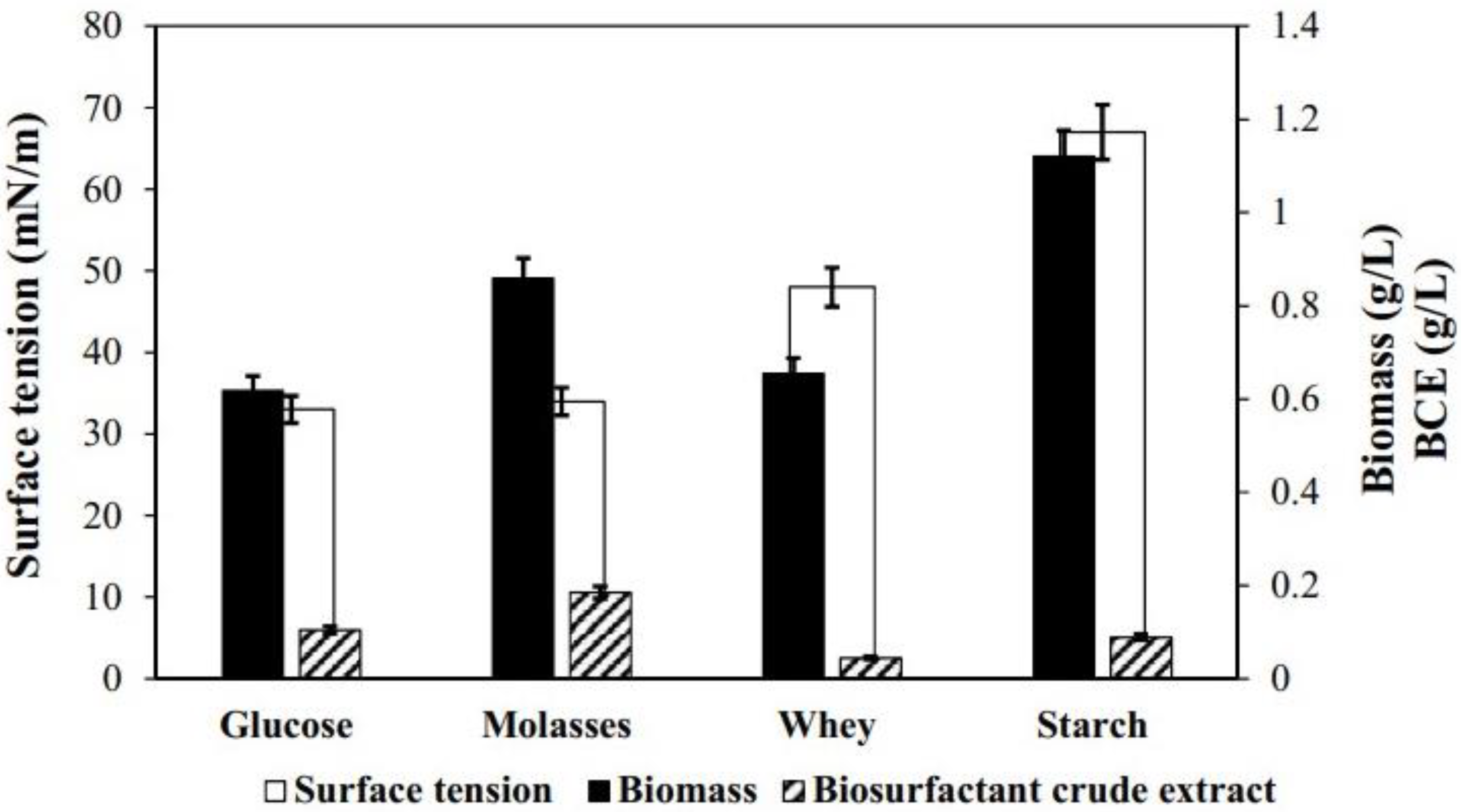
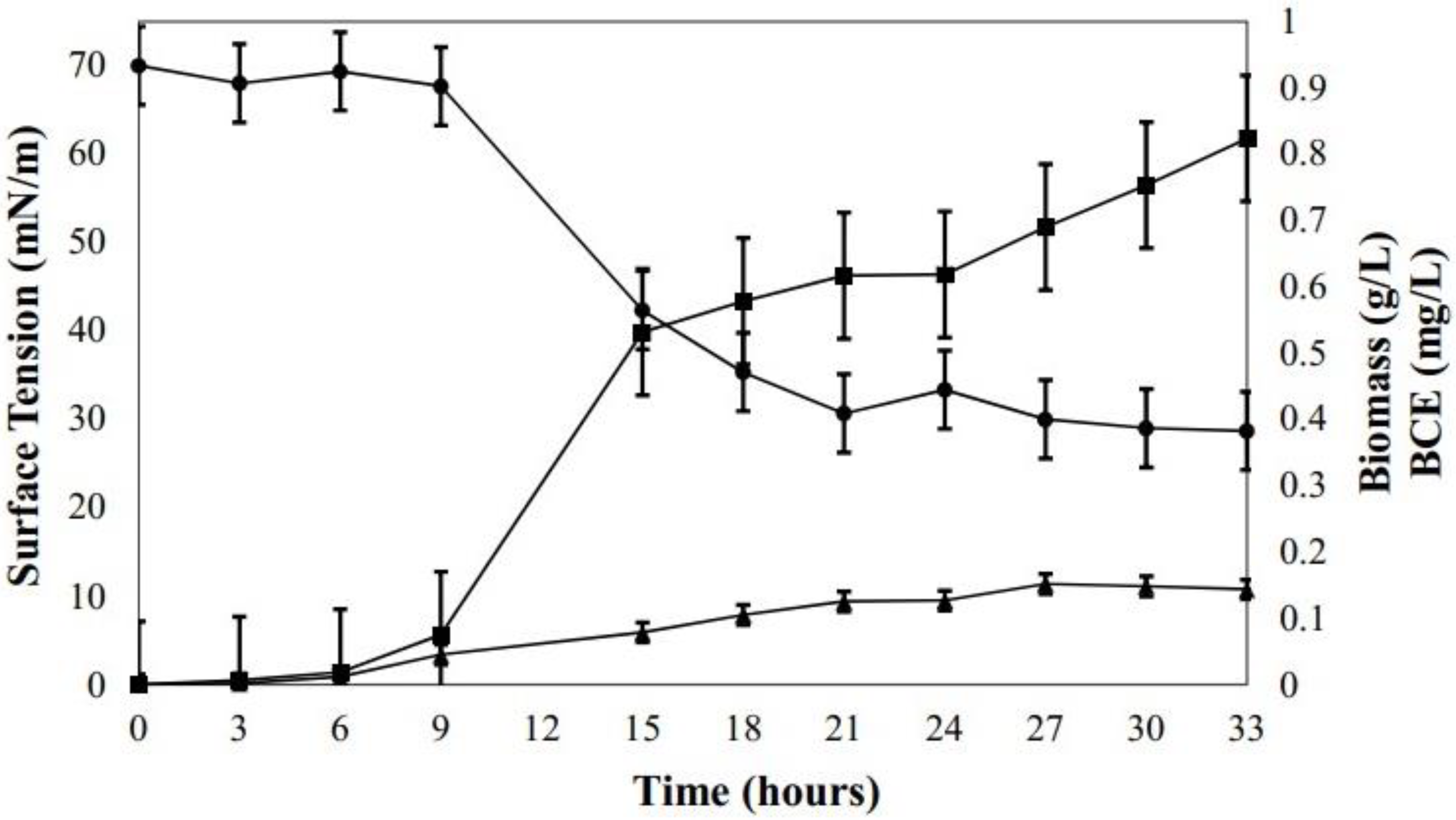
3.1. Production of Biosurfactant
3.2. Physical-Chemical and Biological Properties of BCE
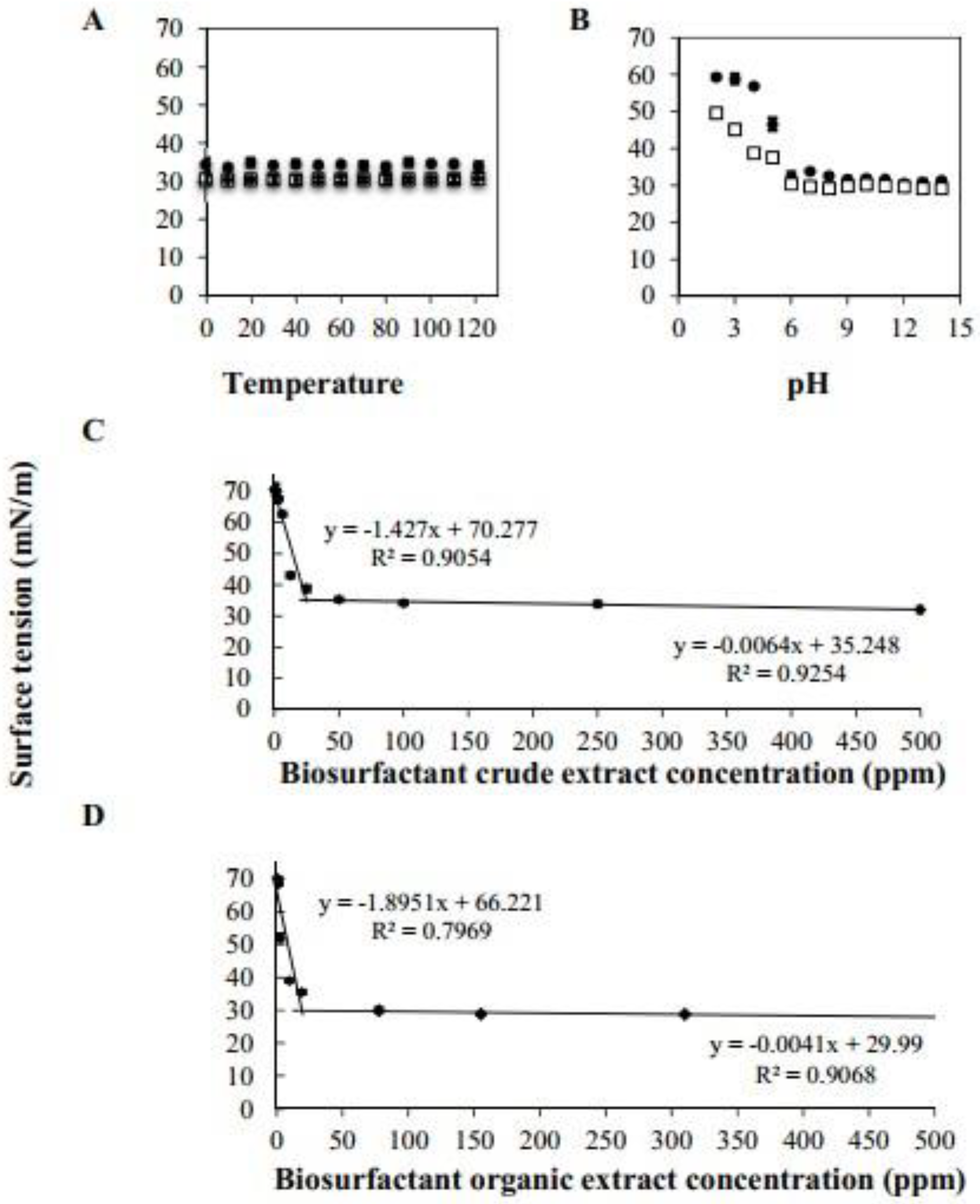
3.2.1. Effect of pH and Temperature on BCE Action
3.2.2. Emulsifying Activity
3.2.3. Determination of Critical Micellar Concentration (CMC)
3.3. Antiadhesion Assay on Polystyrene Surface
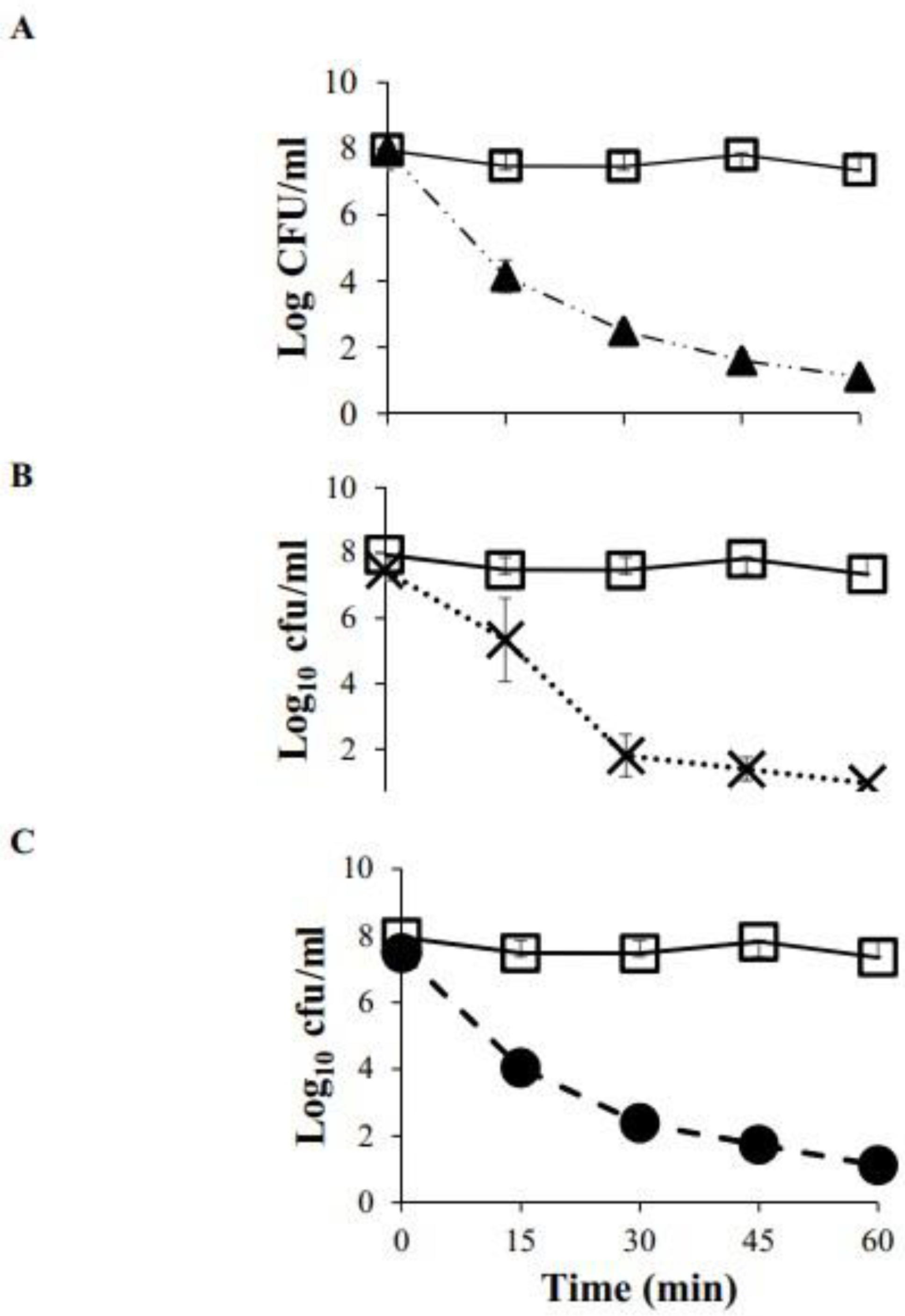
3.4. Evaluation of Antimicrobial Activity
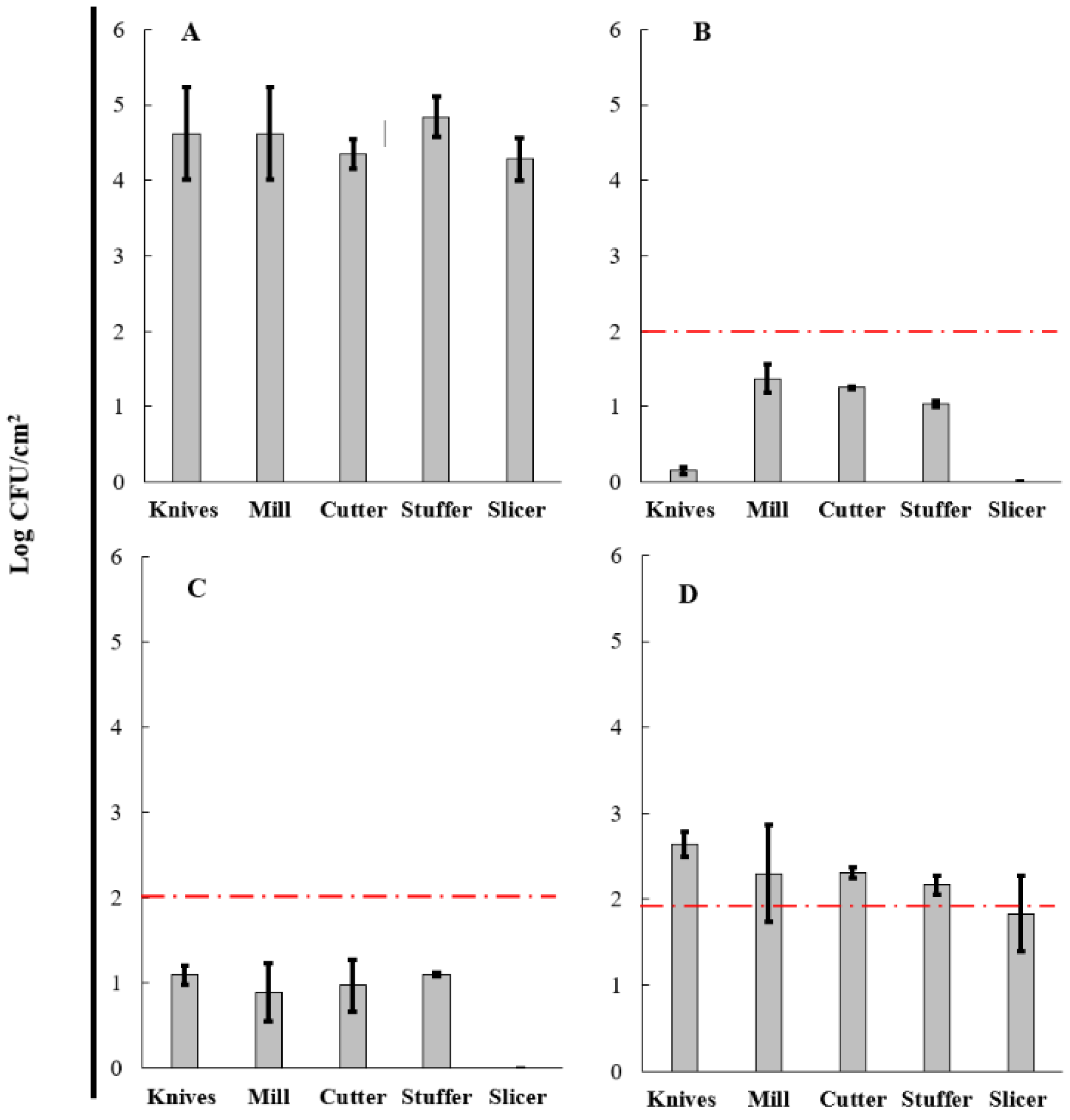
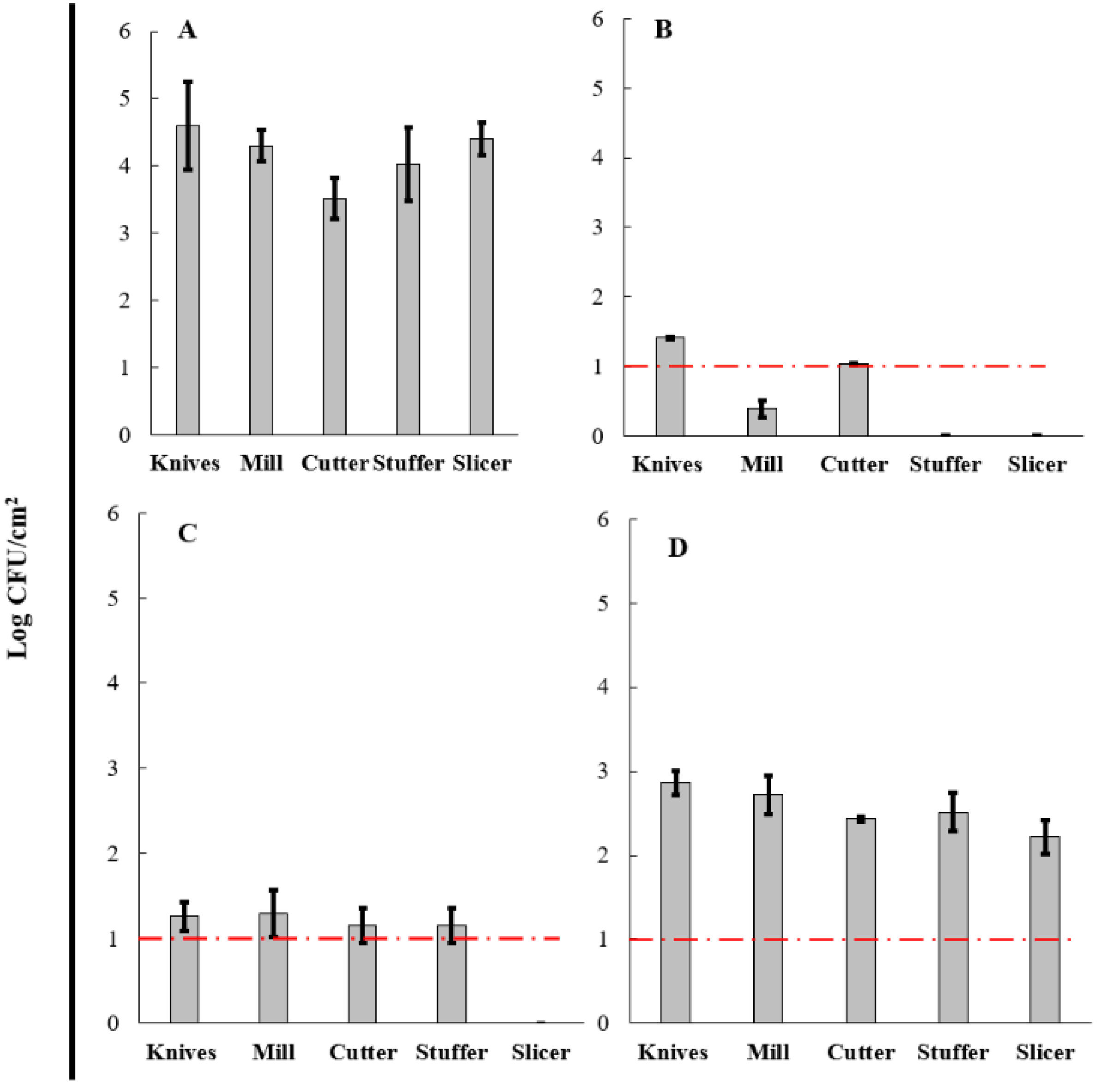
3.5. Evaluation of BCE in a Cleaning-Out-Of-Place (COP) Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madoumier, M.; Azzaro-Pantel, C.; Gésan-Guiziou, G. Including cleaning and production phases in the eco-design of a milk evaporation process. Food Bioprod. Process. 2020, 123, 427–436. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, V.P.; Harika, K.; Narasimhaswamy, N.; Chawla, K. Detection of biofilm production and its impact on antibiotic resistance profile of bacterial isolates from chronic wound infections. J. Glob. Infect. Dis. 2020, 12, 129–134. [Google Scholar] [CrossRef]
- Yuan, Y.; Qu, K.; Tan, D.; Li, X.; Wang, L.; Cong, C.; Xiu, Z.; Xu, Y. Isolation and characterization of a bacteriophage and its potential to disrupt multi-drug resistant Pseudomonas aeruginosa biofilms. Microb. Pathog. 2019, 128, 329–336. [Google Scholar] [CrossRef]
- Nitschke, M.; Silva, S.S.E. Recent food applications of microbial surfactants. Crit. Rev. Food Sci. Nutr. 2018, 58, 631–638. [Google Scholar] [CrossRef]
- Liu, P.; Wang, G.; Ruan, Q.; Tang, K.; Chu, P.K. Plasma-activated interfaces for biomedical engineering. Bioact. Mater. 2021, 6, 2134–2143. [Google Scholar] [CrossRef] [PubMed]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety—A Review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Olaniran, A.O. Production and potential biotechnological applications of microbial surfactants: An overview. Saudi J. Biol. Sci. 2020, 28, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Fei, D.; Zhou, G.; Yu, Z.; Gang, H.; Liu, J.; Yang, S.; Ye, R.; Mu, B. Low-Toxic and Nonirritant Biosurfactant Surfactin and its Performances in Detergent Formulations. J. Surfactants Deterg. 2019, 23, 109–118. [Google Scholar] [CrossRef]
- Paulo, A.M.S.; Aydin, R.; Dimitrov, M.R.; Vreeling, H.; Cavaleiro, A.; Garcia-Encina, P.A.; Stams, A.J.M.; Plugge, C.M. Sodium lauryl ether sulfate (SLES) degradation by nitrate-reducing bacteria. Appl. Microbiol. Biotechnol. 2017, 101, 5163–5173. [Google Scholar] [CrossRef]
- Rissoli, R.Z.; Abdalla, F.C.; Costa, M.J.; Rantin, F.T.; McKenzie, D.J.; Kalinin, A.L. Effects of glyphosate and the glyphosate based herbicides Roundup Original® and Roundup Transorb® on respiratory morphophysiology of bullfrog tadpoles. Chemosphere 2016, 156, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Latef, S.E.; AL-Azawey, A.S.N. Study of effect of some surfactants on water quality of Al-Hilla river, Iraq. Plant Arch. 2020, 20, 99–105. [Google Scholar]
- Dey, G.; Bharti, R.; Sen, R.; Mandal, M. Microbial amphiphiles: A class of promising new-generation anticancer agents. Drug Discov. Today 2015, 20, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Nounou, M.I.; Zaghloul, T.I.; Ahmed, N.A.; Eid, A.A.; El-Khordagui, L.K. Skin permeability enhancement by Bacillus subtilis alkaline protease: Application to transdermal drug delivery. Int. J. Pharm. 2017, 529, 423–432. [Google Scholar] [CrossRef]
- De Giani, A.; Zampolli, J.; Di Gennaro, P. Recent Trends on Biosurfactants with Antimicrobial Activity Produced by Bacteria Associated with Human Health: Different Perspectives on Their Properties, Challenges, and Potential Applications. Front. Microbiol. 2021, 12, 655150. [Google Scholar] [CrossRef]
- Mohsin, M.Z.; Omer, R.; Huang, J.; Mohsin, A.; Guo, M.; Qian, J.; Zhuang, Y. Advances in engineered Bacillus subtilis biofilms and spores, and their applications in bioremediation, biocatalysis, and biomaterials. Synth. Syst. Biotechnol. 2021, 6, 180–191. [Google Scholar] [CrossRef]
- Théatre, A.; Cano-Prieto, C.; Bartolini, M.; Laurin, Y.; Deleu, M.; Niehren, J.; Fida, T.; Gerbinet, S.; Alanjary, M.; Medema, M.H.; et al. The Surfactin-Like Lipopeptides from Bacillus spp.: Natural Biodiversity and Synthetic Biology for a Broader Application Range. Front. Bioeng. Biotechnol. 2021, 9, 623701. [Google Scholar] [CrossRef]
- Coronel-León, J.; Marqués, A.M.; Bastida, J.; Manresa, A. Optimizing the production of the biosurfactant lichenysin and its application in biofilm control. J. Appl. Microbiol. 2016, 120, 99–111. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.-S.; Wong, J.W.C.; Bui, X.-T. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb. Cell Factories 2021, 20, 1–13. [Google Scholar] [CrossRef]
- Zainudin, M.H.M.; Zulkarnain, A.; Azmi, A.S.; Muniandy, S.; Sakai, K.; Shirai, Y.; Hassan, M.A. Enhancement of Agro-Industrial Waste Composting Process via the Microbial Inoculation: A Brief Review. Agronomy 2022, 12, 198. [Google Scholar] [CrossRef]
- Valenzuela Ruiz, V.; Gálvez Gamboa, G.T.; Villa Rodríguez, E.D.; Parra Cota, F.I.; Santoyo, G.; Santos-Villalobos, S.D.L. Lipopeptides produced by biological control agents of the genus Bacillus: A review of analytical tools used for their study. Rev. Mex. De Cienc. Agrícolas 2020, 11, 419–432. [Google Scholar] [CrossRef]
- Serrano, L.; Moreno, A.S.; Castillo, D.S.D.; Bonilla, J.; Romero, C.A.; Galarza, L.L.; Coronel-León, J.R. Biosurfactants synthesized by endophytic Bacillus strains as control of Moniliophthora perniciosa and Moniliophthora roreri Lizette. Sci. Agric. 2021, 78, 1–11. [Google Scholar] [CrossRef]
- Coronel-León, J.; de Grau, G.; Grau-Campistany, A.; Farfan, M.; Rabanal, F.; Manresa, A.; Marqués, A.M. Biosurfactant production by AL 1.1, a Bacillus licheniformis strain isolated from Antarctica: Production, chemical characterization and properties. Ann. Microbiol. 2015, 65, 2065–2078. [Google Scholar] [CrossRef]
- Romero-Peña, M.; Ng, E.K.; Ghosh, S. Development of thermally stable coarse water-in-oil emulsions as potential DNA bioreactors. J. Dispers. Sci. Technol. 2021, 42, 2075–2084. [Google Scholar] [CrossRef]
- Coronel-León, J.; Pinazo, A.; Pérez, L.; Espuny, M.J.; Marqués, A.M.; Manresa, A. Lichenysin-geminal amino acid-based surfactants: Synergistic action of an unconventional antimicrobial mixture. Colloids Surf. B Biointerfaces 2017, 149, 38–47. [Google Scholar] [CrossRef]
- Mouafo, T.H.; Mbawala, A.; Ndjouenkeu, R. Effect of Different Carbon Sources on Biosurfactants’ Production by Three Strains of Lactobacillus spp. BioMed Res. Int. 2018, 2018, 5034783. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Jiang, H. Microbial production of value-added bioproducts and enzymes from molasses, a by-product of sugar industry. Food Chem. 2021, 346, 128860. [Google Scholar] [CrossRef]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Varjani, S.; Taherzadeh, M.J.; Chang, J.-S.; Ng, H.Y.; Wong, J.W.; Kim, S.-H. Production of biosurfactants from agro-industrial waste and waste cooking oil in a circular bioeconomy: An overview. Bioresour. Technol. 2022, 343, 126059. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, J.; Verma, N. Statistical optimization and comparative study of lipopeptides produced by Bacillus amyloliquefaciens SAS-1 and Bacillus subtilis BR-15. Biocatal. Agric. Biotechnol. 2019, 25, 101575. [Google Scholar] [CrossRef]
- Fonseca, R.R.; Silva, A.J.R.; De França, F.P.; Cardoso, V.L.; Sérvulo, E.F.C. Optimizing carbon/nitrogen ratio for biosurfactant production by a Bacillus subtilis strain. Appl. Biochem. Biotechnol. 2007, 136–140, 471–486. [Google Scholar] [CrossRef]
- Heryani, H.; Putra, M.D. Dataset on potential large scale production of biosurfactant using Bacillus sp. Data Brief 2017, 13, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Djordjević, M.; Šereš, Z.; Maravić, N.; Djordjević, M. Chapter 3. Molasses: Desugarization Processes and Purification Treatments. In Molasses: Forms, Production and Uses; Maddison, K., Fuller, R., Eds.; Nova Science Publishers: Hauppage, NY, USA, 2019; pp. 97–123. [Google Scholar]
- Karlapudi, A.P.; Venkateswarulu, T.; Tammineedi, J.; Kanumuri, L.; Ravuru, B.K.; Dirisala, V.R.; Kodali, V.P. Role of biosurfactants in bioremediation of oil pollution-a review. Petroleum 2018, 4, 241–249. [Google Scholar] [CrossRef]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Priyadharsini, G.B.; Poulose, N.; Selvin, J. Production of Lipopeptide Biosurfactant by a Marine Nesterenkonia sp. and Its Application in Food Industry. Front. Microbiol. 2017, 8, 1138. [Google Scholar] [CrossRef]
- Balan, S.S.; Kumar, C.G.; Jayalakshmi, S. Pontifactin, a new lipopeptide biosurfactant produced by a marine Pontibacter korlensis strain SBK-47: Purification, characterization and its biological evaluation. Process Biochem. 2016, 51, 2198–2207. [Google Scholar] [CrossRef]
- Hentati, D.; Chebbi, A.; Hadrich, F.; Frikha, I.; Rabanal, F.; Sayadi, S.; Manresa, A.; Chamkha, M. Production, characterization and biotechnological potential of lipopeptide biosurfactants from a novel marine Bacillus stratosphericus strain FLU5. Ecotoxicol. Environ. Saf. 2019, 167, 441–449. [Google Scholar] [CrossRef]
- Ahire, J.J.; Kashikar, M.S.; Lakshmi, S.G.; Madempudi, R. Identification and characterization of antimicrobial peptide produced by indigenously isolated Bacillus paralicheniformis UBBLi30 strain. 3 Biotech 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Schultz, J.; Rosado, A.S. Extreme environments: A source of biosurfactants for biotechnological applications. Extremophiles 2019, 24, 189–206. [Google Scholar] [CrossRef]
- Suryanti, V.; Handayani, D.S.; Marliyana, S.D.; Suratmi, S. Physicochemical Properties of Biosurfactant Produced by Pseudomonas fluorescens Grown on Whey Tofu. IOP Conf. Ser. Mater. Sci. Eng. 2017, 176, 012003. [Google Scholar] [CrossRef]
- Essien, E.; Antia, B. Lagenaria siceraria (Mol.) Standley. Properties of Seed Oils and Variability in Fatty Acids Composition of Ten Cultivars Essential Oils and Biological Activities of Medical-Aromatic Plants of Nigeria View Project. 2013. Available online: https://www.researchgate.net/publication/318661738 (accessed on 3 February 2022).
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Koim-Puchowska, B.; Kłosowski, G.; Mikulski, D.; Menka, A. Evaluation of various methods of selection of B. subtilis strains capable of secreting surface-active compounds. PLoS ONE 2019, 14, e0225108. [Google Scholar] [CrossRef] [PubMed]
- Ezeofor, C.O. Effect of Change in Hydrophilic-Lipophilic Balance (HLB) on the State of Stable Invert Emulsion Drilling Fluid. Master’s Thesis, University of Cagliari, Cagliari, Italy, 2015. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena Surfactants and Interfacial Phenomena; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Zhao, Y.; Yang, S.Z.; Mu, B.Z. Quantitative Analyses of the Isoforms of Surfactin Produced by Bacillus subtilis HSO 121 Using GC-MS. Anal. Sci. 2012, 28, 789–793. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22878634 (accessed on 20 February 2022). [CrossRef] [PubMed]
- Slivinski, C.T.; Mallmann, E.; de Araújo, J.M.; Mitchell, D.A.; Krieger, N. Production of surfactin by Bacillus pumilus UFPEDA 448 in solid-state fermentation using a medium based on okara with sugarcane bagasse as a bulking agent. Process. Biochem. 2012, 47, 1848–1855. [Google Scholar] [CrossRef]
- Ładniak, A.; Jurak, M.; Palusińska-Szysz, M.; Wiącek, A.E. The Influence of Polysaccharides/TiO2 on the Model Membranes of Dipalmitoylphosphatidylglycerol and Bacterial Lipids. Molecules 2022, 27, 343. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Douglas, S.I.; Stanley, H.O. A Review on Microbial Surfactants: Production, Classifications, Properties and Characterization. J. Adv. Microbiol. 2019, 18, 1–22. [Google Scholar] [CrossRef]
- Industry Coalition for the SIDS Assessment of LAS. OECD SIDS LINEAR ALKYLBENZENE SULFONATE (LAS). UNEP PUBLICATIONS. 2005, pp. 1–357. Available online: https://hpvchemicals.oecd.org/ui/handler.axd?id=5b837fb0-350c-4742-914e-5f6513df120a (accessed on 4 March 2022).
- Sulek, M.W.; Wasilewski, T.; Kurzydłowski, K.J. The Effect of Concentration on Lubricating Properties of Aqueous Solutions of Sodium Lauryl Sulfate and Ethoxylated Sodium Lauryl Sulfate. Tribol. Lett. 2010, 40, 337–345. [Google Scholar] [CrossRef]
- Yuan, L.; Hansen, M.F.; Roeder, H.L.; Wang, N.; Burmølle, M.; He, G. Mixed-species biofilms in the food industry: Current knowledge and novel control strategies. Crit. Rev. Food Sci. Nutr. 2019, 60, 2277–2293. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, P. Disinfectant-like activity of lipopeptide biosurfactant produced by Bacillus tequilensis strain SDS21. Colloids Surf. B Biointerfaces 2020, 185, 110514. [Google Scholar] [CrossRef]
- de Araujo, L.V.; Guimarães, C.R.; Marquita, R.L.D.S.; Santiago, V.M.; de Souza, M.P.; Nitschke, M.; Freire, D.M.G. Rhamnolipid and surfactin: Anti-adhesion/antibiofilm and antimicrobial effects. Food Control 2016, 63, 171–178. [Google Scholar] [CrossRef]
- Smith, M.; Gandolfi, S.; Coshall, P.M.; Rahman, P.K.S.M. Biosurfactants: A COVID-19 Perspective. Front. Microbiol. 2020, 11, 1341. [Google Scholar] [CrossRef]
- American Public Health Association; Andrew, D.; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005; APHA-AWWA-WEF. [Google Scholar]
- Sneed, J.; Strohbehn, C.; Gilmore, S.A.; Mendonca, A. Microbiological evaluation of foodservice contact surfaces in Iowa assisted-living facilities. J. Am. Diet. Assoc. 2004, 104, 1722–1724. [Google Scholar] [CrossRef] [PubMed]
- Aranda, F.J.; Teruel, J.A.; Ortiz, A. Further aspects on the hemolytic activity of the antibiotic lipopeptide iturin A. Biochim. Biophys. Acta BBA Biomembr. 2005, 1713, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Coronel, J.R.; Marqués, A.; Manresa, A.; Aranda, F.J.; Teruel, J.A.; Ortiz, A. Interaction of the Lipopeptide Biosurfactant Lichenysin with Phosphatidylcholine Model Membranes. Langmuir 2017, 33, 9997–10005. [Google Scholar] [CrossRef] [PubMed]
- Brasil, C.C.B.; Barin, J.S.; Jacob-Lopes, E.; Menezes, C.R.; Zepka, L.Q.; Wagner, R.; Campagnol, P.C.B.; Cichoski, A.J. Single step non-thermal cleaning/sanitation of knives used in meat industry with ultrasound. Food Res. Int. 2017, 91, 133–139. [Google Scholar] [CrossRef] [PubMed]

| Raw Material | % Composition |
|---|---|
| Lean pork | 40 |
| Lean beef | 20 |
| Fat | 15 |
| Pork leather | 15 |
| Isolated soy protein | 5 |
| Nitrites | 1 |
| Seasonings | 4 |
| Surfaces | (%) Reduction in Aerobic Mesophilic Microorganisms | (%) Reduction in Total Coliforms | ||||
|---|---|---|---|---|---|---|
| COP-A | COP-B | COP-C | COP-A | COP-B | COP-C | |
| Knives | 96.75 | 76.13 | 42.94 | 66.88 | 70.45 | 33.02 |
| Mill | 69.13 | 79.95 | 48.08 | 90.95 | 69.91 | 36.81 |
| Cutter | 71.28 | 77.76 | 46.90 | 70.46 | 67.24 | 30.95 |
| Stuffer | 78.53 | 77.34 | 55.09 | 100.00 | 71.36 | 37.60 |
| Slicer | 100.00 | 100.00 | 57.35 | 100.00 | 100.00 | 49.55 |
| Average reduction | 83.14 | 82.24 | 50.07 | 85.66 | 75.79 | 35.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz Mendoza, I.; Villavicencio-Vasquez, M.; Aguayo, P.; Coello Montoya, D.; Plaza, L.; Romero-Peña, M.; Marqués, A.M.; Coronel-León, J. Biosurfactant from Bacillus subtilis DS03: Properties and Application in Cleaning Out Place System in a Pilot Sausages Processing. Microorganisms 2022, 10, 1518. https://doi.org/10.3390/microorganisms10081518
Cruz Mendoza I, Villavicencio-Vasquez M, Aguayo P, Coello Montoya D, Plaza L, Romero-Peña M, Marqués AM, Coronel-León J. Biosurfactant from Bacillus subtilis DS03: Properties and Application in Cleaning Out Place System in a Pilot Sausages Processing. Microorganisms. 2022; 10(8):1518. https://doi.org/10.3390/microorganisms10081518
Chicago/Turabian StyleCruz Mendoza, Iana, Mirian Villavicencio-Vasquez, Paola Aguayo, Diana Coello Montoya, Luis Plaza, María Romero-Peña, Ana M. Marqués, and Jonathan Coronel-León. 2022. "Biosurfactant from Bacillus subtilis DS03: Properties and Application in Cleaning Out Place System in a Pilot Sausages Processing" Microorganisms 10, no. 8: 1518. https://doi.org/10.3390/microorganisms10081518
APA StyleCruz Mendoza, I., Villavicencio-Vasquez, M., Aguayo, P., Coello Montoya, D., Plaza, L., Romero-Peña, M., Marqués, A. M., & Coronel-León, J. (2022). Biosurfactant from Bacillus subtilis DS03: Properties and Application in Cleaning Out Place System in a Pilot Sausages Processing. Microorganisms, 10(8), 1518. https://doi.org/10.3390/microorganisms10081518






